Abstract
We previously reported that 17β – estradiol (E2) is pronociceptive in a visceral pain model in the rat. Subcutaneously (s.c.) administered E2 reversed the decrease in the colorectal distention (CRD)-evoked visceromotor response produced by ovariectomy (OVx) and CRD-induced nociceptive responses were greater in proestrous rats compared to met/diestrous rats. The site of action, the type of estrogen receptors activated and the possible intracellular signaling pathway involved are yet to be established. In the present study, intrathecal (i.t.) E2 administered to OVx rats mimicked the effects of s.c. E2, suggesting spinal E2 receptors are involved. This is further supported by the observations that the anti-estrogen ICI 182,780 injected i.t. in intact female rats significantly decreased the visceromotor response to CRD, the response of colonic afferents was not affected by OVx and colonic afferents did not label for estrogen receptor α (ERα). The ERα selective agonist, 4,4',4"-[4-propyl-(1H)-pyrazole-1,3,5-triyl]tris-phenol (PPT; s.c. or i.t.) facilitated the visceromotor response similar to E2, suggesting ERα activation is involved in mediating the pronociceptive effect of E2. PPT (s.c. or i.t.) increased the response of spinal dorsal horn neurons to CRD, indicating a spinal site of action. In addition, s.c. E2 or PPT increased CRD-induced spinal extracellular-signal-regulated kinase (ERK) phosphorylation that was not observed in OVx rats and a mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitor blocked facilitation of the visceromotor response by PPT. Taken together, the present study demonstrates that spinal ERα mediates the pronociceptive effect of E2 on visceral signal processing through activation of the MAPK pathway.
Keywords: colorectal distention, visceromotor response, gonadal hormones, estrogen receptor alpha, spinal cord, visceral pain, pERK, dorsal horn neurons, estradiol, PPT, colonic afferent
Introduction
The role of estrogens in nociceptive processing is unclear. Many studies suggest an increase in plasma or local estrogen levels parallel an increase in nociceptive sensitivity [3,7,8,13,21,41,47,63]. Consistent with these observations, our previous studies showed the response to colorectal distention (CRD) is greater in rats in proestrus compared to diestrus, and short-term ovariectomy (less than 3 weeks) decreases sensitivity to CRD that is reversed by systemically administrated estradiol (E2) [34–36]. In contrast, long-term loss of gonadal hormones by ovariectomy (at least 6 weeks) produced somatic and visceral hyperalgesia that was reversed by E2 replacement and E2 is antinociceptive in some somatic pain tests [51,66,67,73].
The classical estrogen receptors alpha (ERα) and beta (ERβ) are nuclear receptors. Activation of both receptors initiates transcriptional changes through direct or indirect interaction with promoters of target genes modulating cellular function on a time scale of hours to days [18,52]. E2 also elicits rapid effects via membrane bound receptors by triggering rapid activation of intracellular signaling transduction pathways including PLC/IP3, PI3K/AKT and MAPK (ERK, JNK and p38), which modulate receptor function, gene expression and cell proliferation [11,33,46,55,90]. Furthermore, activation of different ERs initiates different signaling cascades in different cells. Activation of ERα induces cAMP/ERK and PI3K/AKT activation in breast cancer and endothelial cells [27] while ERβ activation increases ERK and JNK phosphorylation in CHO cells [62] and activates p38 in human colon cancer cells [1]. Given the diverse effect of differential ER activation in different cell types, it is of interest to investigate the signaling cascade involved in the pronociceptive effect of E2.
ERα and ERβ are both expressed in the peripheral nervous system, spinal cord and supraspinal sites [20,58,69,74,81–83]. In the spinal cord, ERα is expressed predominantly in the superficial dorsal horn, localized to modulate nociceptive processing. ERβ is denser in deeper laminae, suggesting a differential role of these receptors in nociceptive processing. However, there is no clear consensus as to the relative contribution of the different ERs to nociceptive processing.
In the present study, we focus on the role of spinal ERα in modulating visceral nociception. We hypothesize that spinal ERα mediates E2-induced visceral pronociception. The intracellular signaling pathway that could be initiated by ERα activation was also explored.
Methods
All experimental protocols were approved by the University of Maryland Dental School Institutional Animal Care and Use Committee and adhered to guidelines for experimental pain in animals published by the International Association for the Study of Pain. All survival surgical procedures were done under aseptic conditions as stipulated by approved protocols. In this study we focused on the lumbosacral spinal cord since we previously reported this is the main spinal site involved in processing acute nociceptive colonic stimuli [78,79].
