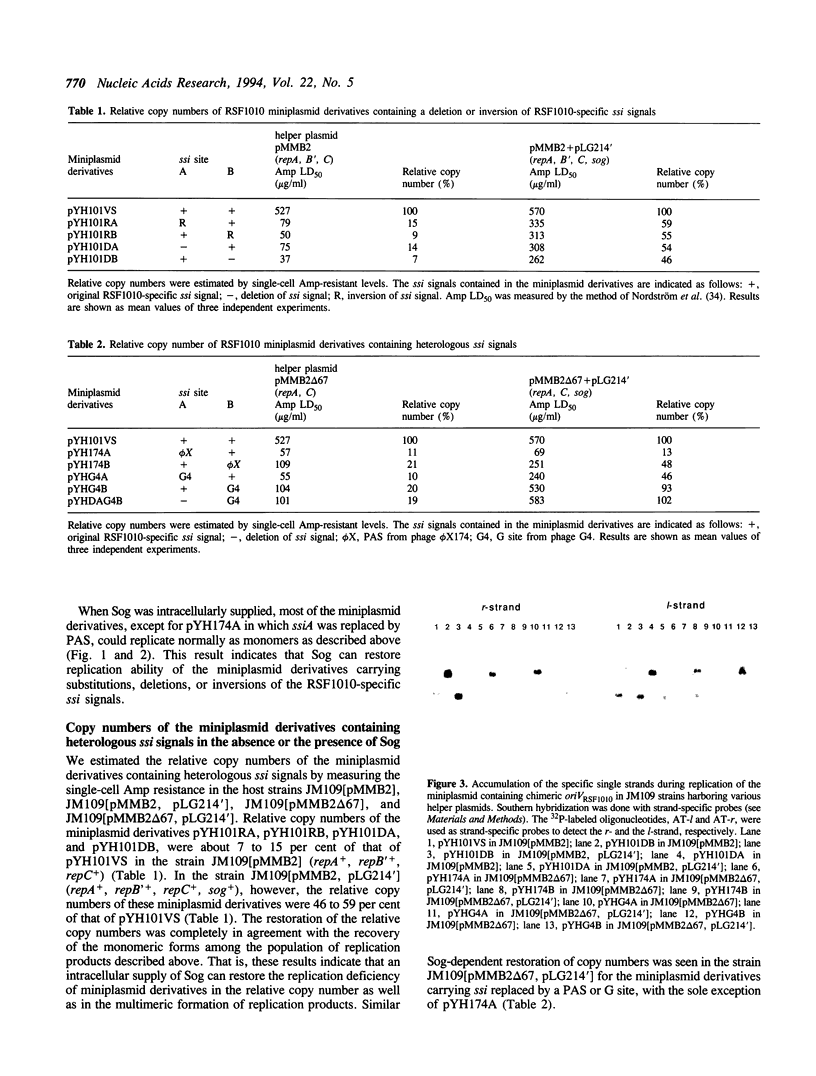
Abstract
The broad host-range plasmid RSF1010 contains two oppositely oriented priming signals, ssiA and ssiB, for DNA synthesis dependent on the origin of vegetative DNA replication (oriV). If either ssiA or ssiB was deleted or inverted, the RSF1010 miniplasmids containing engineered oriVs were maintained at low copy numbers, replicated abnormally as dimers, and accumulated specific single strands in the Escherichia coli strain supplying the three RSF1010-encoded RepA, RepB', and RepC proteins. Interestingly, an additional intracellular supply of the Sog primase (the sog gene product of plasmid CoIIb-P9) reversed the replication deficiency of these miniplasmids with respect to all three aspects described above. These were also true for the RSF1010 miniplasmids in which either ssiA or ssiB was replaced by the primosome assembly site (PAS) or by the G4-type ssi signal (G site). Furthermore, comparative analysis of the functional contribution of the two oppositely oriented ssi signals to the DNA replication of RSF1010 showed that, irrespective of their types, ssi signals conducting the initiation of DNA chain elongation away from the iterons were functionally more important than ones in the inverted orientation. We consider that this functional difference reflects the inherent properties of the initiation mechanism of RSF1010 DNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Low R. L., Kornberg A. Movement and site selection for priming by the primosome in phage phi X174 DNA replication. Proc Natl Acad Sci U S A. 1981 Feb;78(2):707–711. doi: 10.1073/pnas.78.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Comparison of the deoxyribonucleic acid molecular weights and homologies of plasmids conferring linked resistance to streptomycin and sulfonamides. J Bacteriol. 1974 Nov;120(2):618–630. doi: 10.1128/jb.120.2.618-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander R., Krabbe M., Nordström K. Mapping of the in vivo start site for leading strand DNA synthesis in plasmid R1. EMBO J. 1992 Dec;11(12):4481–4487. doi: 10.1002/j.1460-2075.1992.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P., Rowen L., Kornberg A. The RNA primer synthesized by primase to initiate phage G4 DNA replication. J Biol Chem. 1978 Feb 10;253(3):765–769. [PubMed] [Google Scholar]
- Boulnois G. J., Wilkins B. M. A novel priming system for conjugal synthesis of an IncI alpha plasmid in recipients. Mol Gen Genet. 1979 Oct 1;175(3):275–279. doi: 10.1007/BF00397227. [DOI] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B. E., Baker T. A., Kornberg A. In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome. J Biol Chem. 1987 Jul 25;262(21):10327–10334. [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring V., Scholz P., Scherzinger E., Frey J., Derbyshire K., Hatfull G., Willetts N. S., Bagdasarian M. Protein RepC is involved in copy number control of the broad host range plasmid RSF1010. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6090–6094. doi: 10.1073/pnas.82.18.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y., Nakamura T., Tanaka K., Higashi A., Sakai H., Komano T., Bagdasarian M. DnaG-dependent priming signals can substitute for the two essential DNA initiation signals in oriV of the broad host-range plasmid RSF1010. Nucleic Acids Res. 1992 Apr 11;20(7):1733–1737. doi: 10.1093/nar/20.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y., Sakai H., Hiasa H., Tanaka K., Komano T., Bagdasarian M. Functional division and reconstruction of a plasmid replication origin: molecular dissection of the oriV of the broad-host-range plasmid RSF1010. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):179–183. doi: 10.1073/pnas.88.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y., Sakai H., Komano T., Bagdasarian M. RepB' is required in trans for the two single-strand DNA initiation signals in oriV of plasmid RSF1010. Gene. 1989 Aug 1;80(1):155–159. doi: 10.1016/0378-1119(89)90261-8. [DOI] [PubMed] [Google Scholar]
