Abstract
Fragile X mental retardation protein (FMRP) is highly enriched in neurons and binds to approximately 4% of mRNAs in mammalian brain. Its loss is a hallmark of fragile X syndrome (FXS), the most common form of mental retardation. In this review we discuss the mutation in the fragile X mental retardation-1 gene (FMR1), that leads to FXS, the role FMRP plays in neuronal cells, experiments from our own laboratory that demonstrate reductions of FMRP in additional psychiatric disorders (autism, schizophrenia, bipolar disorder, and major depressive disorder), and potential therapies to ameliorate the loss of FMRP.
Keywords: Fragile X mental retardation protein, brain, autism, schizophrenia, dendrite, metabotropic glutamate receptor
1. Fragile X syndrome and the fragile X mental retardation gene
Fragile X syndrome (FXS), is the most common inherited form of mental retardation which affects approximately 1:4,500 males and 1:9,000 females (Huber, 2006). Subjects with FXS display learning difficulties, delayed language acquisition, impairment of fine motor skills, and behavioral deficits reminiscent of autism including repetitive behavior, decreased attention, and poor eye contact (Hagerman, 1996). Seizures are another common feature of FXS, affecting approximately 20% of patients (Partington, 1984). More than 80% of males with FXS also display macroorchidism (Bardoni et al., 2001). All cases of FXS are the result of an abnormality of the fragile X mental retardation 1 gene.
The fragile X mental retardation 1 (FMR1) gene is located to the X chromosome and mutations in this gene are almost entirely responsible for the development of FXS. The gene was first identified in 1991 (Verkerk et al., 1991). FXS is caused by an expansion of a CGG repeat in the 5′ untranslated portion of the gene. In the normal form of the gene there are anywhere from 5–55 CGG repeats (Fu et al., 1991). Individuals with between 56–200 repeat premutations of the gene, which lack methylation, do not display obvious clinical symptoms of FXS but are found in FXS families (Bardoni et al., 2001). However, in individuals with the full mutation of over 200 repeats, there is extensive methylation, including the CpG islands in the gene’s promoter region, resulting in transcriptional silencing of the gene (Pieretti et al., 1991). Expansion from premutation to the full mutation occurs only during maternal transmission (Oostra and Willemsen, 2009). These individuals do not produce the gene product, fragile X mental retardation protein (FMRP) and display the clinical symptoms of FXS.
Carriers of the premutation are at risk for developing a separate disorder called Fragile X-associated tremor/ataxia syndrome (FXTAS). FXTAS is a progressive neurodegenerative disorder characterized by action tremor and ataxia. Advanced or severe cases also display cognitive decline (Hagerman and Hagerman, 2007). More than one third of premutation carriers over age 50 display symptoms of FXTAS, and by age 70 more than 50% of male carriers show FXTAS (Jacquemont et al., 2004).
2. Fragile X Mental Retardation Protein
FMRP is an RNA binding protein that is highly expressed in neurons (Devys et al., 1993) and glial cells (Pacey and Doering, 2007) and functions primarily as a regulator of translation. FMRP contains both nuclear localization and export domains allowing it to move between the nucleus and the cytoplasm (Eberhart et al., 1996; Sittler et al., 1996). However, in neurons, the vast majority of FMRP is localized to the cytoplasm with primary sublocalization to the dendrites, spines, and soma (Bakker et al., 2000; Weiler et al., 1997). FMRP associates, in an mRNA dependent manner, with large polyribosome complexes (Ceman et al., 1999; Feng et al., 1997; Willemsen et al., 1996) and smaller mRNA ribonucleoprotein complexes (mRNP), and dendritic “RNA granules” which are complexes of ribosomes, RNA-binding proteins, and RNAs. The RNA granules travel on microtubules to the dendrites and are believed to be translationally arrested (Antar et al., 2005; Kanai et al., 2004). Antar et al. (2004) demonstrated that mGluR5 activation increased the presence of FMRP to dendrites of cultured hippocampal neurons, and this increase was not due to increased synthesis of mRNA. A further study (Antar et al., 2005) showed that FMRP-associated RNA granules also increased in the dendrites in response to glutamatergic signaling and that this increase was reduced if microtubule dynamics were disrupted.