Intact and ovariectomized (OVx) adult female Sprague-Dawley rats (225–250 g) were purchased from Harlan (Frederick, MD). Ovariectomized rats were tested between 10 and 14 days following surgery. Only OVx rats were treated with E2. We have previously reported that 7 days following OVx, the plasma E2 concentration was below 5 pg/ml and approximately 100 pg/ml 48 hours following subcutaneous injection of 50 μg E2 [34].
Rats were double-housed in a room maintained at 25 °C under 12 h-12 h alternating light-dark cycle with free access to food and water.
Electromyogram (EMG) electrodes and intrathecal catheters
For surgical implantation of EMG electrodes alone, rats were anesthetized with isofluorane. For implantation of intrathecal catheters and EMG electrodes, rats were anesthetized with a cocktail of 55 mg/kg ketamine, 5.5 mg/kg xylazine, 1.1 mg/kg acepromazine. Electromyogram electrodes made from Teflon coated 32 g stainless steel wire (Cooner Wire Co., CA) were implanted in the lateral abdominal muscle and passed subcutaneously to be exteriorized at the back of the neck. Following EMG electrode placement, the rat was placed into a stereotaxic apparatus. The atlantooccipital membrane was exposed and incised. A catheter made of 32 g polyethylene tubing (ReCathCo, Allison Park, PA) was inserted 7.8 cm in the subdural space to reach to the lumbosacral spinal cord (L6–S2). The catheter and electrode leads were exteriorized at the back of the neck. Rats were subsequently singly housed and allowed to recover from surgery for 5–7 days.
Visceromotor response
All rats were fasted 24 hours (water ad libitum) prior to testing to facilitate balloon placement. On the day of experiment, rats were briefly sedated with isoflurane and a 6 cm balloon made from the finger of a latex glove attached to Tygon tubing was inserted through the anus into the colon. Rats were loosely restrained in rodent restrainers and allowed 30 minutes to recover from the isofluorane. Colorectal distention (CRD; graded intensity trials: 20, 40, 60, 80 mmHg, or single intensity trials: 60 mmHg; 20 sec duration, 3 minute interstimulus interval) was produced by inflating the distention balloon with air. The pressure was monitored and kept constant by a pressure controller/timing device.
The electromyogram was recorded with a CED 1401plus and analyzed using Spike 2 for windows software (Cambridge Electronic Design, UK). The electromyogram was rectified and the area under the curve (AUC) for the 20 seconds prior to distention subtracted from the AUC during the 20 second distention to derive the visceromotor response (vmr).
Electrophysiology
Rats were anesthetized with Nembutal (50 mg/kg, i.p.). The left jugular vein was catheterized for continuous infusion of Nembutal at a rate of 5–10 mg/kg/hr. The left carotid artery was catheterized for continuous arterial blood pressure monitoring and bolus administration of pancuronium bromide (0.2 mg/kg/hr). A tracheal cannula was inserted for artificial ventilation. End-tidal CO2 was maintained at 3.5–4.5%. Body temperature was maintained with a circulating water heating pad and overhead lamp. The rat was placed in a head holder and suspended with thoracic vertebral and ishial clamps. The LS (L6-S2) spinal cord segments or dorsal roots were exposed by laminectomy. The dura matter was cut and the spinal cord was bathed in the warm paraffin oil. The distention balloon was placed into the colorectum and the rat left undisturbed for 1 hour before recording.
Primary afferents
A pair of silver electrodes was used for recording. The dorsal roots were cut near the root entry zone and the cut proximal end was carefully split into fine filaments until a single CRD responsive fiber could be isolated. Signals were amplified (model 1800 AC amplifier; A–M systems, Carlsborg, WA) and passed through a dual time and voltage window discriminator (DDIS-1; BAK Electronics, Germantown, MD) to isolate a single unit. Data were collected with a CED micro 1401 and Spike 2 for Windows software for online and offline analysis. Responses to graded intensities of CRD were recorded. The activity was quantified as the mean discharge frequency during the 20 sec of the CRD stimulus minus spontaneous activity determined in the preceding 20 sec. The data are expressed as mean ± SEM.
