Abstract
A class of oligonucleotides which binds to naturally-occurring duplex DNA sites at physiologic pH to form triple helical structures was used as transcription attenuators in an in vitro transcription assay. Oligonucleotides were designed to form triple helices with a purine-rich, double-stranded target by binding in the major groove in an orientation anti-parallel to the most purine-rich strand of the target. A 45 base-pair purine-rich region located within the gag gene of Friend Murine Leukemia Virus (FMLV) was used as the duplex target. The target DNA was inserted by molecular cloning downstream of either the bacterial T7- or T3 promoter. The sequence-specific interaction of the triple helix-forming oligonucleotide (TFO) with the FMLV target was confirmed by DNAse I footprint analysis. The affinity of the TFO, as measured by the equilibrium dissociation constant of the TFO for the duplex, was determined by band shift analysis. When a TFO was allowed to form a triple helix with the target duplex in well-defined buffer conditions before the transcription reaction, truncated transcripts of a predicted size were observed. Attenuation of transcription was observed only when buffer conditions favorable to triple helix formation were used. In addition, oligonucleotides containing a high percentage of guanosine residues were able to inhibit mRNA production of the bacterial T7 polymerase by a mechanism independent of transcription attenuation. The ability of an oligonucleotide-directed triple helical structure to slow down, or even completely stop, RNA chain elongation may expand the utility of triple helix technology in the area of gene regulation.
Full text
PDF


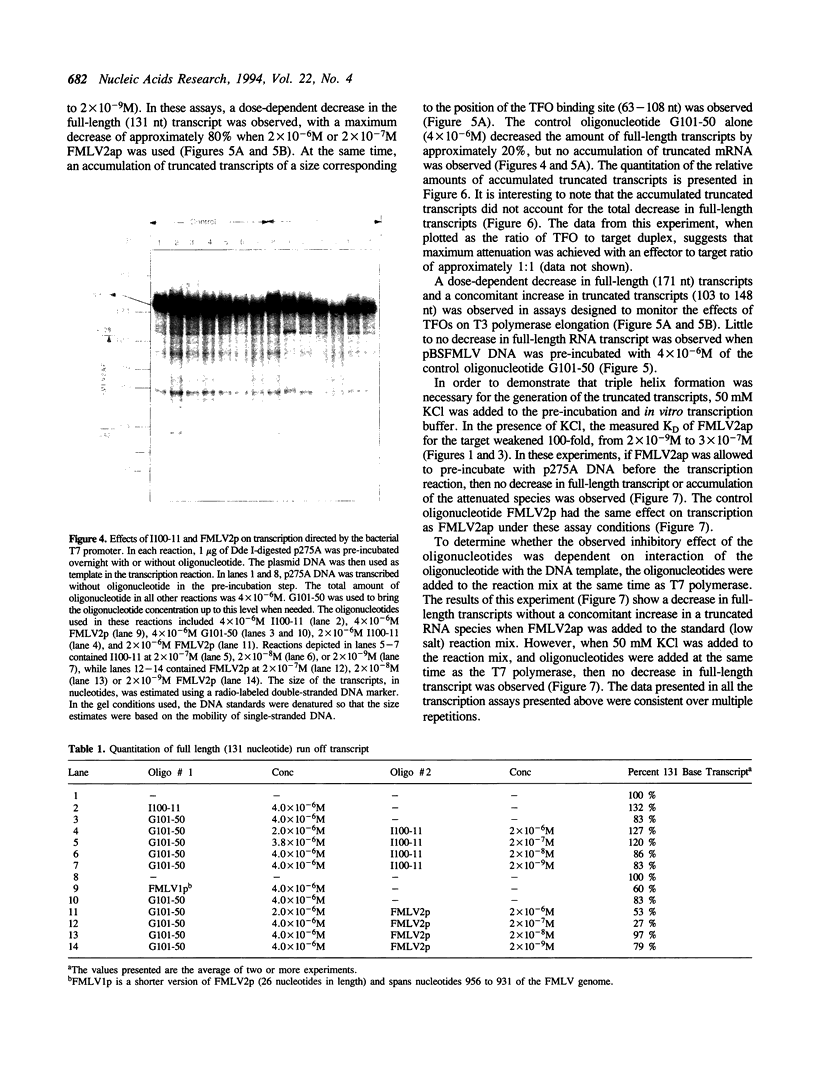
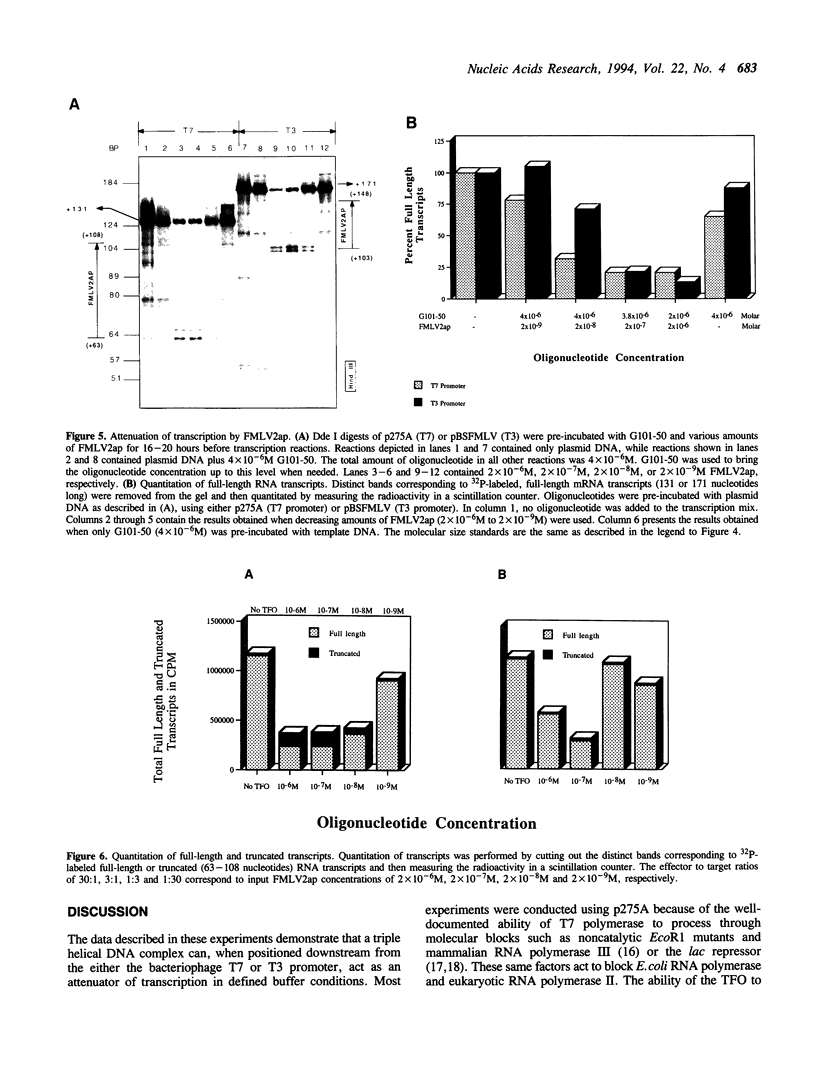


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal P. A., Dervan P. B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991 Mar 15;251(4999):1360–1363. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Cooney M., Czernuszewicz G., Postel E. H., Flint S. J., Hogan M. E. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988 Jul 22;241(4864):456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- Deuschle U., Gentz R., Bujard H. lac Repressor blocks transcribing RNA polymerase and terminates transcription. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4134–4137. doi: 10.1073/pnas.83.12.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle U., Hipskind R. A., Bujard H. RNA polymerase II transcription blocked by Escherichia coli lac repressor. Science. 