FMRP has been shown to bind approximately 4% of mRNA expressed in mammalian brain including its own message (Bassell and Warren, 2008). Specific mRNA targets of FMRP or other components of the RNP include myelin basic protein (MBP); microtubule-associated protein 1B (MAP1B), calcium/calmodulin protein kinase II alpha (CAMK2A), activity-regulated cytoskeletal-associated protein (ARC), ras related C3 botulinum toxin substrate 1 (RAC1), AMAP receptor subunits GluR1 and GluR2, and SAP90/PSD-95-associated protein 4 (SAPAP4) (Brown et al., 1998, 2000, 2001; Castets et al., 2005; Hou et al., 2006; Muddashetty et al., 2007; Zalfa et al., 2003). At the dendrites, FMRP may have a primary function as a transcriptional repressor. In the dendrites of Fmr1 knockout (KO) mice, there is increased protein synthesis for a number of proteins including PSD-95, Arc, and GluR1 (Hou et al., 2006; Muddashetty et al., 2007; Zalfa et al., 2007).
Microarray experiments also have identified genes that display altered expression in the absence of FMRP. In a study using lymphoblastoid cell lines from males with Fragile X syndrome there were 90 genes that showed significantly altered expression of at least 1.5 fold (Bittel et al., 2007). Quantitative real time polymerase chain reaction (qRT-PCR) confirmed altered expression of a number of genes including MAP1B, gamma-aminobutyric acid receptor subunit delta (GABRd), and unc-13 homolog B (UNC13B) (Bittel et al., 2007). UNC13B is a presynaptic protein that interacts with syntaxin 1 and 2 to promote priming of synaptic vesicles (Betz et al., 1997; Richmond et al., 2001). MAP1B codes for a precursor protein that undergoes proteolytic cleavage to form the MAP1B heavy chain and L1 light chains (Hammarback et al., 1991). As microtubule assembly is an important step in neurogenesis, impairment of MAP1B expression may affect normal brain development and neuronal plasticity. The GABRd subunit, combines with other GABAA receptor subunits to form a ligand-gated chloride channel (Windpassinger et al., 2002). Interestingly, GABRd mRNA has also been shown to be downregulated in hippocampus and neocortex of Fmr1 KO mice (Gantois et al., 2006). Other GABAA receptor subunits have been shown to display reduced expression in animal models of FXS including GABRa1, GABRa4, GABRβ1, GABRβ2, GABR 1, and GABR 2 (D’Hulst et al., 2006; El Idrissi et al., 2005). As GABA is the main inhibitory neurotransmitter in brain, disruption of GABA signaling could possibly explain seizures that are often comorbid with FXS.
3. FMRP is reduced in brains of subjects with autism
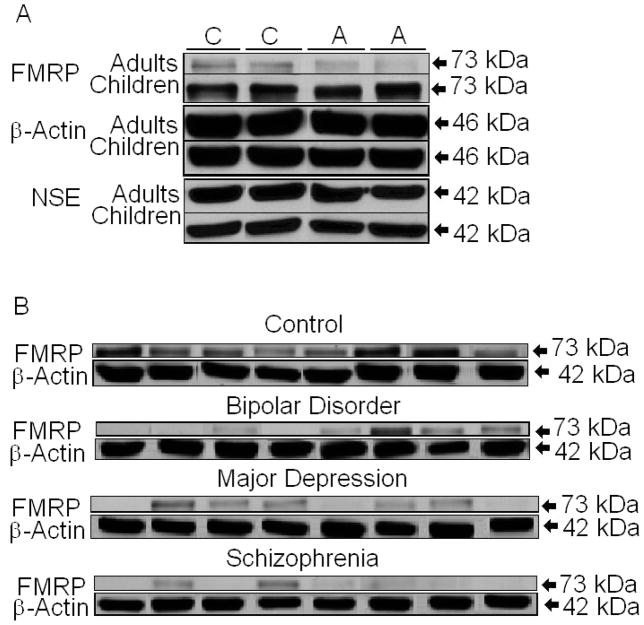
As previously mentioned, there are behavioral deficits in common between subjects with autism and subjects with FXS. Moreover, up to 30% of subjects with FXS are comorbid for autism while 2–3% of subjects with autism display comorbid FXS (Kau et al., 2004; Hagerman et al., 2005). Our laboratory was interested in investigating whether subjects with autism also displayed reductions in FMRP. We examined FMRP protein expression in two brain regions: cerebellar vermis and superior frontal cortex [Brodmann’s Area 9 (BA9)], two regions that show extensive pathology in subjects with autism (Bauman and Kemper, 1994, 2005). For all experiments, FMRP was normalized against both neuronal specific enolase (NSE) and β-actin in order to ensure that the observed changes were specific for FMRP. In cerebellar vermis of adult subjects with autism, there was a significant reduction in levels of FMRP when compared with matched controls (Figure 1A; Fatemi et al., 2010a). In contrast there was no significant difference in FMRP levels in vermis between children with autism and matched child controls (Figure 1A; Fatemi et al., 2010a). In BA9 of adults, there was also a significant reduction in FMRP protein expression (Fatemi, unpublished observations). As with cerebellar vermis, there was no change in FMRP expression in BA9 of children with autism (Fatemi, unpublished results).