- Honda Y., Sakai H., Komano T. Two single-strand DNA initiation signals located in the oriV region of plasmid RSF1010. Gene. 1988 Sep 7;68(2):221–228. doi: 10.1016/0378-1119(88)90024-8. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Marians K. J. The Escherichia coli primosome can translocate actively in either direction along a DNA strand. J Biol Chem. 1989 Aug 25;264(24):14531–14542. [PubMed] [Google Scholar]
- Masai H., Arai K. Initiation of lagging-strand synthesis for pBR322 plasmid DNA replication in vitro is dependent on primosomal protein i encoded by dnaT. J Biol Chem. 1988 Oct 15;263(29):15016–15023. [PubMed] [Google Scholar]
- Masai H., Arai K. Leading strand synthesis of R1 plasmid replication in vitro is primed by primase alone at a specific site downstream of oriR. J Biol Chem. 1989 May 15;264(14):8082–8090. [PubMed] [Google Scholar]
- Meyer R., Hinds M., Brasch M. Properties of R1162, a broad-host-range, high-copy-number plasmid. J Bacteriol. 1982 May;150(2):552–562. doi: 10.1128/jb.150.2.552-562.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N., Masai H., Inuzuka M., Miyazaki C., Ohtsubo E., Itoh T., Sasamoto S., Matsui M., Ishizaki R., Arai K. Identification of eleven single-strand initiation sequences (ssi) for priming of DNA replication in the F, R6K, R100 and ColE2 plasmids. Gene. 1991 Dec 1;108(1):15–22. doi: 10.1016/0378-1119(91)90482-q. [DOI] [PubMed] [Google Scholar]
- Sakai H., Komano T. DNA synthesis of plasmids in Escherichia coli. Biotechnol Genet Eng Rev. 1992;10:209–252. doi: 10.1080/02648725.1992.10647889. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Bagdasarian M. M., Scholz P., Lurz R., Rückert B., Bagdasarian M. Replication of the broad host range plasmid RSF1010: requirement for three plasmid-encoded proteins. Proc Natl Acad Sci U S A. 1984 Feb;81(3):654–658. doi: 10.1073/pnas.81.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Haring V., Lurz R., Otto S. Plasmid RSF1010 DNA replication in vitro promoted by purified RSF1010 RepA, RepB and RepC proteins. Nucleic Acids Res. 1991 Mar 25;19(6):1203–1211. doi: 10.1093/nar/19.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz P., Haring V., Scherzinger E., Lurz R., Bagdasarian M. M., Schuster H., Bagdasarian M. Replication determinants of the broad host-range plasmid RSF1010. Basic Life Sci. 1985;30:243–259. doi: 10.1007/978-1-4613-2447-8_20. [DOI] [PubMed] [Google Scholar]
- Scholz P., Haring V., Wittmann-Liebold B., Ashman K., Bagdasarian M., Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989 Feb 20;75(2):271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sakai T., Honda Y., Hiasa H., Sakai H., Komano T. Plasmid Co1IB contains an ssi signal close to the replication origin. Plasmid. 1991 Mar;25(2):125–130. doi: 10.1016/0147-619x(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M., Boulnois G. J., Lanka E. A plasmid DNA primase active in discontinuous bacterial DNA replication. Nature. 1981 Mar 19;290(5803):217–221. doi: 10.1038/290217a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhou H. S., Byrd C., Meyer R. J. Probing the activation of the replicative origin of broad host-range plasmid R1162 with Tus, the E.coli anti-helicase protein. Nucleic Acids Res. 1991 Oct 11;19(19):5379–5383. doi: 10.1093/nar/19.19.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. S., Meyer R. J. Deletion of sites for initiation of DNA synthesis in the origin of broad host-range plasmid R1162. J Mol Biol. 1990 Aug 5;214(3):685–697. doi: 10.1016/0022-2836(90)90286-u. [DOI] [PubMed] [Google Scholar]
- de Graaff J., Crosa J. H., Heffron F., Falkow S. Replication of the nonconjugative plasmid RSF1010 in Escherichia coli K-12. J Bacteriol. 1978 Jun;134(3):1117–1122. doi: 10.1128/jb.134.3.1117-1122.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]