Dorsal horn neurons
The surgery was done as described above. Tungsten microelectrodes (1–2 MΩ; Micro probe, Potomac, MD) were used for extracellular single-unit recording in the LS spinal segments (0–1.5 mm lateral to midline, 500–1500 μm ventral to spinal cord dorsum). Visceroceptive neurons showing excitatory responses to 80 mmHg CRD were classified as Abrupt or Sustained [86]. Abrupt neurons ceased responding within 4 seconds of terminating the stimulus; activity dropping below the mean plus 2 standard deviations of the background spontaneous activity (20 sec prior to distention) for 2 sec. Sustained neurons had an afterdischarge that persisted longer than 4 seconds. Neurons were tested with two graded intensity distention trials (20, 40, 60 and 80 mmHg, 20 sec duration, 3 minute interstimulus interval). The mean response at each pressure was used as the response from that cell.
Western blots
Rats were briefly sedated with isoflurane for inserting the distention balloons and then loosely restrained in tubes and allowed 30 minutes to recover. Rats were distended 48 hours after injection of E2 or 5 minutes, 2 hours, or 4 hours following injection of PPT. Rats were distended for 30 minutes (80 mmHg, 20 sec duration, 3 minute interstimulus interval). Rats were subsequently overdosed with Nembutal (100 mg/kg) and decapitated. The spinal cord was removed by pressure ejection with 10 ml ice cold saline. The L6 to S2 region of the spinal cord was isolated, snap frozen and kept at −80 °C until use. The ventral part of the spinal cord was removed. To generate the whole lysate protein, tissue was sonicated in RIPA buffer containing (50 mM Tris-HCl, pH 8.0; 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS, 1 mM Na3VO4, 1 U/ml aprotinin, 20 μg/ml leupetin, and 20 μg/ml pepstatin A). The homogenate was centrifuged at 14,000 g for 10 minutes at 4 °C and the supernatant was collected. Cytosolic and nuclear proteins were prepared by differential centrifugation [28] with modification. Briefly, tissue was homogenized in RIPA buffer without Triton X-100 and deoxycholic acid. The homogenate was centrifuged at 6000g for 20 minutes at 4°C. The above process was repeated and the resulting supernatants were combined and used as cytoplasmic extracts. RIPA buffer was added to the nuclear pellets, the mixture was incubated on ice for 1 hour before being centrifuged at 21000g for 5minutes. The resulting supernatants were used as nuclear extracts. The specificity of subcellular fractions was determined by probing parallel Western blots with histone H2A antibody (abcam, Cambridge, MA).
The protein concentration was measured using the Bradford method. Protein samples were loaded (25–40 μg per lane) and separated on a 4–12% Bis-Tris gel and transferred to a nitrocellulose membrane. After incubating in blocking buffer (Pierce) for 1 hour, the membrane was incubated with pERK1/2 (1:500; Cell Signaling Technology, cat no. 9101) at 4 °C overnight. The membrane was then washed with TBST for 30 minutes and incubated for 1 hour with a HRP conjugated secondary antibody (1:3000, Santa Cruz Biotechnology, Santa Cruz, CA). The membrane was washed with TBST for 30 minutes before the bands were viewed using HRP chemiluminescence and film. Band density was determined using ImageJ software (NIH). The blots were further incubated in stripping buffer (Pierce Biotechnology, Inc. IL) for 30 minutes at 50 °C and reprobed with anti-ERK antibody (1:1000, Cell Signaling Technology, cat no. 9102).
Retrograde labeling of colonic afferents and immunocytochemistry
Intact female rats were anesthetized with the rat cocktail. A laparotomy exposed the descending colon and rectum or bladder. Cholera toxin subunit B conjugated to Alexa Fluor 594 (ctb-594; 25 μl total, 1 mg/ml saline) was injected in 8–10 sites in the colon wall or 5–7 sites in the bladder wall. Seven days later rats were euthanized with isofluorane and perfused with saline followed by 500 ml 4% paraformaldehyde. The T13-L2 and L6-S2 DRG were removed, post fixed overnight and transferred to 30% sucrose. The DRG were cut at 20 μm on a cryostat, every third section saved on the same slide. Briefly, sections were incubated in rabbit anti-ERa (1:1000, Upstate Biotechnology, Lake Placid, NY) followed by donkey anti-rabbit IgG conjugated to Alexa fluor 488 (1:1000, Molecular Probes, Carlsbad, CA). Sections were coverslipped with Prolong Gold (Molecular Probes, Carlsbad, CA). One slide from each pair of ganglia from T13-L2 and L6-S2 was analyzed. Sections were examined at 400× using a Nikon microscope equipped for epifluorescence and filter sets for simultaneous viewing of Alexa fluor 488 and 594 as well as filters for viewing each fluorophore individually. Every ctb labeled cell was counted (only cells with a visible nucleus) and then examined for expression of ERα.