Figure 1.
Reduction of FMRP is subjects with autism (A), and subjects with bipolar disorder, major depression, and schizophrenia (B) vs. controls. A: Expression of FMRP, β-actin, and neuronal specific enolase (NSE) in cerebellar vermis from subjects with autism (A) and control subjects (C). B: Expression of FMRP and β-actin in lateral cerebellum of subjects with bipolar disorder, major depression, and schizophrenia. Part A reprinted from Anatomical Record, Fatemi, S.H., Folsom, T.D., Kneeland, R.E., Liesch, S.B., Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism, Figures 1 and 2 Copyright (2010), with permission from John Wiley and Sons. Part B reprinted from Schizophrenia Research, 124(1-3):246-247, Fatemi, S.H., Kneeland, R.E., Liesch, S.B., Folsom, T.D., Fragile X mental retardation protein levels are decreased in major psychiatric disorders, page 247, Figure 1, Copyright (2010), with permission from Elsevier.
In addition to FMRP, we also investigated protein levels of metabotropic glutamate receptor 5 (mGluR5) and gamma-aminobutyric acid (GABA) A receptor, beta 3 (GABRβ3) in both vermis and BA9. Activation of group 1 metabotropic glutamate receptors (including mGluR5) result in increased synthesis of synaptic proteins (Weiler and Greenough, 1993). In the absence of FMRP, processes that depend upon protein synthesis such as epileptiform discharges (Chuang et al., 2005) and improper regulation of long term depression (Hou et al., 2006) are enhanced, suggesting that protein synthesis resulting from mGluR-stimulation is inhibited by FMRP. In animal models of FXS, inhibitors of mGluR5 have been shown to rescue several FXS phenotypes (de Vrij et al., 2008; Yan et al., 2005) as does reduction in mGluR5 expression (Dölen et al., 2007). However, expression of mGluR5 does not appear to be altered in Fmr1 KO mice (Price et al., 2007; Zhang and Alger, 2010). A recent study found that there was no change in mGluR1, mGluR5, or endocannabinoid receptor expression in hippocampi of Fmr1 KO mice when compared with wild type (Zhang and Alger, 2010). Price et al (2007) also found that in spinal cord of Fmr1 KO mice there was no difference in mGluR5 expression compared with wild type. These studies suggest that mGluR5 activation may be independent of FMRP action, at least in Fmr1 KO mice.
GABAA receptors are also known to be targets of FMRP as animal models for FXS have shown reduction in multiple GABAA receptor subunits (Figure 2; D’Hulst et al., 2006; El Idrissi et al., 2005; Gantois et al., 2006). mGluR5 was measured as a dimer (224 kDa) and total protein (dimer plus 112 kDa monomer). In vermis of children with autism there was a significant increase in mGluR5 dimer and total mGluR5 protein when compared with healthy controls (Fatemi et al., 2010a). Similarly, in BA9, we also observed significant increases in mGluR5 dimer and total mGluR5 in children with autism (Fatemi, unpublished results). Interestingly, in vermis of children with autism there was an increase in the ratio of dimerized mGluR5 to total mGluR5 (Fatemi et al., 2010a) There were no significant differences in mGluR5 protein in BA9 and vermis of adults with autism vs. control subjects. Finally, in vermis, but not BA9, we observed a significant reduction in protein for GABRβ3 when compared with controls (Fatemi et al., 2010a). These results persuaded us to look for potential involvement of FMRP in other psychiatric disorders. Thus, we pursued measuring levels of FMRP in three other disorders: schizophrenia, bipolar disorder, and major depressive disorder.