1990 Apr 27;248(4954):480–483. doi: 10.1126/science.2158670. [DOI] [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- Hanvey J. C., Shimizu M., Wells R. D. Site-specific inhibition of EcoRI restriction/modification enzymes by a DNA triple helix. Nucleic Acids Res. 1990 Jan 11;18(1):157–161. doi: 10.1093/nar/18.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R., Gaffney B. L., Wang C., Jones R. A., Breslauer K. J. Thermodynamics and structure of a DNA tetraplex: a spectroscopic and calorimetric study of the tetramolecular complexes of d(TG3T) and d(TG3T2G3T). Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8832–8836. doi: 10.1073/pnas.89.18.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd Inhibition of T7 RNA polymerase initiation by triple-helical DNA complexes: a model for artificial gene repression. Biochemistry. 1992 Aug 25;31(33):7587–7594. doi: 10.1021/bi00148a021. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Wold B., Dervan P. B. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science. 1989 Aug 18;245(4919):725–730. doi: 10.1126/science.2549631. [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Beaton G., Stein C. A., Matsukura M., Caruthers M. H. Inhibition of human immunodeficiency virus activity by phosphorodithioate oligodeoxycytidine. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6265–6269. doi: 10.1073/pnas.89.14.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M., Rieger M., Jayaraman K. Large-scale synthesis of triple helix forming oligonucleotides using a controlled-pore glass support. Biotechniques. 1993 Dec;15(6):1004-6, 1008, 1010. [PubMed] [Google Scholar]
- Orson F. M., Thomas D. W., McShan W. M., Kessler D. J., Hogan M. E. Oligonucleotide inhibition of IL2R alpha mRNA transcription by promoter region collinear triplex formation in lymphocytes. Nucleic Acids Res. 1991 Jun 25;19(12):3435–3441. doi: 10.1093/nar/19.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavco P. A., Steege D. A. Characterization of elongating T7 and SP6 RNA polymerases and their response to a roadblock generated by a site-specific DNA binding protein. Nucleic Acids Res. 1991 Sep 11;19(17):4639–4646. doi: 10.1093/nar/19.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. L., Krawczyk S. H., Matteucci M. D., Toole J. J. Triple helix formation inhibits transcription elongation in vitro. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10023–10026. doi: 10.1073/pnas.88.22.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendegui J. G., Vasquez K. M., Tinsley J. H., Kessler D. J., Hogan M. E. In vivo stability and kinetics of absorption and disposition of 3' phosphopropyl amine oligonucleotides. Nucleic Acids Res. 1992 Jan 25;20(2):307–314. doi: 10.1093/nar/20.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]