Drugs
17β-E2 benzoate (E2; Sigma) was dissolved in safflower oil (100 μl injection volume, 500 μg/ml) for subcutaneous injection. For intrathecal injection, water soluble E2 (Sigma, 0.46, 4.6 and 46 ng in 10μl) was dissolved in normal saline. The ERα selective agonist 4,4',4”-[4-propyl-(1H)-pyrazole-1,3,5-triyl]tris-phenol (PPT; Sigma, 1, 3mg/kg, 100 μl, s.c.; 50 ng/5 μl, i.t.) was dissolved in DMSO (100% and 0.1%, respectively). The MEK inhibitor PD98059 (Sigma) was dissolved in 4% DMSO to a final concentration of 1μg /5 μl. The steroidal anti-estrogen ICI 182,780 (Tocris, 10 nmol) was dissolved in 10% ethanol. Control rats were injected with the corresponding vehicle.
Data analysis
Data were analyzed using the t-test or one or two-way repeated measures (RM) ANOVA followed by Student-Newman-Keuls Method for multiple comparisons as appropriate. p< 0.05 was considered significant. NS, not significant.
Results
Estrogen does not modulate the activity of colonic afferents
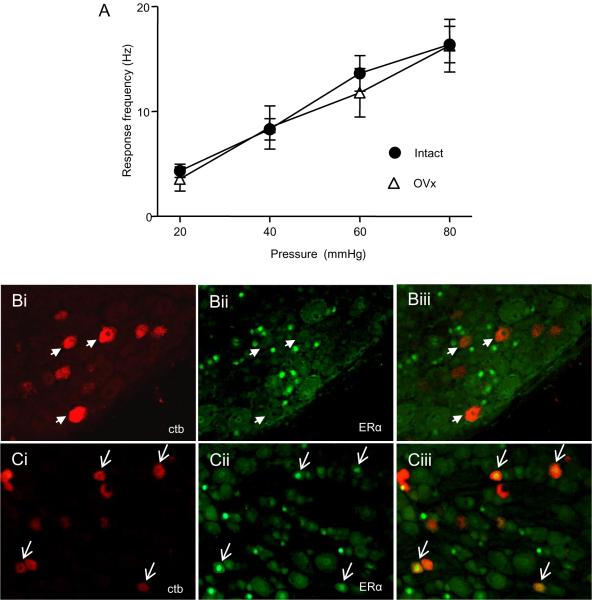
We previously reported that ovariectomy decreased the magnitude of the visceromotor response which was reversed by E2, but the site of action was not determined [34,35]. One possibility is that estrogen modulates colonic afferent activity. To test this hypothesis, single unit recordings were made from dorsal roots that were decentralized from the spinal cord. There was no difference in the stimulus response functions from LS colonic afferents recorded from intact females and ovariectomized females (two way RM ANOVA: pressure, p<0.001; treatment, interaction, NS; Figure 1A). This was supported by a double labeling experiment in which colonic afferents retrogradely labeled with ctb-594 were double labeled for estrogen receptor α (ERα; Figure 1 B). Zero out of 499 colonic afferents double labeled for ERα although many unidentified neurons in the same sections were labeled for ERα. As a positive control, bladder afferents which were reported to express ERα [6] were retrogradely labeled following injection of ctb. Approximately 20% of retrogradely labeled bladder afferents double labeled for ERα (Figure 1C). These data suggest the previously reported effects of systemically administered E2 on colorectal sensitivity and nociceptive processing were centrally mediated.
Figure 1.
A: Colonic afferents are unresponsive to modulation by estradiol. A: There was no difference in the stimulus response functions of colonic afferents to graded intensities of CRD between intact and OVx rats, n=18–38/group. B: Photomicrographs of the same field from an L6 DRG showing colonic afferents retrogradely labeled with ctb-594 (Bi); the same field labeled for ERα (Bii); and the merged image showing a lack of double labeled cells (Biii). Arrowheads point to examples of colonic afferents that do not express ERα. C: Photomicrographs of the same field from an L6 DRG showing bladder afferents retrogradely labeled with ctb-594 (Ci); the same field labeled for ERα (Cii); and the merged image showing several double labeled cells (Ciii). Arrowheads point to bladder afferents that express ERα.