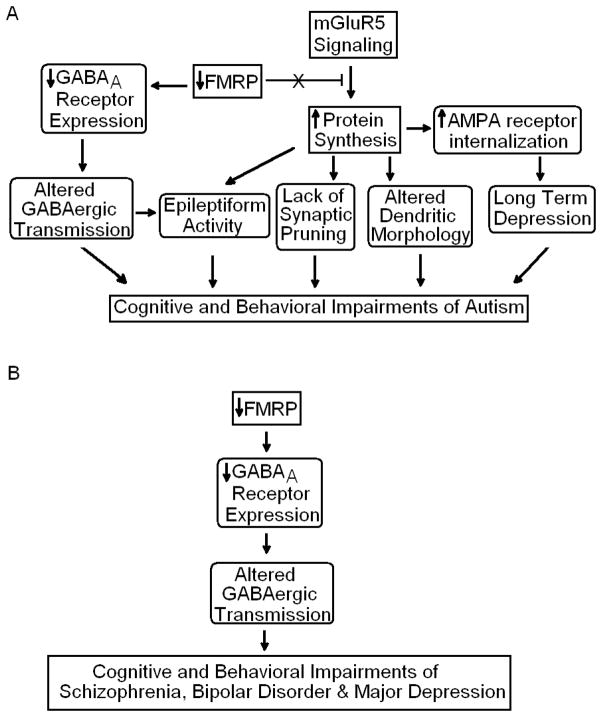
Figure 2.
The effects of reduced FMRP in major mental disorders. Evidence from animal studies suggests that a reduction in FMRP leads to a reduction in a number of GABAA receptor subunits. This reduction could potentially lead to altered GABAergic transmission and GABA/glutamate balance in the brain potentially explaining increased seizure and cognitive disturbances of subjects with FXS and other psychiatric disorders. FMRP normally acts as an inhibitor of protein synthesis resulting from mGluR5 activation. In the absence of FMRP there is increased protein synthesis. The increased protein synthesis results in increased internalization of AMPA receptors ultimately leading to long term depression. Moreover, increased protein synthesis may be responsible for altered morphology of dendrites, epileptiform activity, and lack of synaptic pruning in autism (A) or altered GABA transmission and subsequent deficits in cognition in schizophrenia, bipolar disorder and major depression (B).
4. FMRP is reduced in lateral cerebellum in subjects with schizophrenia and mood disorders
Studies examining the FMR1 gene and a possible association with schizophrenia have found that mutations in the FMR1 gene do not seem to confer a greater risk for the development of schizophrenia (Ashworth et al., 1996; Jnsson et al., 1995). However, a small number of case reports have identified individuals who display psychosis also have FMR1 mutations (Ashworth et al., 1996; Jnsson et al., 1995; Khin et al., 1998). Thus far, there have been no findings showing an association between FMR1 and either bipolar disorder or major depressive disorder (MDD).
Our laboratory studied protein levels of FMRP in lateral cerebella of subjects with schizophrenia, bipolar disorder, and MDD, and healthy controls from the Stanley Neuropathology Consortium. As with our studies with subjects with autism, all FMRP measurements were normalized against β-actin. Analysis of variance (ANOVA) showed a significant difference between the four means (Fatemi et al., 2010b). Individual comparisons were subsequently made and we observed significant reductions in FMRP in subjects with schizophrenia, bipolar disorder, and MDD when compared with controls (Figure 1B; Fatemi et al., 2010b). These changes were specific for FMRP as there were no significant differences in expression of β-actin (Figure 1B). Moreover, analysis of confounding variables found that none of them had an impact on FMRP expression (Fatemi et al., 2010b).
5. Implications for involvement of FMRP in psychiatric disease
Our laboratory found reductions in FMRP in autism, schizophrenia, bipolar disorder, and MDD. These results are significant as they are the first to demonstrate that FMRP is reduced in brains of subjects that have not been diagnosed with FXS. Cognitive deficits are common to members of these four diagnostic groups and GABAergic dysfunction is likely to contribute to these deficits. Fmr1 knockout mice and Drosophila display reduced expression of GABAA receptors (D’Hulst et al., 2006; El Idrissi et al., 2005; Gantois et al., 2006). Reduced FMRP expression in subjects with autism, bipolar disorder, schizophrenia and MDD could potentially explain the observed reductions of GABAA and GABAB receptor expression in postmortem brain studies performed by our laboratory (Fatemi et al., 2009a,b, 2010c, unpublished observations). GABAergic dysfunction in these four disorders has been demonstrated in postmortem studies by altered expression mRNA and protein of glutamic acid decarboxylase 65 and 67 kDa (GAD65/67) (Akbarian et al., 1995; Fatemi et al., 2002, 2005; Guidotti et al., 2000; Yip et al., 2007, 2008), and GABAA and GABAB receptors (Blatt, 2005; Duncan et al., 2010; Fatemi et al., 2009a,b, 2010c, unpublished observations; Oblak et al., 2010a,b; Ghose et al., 2011). Presence of seizure disorder in subjects with autism (as well as those with FXS) may also contribute to cognitive dysfunction. However, aside from individuals in our population sample diagnosed with autism, none of the subjects diagnosed with bipolar disorder, schizophrenia, or major depression were comorbid for seizure disorder.
Glutamatergic signaling is also affected by loss of FMRP. Stimulation of group 1 metabotropic glutamate receptors (mGluR) results in signaling cascades post-synaptically, causing increased protein synthesis (Weiler and Greenough, 1993, 1999). In contrast, there is evidence that FMRP acts as a negative regulator of protein synthesis (Dölen et al., 2007). Multiple phenomena observed in Fmr1 KO mice including long term depression, increased density of long, thin dendritic spines, and epileptiform activity are dependent on both mGluR activity and protein synthesis (Dölen and Bear, 2008). It has been hypothesized that reduction in FMRP expression leads to unregulated protein synthesis induced by group 1 mGluRs, which in turn is responsible for the multiple physical and cognitive pathologies of FXS (Figure 2; Bear, 2004; Dölen and Bear, 2008).
The reduced expression of FMRP may also have consequences for synaptic plasticity. A consistent feature in both subjects with FXS and Fmr1 KO mice is the presence of dendrites with abundance of long, thin spines which suggest an immature morphology (Figure 2; Grossman et al., 2006; Irwin et al., 2002; Meredith et al., 2007). Interestingly, Vanderklish and Edelman (2002) found that stimulation of group 1 mGluRs of cultured hippocampal neurons resulted in increased length of dendritic spines, further supporting the role of glutamatergic signaling in the pathology of FXS. The increased number of long, thin dendritic spines could potentially result in an abnormally large number of synapses. The large number of synapses may result in cognitive impairments associated with FXS as well as autism. Animal models have provided evidence that FMRP may play a role in synaptic pruning (Figure 2; Pfeiffer and Huber, 2007; Tessier and Broadie, 2008). Tessier and Brodie (2008) found that Drosophila FMRP (dFMRP) is required for axonal pruning of the mushroom body, the primary learning and memory region of Drosophila brain. Similarly, Pfeiffer and Huber (2007) found that overexpression of FMRP in neurons cultured from Fmr1 KO mice resulted in a reduction of synapse number.
6. Potential avenues for treatment
In support of the mGluR theory of FXS, animal experiments have shown that structural and behavioral deficits associated with FXS and presence of seizure can be ameliorated or rescued through the use of lithium and the mGluR5 antagonist MPEP (2-methyl-6-(phenylethynyl)-pyridine) or by reducing levels of mGluR5 (de Vrij et al., 2008; Dölen et al., 2007; Westmark et al., 2009; Yan et al., 2005; Yuskaitis et al., 2010). De Vrij et al (2008) found that treatment with MPEP rescued prepulse inhibition (PPI) of the acoustic startle response in Fmr1 KO mice and reduced the number of dendritic protrusions from cultured hippocampal neurons. MPEP has also been shown to repress seizures in Fmr1 KO mice (Westmark et al., 2009; Yan et al., 2005). Additionally Yan et al., (2005) found that treatment with MPEP reduced center field behavior in the open field test, demonstrating that MPEP could also affect behavioral phenotypes. Dölen et al. (2007) generated Fmr1 KO mice that express 50% as much mGluR5 and found that a number of phenotypes associated with FXS which are common to Fmr1 KO mice were rescued including a reduction in density of dendritic spines of pyramidal cells from the visual cortex and reduced presence of audiogenic seizures. Interestingly, the reduction in mGluR5 also resulted in reduced protein synthesis in the hippocampus (Dölen et al., 2007). Finally, chronic treatment with lithium has been shown to rescue behaviors that are altered in Fmr1 KO mice including open field behavior and passive avoidance (Yuskaitis et al., 2010). These results, taken together, suggest that drugs that affect mGluR5 signaling may serve as potential therapies for treatment of FXS. Recently, MPEP has been shown to reduce repetitive self-grooming in a mouse model of autism (Silverman et al., 2010).