Spinal estrogen receptor activation facilitates the visceromotor response
The effect of intrathecal E2 on the visceromotor response in OVx rats
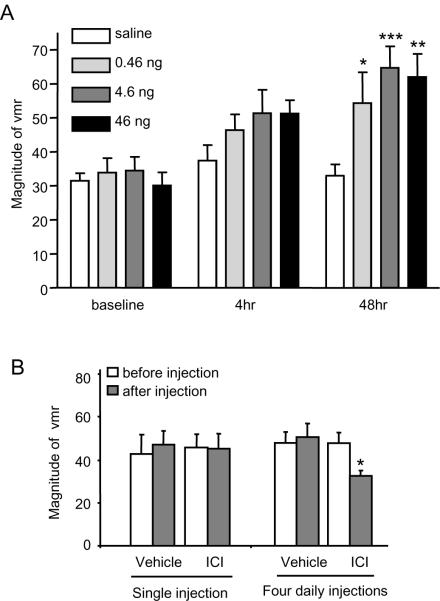
We previously reported that spinal dorsal horn neurons express ERα [74]. To test the hypothesis that E2- induced pronociception is spinally mediated the effect of intrathecally administered E2 (water soluble; 0.46, 4.6, 46 ng) or vehicle (saline) on the visceromotor response to 60 mmHg CRD was examined 4 and 48 hours following injection. Overall, intrathecal E2 facilitated the visceromotor response (two way ANOVA: treatment, p<0.005; time, p<0.001; treatment×time, NS, p>0.1. Figure 2). Forty-eight hours following injection, E2 significantly increased the visceromotor response compared with saline (0.46 ng: p<0.05, n=9; 4.6 ng: p<0.001, n=14; 46 ng: p<0.01, n=8), though no dose-dependency was observed. Four hours post injection, there was a tendency for E2 to increase the magnitude of the visceromotor response, but this only reached significance if the doses of E2 were pooled (t-test: p<0.05).
Figure 2.
Spinal E2 increases visceral nociceptive processing in OVx rats. A: I.t. E2 facilitated the visceromotor response (vmr) for all doses 48 hours post injection (*, **, *** p<0.05, 0.01, 0.005 vs. saline at 48 hours; n=8–14/group). At the earlier time point there was a tendency for E2 to increase the vmr (pooled drug vs. saline, p<0.05). B: a single i.t. injection of ICI 182,780 in intact female rats had no effect on the vmr. Four daily injections of ICI attenuated the vmr. * p<0.05 vs. pre-injection.
The magnitude of the visceromotor response at 48 hours after intrathecal injection of E2 was approximately 60 mV/sec which was comparable to that following systemic injection of E2 [34], although the plasma E2 concentration after intrathecal injection was below the detectable level (<5 pg/ml).
The effect of intrathecal ICI 182,780 on visceromotor response in intact rats
To confirm a spinal site of action of estradiol, an estrogen receptor antagonist or vehicle (10% ETOH) was administered intrathecally to intact female rats. One day following a single i.t. injection of ICI 182,780 (10 nmol) there was no change in the magnitude of the visceromotor response (paired t-test, p=0.8, n=4; Figure 2B). I.t. administration of ICI 182,780 daily for four days significantly decreased the magnitude of the visceromotor response (paired t-test, p<0.05, n=6). One time (n=4) or 4 daily injections (n=11) of vehicle did not induce any change in the magnitude of the visceromotor response. Taken together, the intrathecal agonist and antagonist data support the hypothesis that spinal estrogen receptors mediate the E2-induced increase in visceral nociceptive processing.