A recent study has shown that treatment with lithium resulted in behavioral improvements in subjects with FXS including improved scores on the Aberrant Behavior Checklist-Community Edition, clinical global improvement scale, and the Vineland Adaptive Behavior Scale (Berry Kravis et al., 2008). An open label pilot study using a single dose of fenobam, a selective, potent mGluR5 inhibitor (Porter et al., 2005), in adults with FXS found a 20% improvement over baseline for PPI (Berry-Kravis et al., 2009). Moreover, no significant adverse effects of fenobam on the study subjects were identified (Berry-Kravis et al., 2009). Table 1 summarizes the use of chemical agents that remedy the effects of unchecked mGluR5 signaling. Further studies are required to determine the efficacy and safety of mGluR inhibitors to correct for deficits caused by reduction or absence of FMRP in subjects with major mental disorders.
Table 1.
Summary of agents that are capable of ameliorating effects of unchecked mGluR5 signaling
| Agent | Mode of Action | Animal | Effect | Reference |
|---|---|---|---|---|
| MPEP | mGluR5 inhibitor | Mouse | Correction of PPI | DeVrij et al., 2008 |
| Reduction of dendritic protrusions | DeVrij et al., 2008 | |||
| Repression of seizures | Westmark et al., 2009 | |||
| Rescue of open field behavior | Yan et al., 2005 | |||
| Rescue of repetitive self-grooming behavior | Silverman et al., 2010 | |||
| Lithium | Mood stabilizer | Mouse | Rescue of open field behavior | Yuskaitis et al., 2010 |
| Human | Corrected behavioral deficits in subjects with FXS | Berry-Kravis et al., 2008 | ||
| Fenobam | mGluR5 inhibitor | Human | Correction of PPI | Berry-Kravis et al., 2009 |
7. Conclusions
FXS is the most common form of mental retardation which is caused by an expansion of a CGG repeat in the 5′ untranslated portion of the FMR1 gene. This expansion results in hypermethylation of the FMR1 promoter and consequent loss of its protein product FMRP. Our laboratory has demonstrated for the first time that reduction in FMRP in brain tissue is not specific to FXS but occurs in patients with autism, schizophrenia, bipolar disorder, and major depression. Evidence from animal models suggests that the loss of FMRP and resultant increase in mGluR signaling and protein synthesis may be responsible for the observed pathologies of FXS. The use of mGluR inhibitors may prove to be a safe, effective way in the treatment of FXS and other psychiatric disorders impacted by the loss of FMRP.
Acknowledgments
Grant support for SHF from NICHD and NIMH (1R015HD052074-04, 3R01HD052074-03S1, 1R01MH086000-01A2) is greatly appreciated. Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders, University of Maryland, Baltimore, MD (The role of the NICHD Brain and Tissue Bank is to distribute tissue, and therefore, cannot endorse the studies performed or the interpretation of results); the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; the Autism Tissue Program; and The Stanley Medical Research Institute’s brain collection and is gratefully acknowledged. Reviews of results of FMRP in autism, schizophrenia, bipolar disorder, and major depressive disorder are summarized from the following articles: 1) Fatemi, S.H., Folsom, T.D., Kneeland, R.E., Liesch, S.B., Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism, Anatomical Record, in press, copyright (2010) with permission from John Wiley and Sons and 2) Fatemi, S.H., Kneeland, R.E., Liesch, S.B., Folsom, T.D., Fragile X mental retardation protein levels are decreased in major psychiatric disorders, Schizophrenia Research, 124(1-3):246-247, copyright (2010) with permission from Elsevier.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S. Hossein Fatemi, Email: fatem002@umn.edu.
Timothy D. Folsom, Email: folso013@umn.edu.
References
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile X mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–359. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Ashworth A, Abusaad I, Walsh C, Nanko S, Murray RM, Asherson P, McGuffin P, Gill M, Owen MJ, Collier DA. Linkage analysis of the Fragile X gene FMR-1 and schizophrenia: no evidence for linkage but report of a family with schizophrenia and an unstable triplet repeat. Psychiatr Genet. 1996;6:81–86. doi: 10.1097/00041444-199622000-00008. [DOI] [PubMed] [Google Scholar]
- Bakker CE, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen AT, Oostra BA, Willemsen R. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Exp Cell Res. 2000;258:162–170. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- Bardoni B, Schenck A, Mandel JL. The fragile X mental retardation protein. Brain Res Bull. 2001;56:375–382. doi: 10.1016/s0361-9230(01)00647-5. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism. In: Bauman M, Kemper T, editors. The neurobiology of autism. Johns Hopkins University Press; Baltimore, MD: 1994. pp. 19–145. [Google Scholar]
- Bauman ML, Kemper TL. Structural Brain Anatomy in Autism: What is the Evidence? In: Bauman M, Kemper T, editors. The neurobiology of autism. Johns Hopkins University Press; Baltimore, MD: 2005. pp. 121–135. [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of Fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, Weiler IJ, Greenough WT. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13–1 with the N terminus of syntaxin. J Biol Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Butler MG. Whole genome microarray analysis of gene expression in subjects with fragile X syndrome. Genet Med. 2007;9:464–472. doi: 10.1097/gim.0b013e3180ca9a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ. GABAergic cerebellar system in autism: a neuropathological and developmental perspective. Int Rev Neurobiol. 2005;71:167–178. doi: 10.1016/s0074-7742(05)71007-2. [DOI] [PubMed] [Google Scholar]
- Brown V, Small K, Lakkis L, Feng Y, Gunter C, Wilkinson KD, Warren ST. Purified recombinant Fmrp exhibits selective RNA binding as an intrinsic property of the fragile X mental retardation protein. J Biol Chem. 1998;273:15521–15527. doi: 10.1074/jbc.273.25.15521. [DOI] [PubMed] [Google Scholar]
- Brown V, Ceman S, Jin P, Jin C, Wilkinson KD, Warren ST. Messenger RNAs associated with the fragile X mental retardation protein in mouse brain. Am J Hum Genet. 2000;67:18. [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Castets M, Schaeffer C, Bechara E, Schenck A, Khandjian EW, Luche S, Moine H, Rabilloud T, Mandel JL, Bardoni B. FMRP interferes with the Rac1 pathway and controls actin cytoskeleton dynamics in murine fibroblasts. Hum Mol Genet. 2005;14:835–844. doi: 10.1093/hmg/ddi077. [DOI] [PubMed] [Google Scholar]
- Ceman S, Brown V, Warren ST. Isolation of an FMRP-associated messenger ribonucleoprotein particle and identification of nucleolin and the fragile X related proteins as components of the complex. Mol Cell Biol. 1999;19:7925–7932. doi: 10.1128/mcb.19.12.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Shankaranarayana Rao BS, Smith GB, Auerbach D, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:15008–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Eberhart DE, Malter HE, Feng Y, Warren ST. The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet. 1996;5:1083–1091. doi: 10.1093/hmg/5.8.1083. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABAA receptor expression in the seizure-prone fragile X mouse. Neurosci Lett. 2005;377:141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) Receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009a;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) receptors is altered in brains of Subjects with autism. Cerebellum. 2009b;8:64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Kneeland RE, Liesch SB. Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec. 2010a doi: 10.1002/ar.21299. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Kneeland RE, Liesch SB, Folsom TD. Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr Res. 2010b doi: 10.1016/j.schres.2010.07.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and Protein Levels for GABA(A) alpha 4, alpha 5, beta 1, and GABA(B)R1 Receptors are Altered in Brains from Subjects with Autism. J Autism Dev Disord. 2010c;40:743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. FMRP associates with polyribosomes as an mRNP, and the 1304N mutation of severe fragile X syndrome abolishes this association. Mol Cell. 1997;1:109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST, et al. Variation in the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gantois I, Vandescompele J, Speleman F, Reyniers E, D’Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABAA receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis. 2006;21:346–357. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Ghose S, Winter MK, McCarson KE, Tamminga CA, Enna SJ. The GABAB receptor as a target for antidepressant drug action. Br J Pharmacol. 2011;162:1–17. doi: 10.1111/j.1476-5381.2010.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Elisseou NM, McKinney BC, Greenough WT. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006;1084:158–164. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Physical and behavioral phenotype. In: Hagerman RJ, Cronister A, editors. Diagnosis, Treatment, and Research. Baltimore: The Johns Hopkins University Press, Baltimore, MD; 1996. pp. 3–87. [Google Scholar]
- Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: a model for autism and neurodegeneration. Curr Opin Psychiatry. 2005;18:490–496. doi: 10.1097/01.yco.0000179485.39520.b0. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome-an older face of the fragile X gene. Nat Clin Pract Neurol. 2007;3:107–112. doi: 10.1038/ncpneuro0373. [DOI] [PubMed] [Google Scholar]
- Hammarback JA, Obar RA, Hughes SM, Vallee RB. MAP1B is encoded as a polyprotein that is processed to form a complex N-terminal microtubule-binding domain. Neuron. 1991;7:129–139. doi: 10.1016/0896-6273(91)90081-a. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM. The fragile X-cerebellum connection. TRENDS Neurosci. 2006;29:183–185. doi: 10.1016/j.tins.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ, Patel B, Swain RA, Weiler IJ, Oostra BA, Greenough WT. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile X-knockout mice. Am J Med Genet. 2002;111:140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco CM, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- Jnsson E, Björck E, Wahlström J, Gustavsson P, Sedvall G. Screening for CGG trinucleotide repeat expansion in the fragile X mental retardation 1 gene in schizophrenic patients. Psychiatr Genet. 1995;5:157–160. doi: 10.1097/00041444-199524000-00002. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transcripts RNA; isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Kau AS, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, Kaufmann WE. Social behavior profile in young males with fragile X syndrome: characteristics and specificity. Am J Med Genet A. 2004;126A:9–17. doi: 10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- Khin NA, Tarleton J, Raghu B, Park SK. Clinical description of an adult male with psychosis who showed FMR1 gene methylation mosaicism. Am J Genet. 1998;81:222–224. doi: 10.1002/(sici)1096-8628(19980508)81:3<222::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron. 2007;54:627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelić S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Decreased GABA(B) receptors in the cingulate cortex and fusiform gyrus in autism. J Neurochem. 2010a;114:1414–1423. doi: 10.1111/j.1471-4159.2010.06858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Reduced GABA(A) receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2010b doi: 10.1016/j.brainres.2010.09.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra BA, Willemsen R. FMR1: A gene with three faces. Biochem Biophys Acta. 2009;1790:467–477. doi: 10.1016/j.bbagen.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey LK, Doering LC. Developmental expression of FMRP in the astrocyte lineage: implications for fragile X syndrome. Glia. 2007;55:1601–1609. doi: 10.1002/glia.20573. [DOI] [PubMed] [Google Scholar]
- Partington MW. The fragile X syndrome II: Preliminary data on growth and development in males. Am J Med Genet. 1984;17:175–194. doi: 10.1002/ajmg.1320170111. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci. 2007;27:3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Büttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Mühlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neurospsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittler A, Devys D, Weber C, Mandel JL. Alternative splicing of exon 14 determines nuclear or cytoplasmic localization of fmr1 protein isoforms. Hum Mol Genet. 1996;5:95–102. doi: 10.1093/hmg/5.1.95. [DOI] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci USA. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1 containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome mental retardation. J Neurosci. 1991;27:11624–11634. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci USA. 1993;90:7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Synaptic synthesis of the fragile X protein: possible involvement in synapse maturation and elimination. Am J Med Genet. 1999;83:248–252. doi: 10.1002/(sici)1096-8628(19990402)83:4<248::aid-ajmg3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Westmark CJ, Westmark PR, Malter JS. MPEP reduces seizure severity in Fmr-1 KO mice over expressing human Abeta. Int J Clin Exp Pathol. 2009;3:56–68. [PMC free article] [PubMed] [Google Scholar]
- Willemsen R, Bontekoe C, Tamanini F, Galjaard H, Hoogeveen A, Oostra B. Association of FMRP with ribosomal precursor particles in the nucleus. Biochem Biophys Res Commun. 1996;225:27–33. doi: 10.1006/bbrc.1996.1126. [DOI] [PubMed] [Google Scholar]
- Windpassinger C, Kroisel PM, Wagner K, Petek E. The human gamma-aminobutryric acid A receptor delta (GABRD) gene: molecular characteristics and tissue-specific expression. Gene. 2002;292:25–31. doi: 10.1016/s0378-1119(02)00649-2. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA levels in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86:525–530. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem Pharmacol. 2010;79:632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, Bagni C. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10:578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]