E2 increases ERK activation in the spinal cord
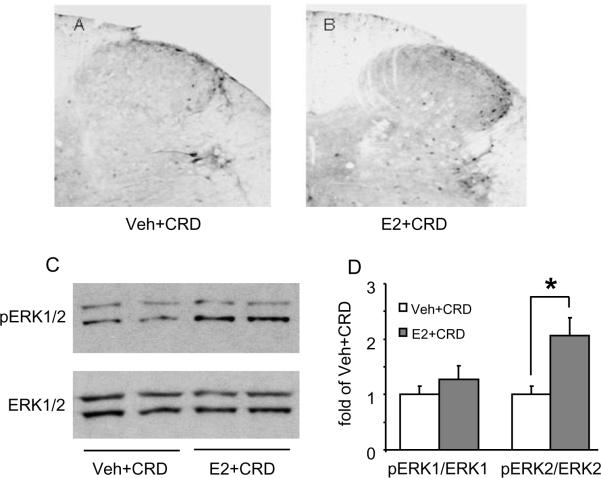
Accumulating lines of evidence suggest Ca2+ influx through NMDA receptors causes activation of protein kinase A (PKA), which further induces phosphorylation of ERK and CREB, and subsequently influences synaptic plasticity [5,31,40,43,85]. CRD-induced visceral signal processing in the spinal cord involves activation of NMDA receptors and E2 facilitates this process [37,74,80], suggesting E2 increases activation of ERK in the spinal cord. To test this hypothesis, the effect of CRD on pERK expression was compared in OVx and 48 hour E2 treated rats. Following noxious CRD only a few pERK-like immunoreactive neurons were scattered in the LS spinal cord in OVx rats (Figure 3A). A greater number of pERK labeled neurons were observed in the superficial and deep layers of the dorsal horn in E2-treated rats (Figure 3B). The increase in phosphorylation of ERK in the spinal cord was quantified by Western blot. The pERK and ERK antibodies recognized two immunoreactive bands, pERK1/2 and ERK1/2, respectively. Noxious CRD increased pERK2 expression in E2-treated rats compared to OVx rats (p<0.05, t-test, n=4–5; Figure 3C,D). Noxious CRD did not alter the level of pERK1 in either group. There was no difference in ERK1/2 expression between the groups. Taken together, these data suggest that E2 increases the CRD-induced phosphorylation of ERK2 in spinal dorsal horn neurons.
Figure 3.
E2 increases the phosphorylation of the Extracellular-Signal-Regulated Kinase (pERK) induced by colorectal distention in OVx rats. A, B: Representative sections of CRD-induced pERK expression in the LS spinal dorsal horn from a vehicle- (A) and E2-treated (B) rat. C: Western blots (C) and quantified data (D) showing that CRD significantly increased the pERK2 level in E2-treated rats. Values represent the pERK1/2 to ERK1/2 ratio normalized to vehicle + CRD rats. * p<0.05 vs. vehicle + CRD.
ERα activation mimics E2 induced facilitation of visceral nociception
Systemic PPT time-dependently increases the visceromotor response
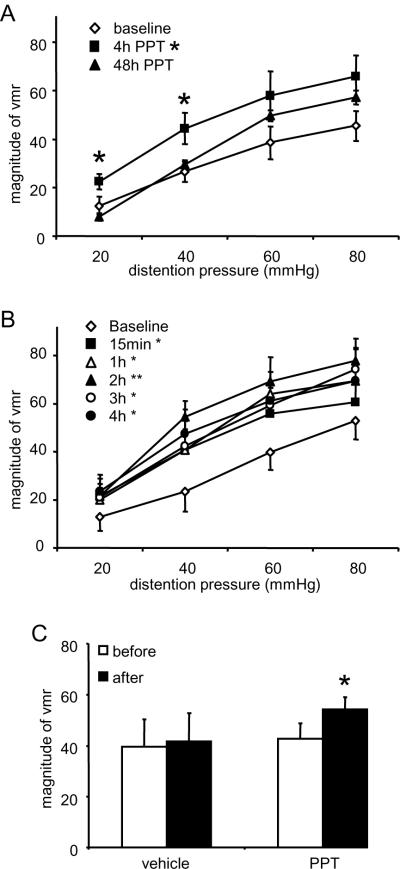
To test whether ERα mediates E2-induced visceral pronociception, the ERα selective agonist PPT (1 mg/kg) was injected subcutaneously to see if it mimics the effect of E2. The dose of PPT for systemic injection was selected based on previous studies [48,87]. The effect of PPT on the visceromotor response was first evaluated four hours and 48 hours post injection, the same time points examined following E2 injection. Four hours after PPT injection the visceromotor response was significantly increased with the response returning towards the baseline level at 48 hours (two way RM ANOVA: time, time × pressure, p<0.05; pressure, p<0.001; n=6; Figure 4A). In a separate group of rats, the visceromotor response was measured at several time points between 15 minutes and 4 hours post PPT administration. PPT significantly increased the visceromotor response at each timepoint measured starting 15 minutes after injection, a facilitation that persisted at least through 4 hours (two way RM ANOVA: time, p<0.05; pressure, p<0.001; interaction, NS; n=6; Figure 4B). The response at four hours was similar to that observed in rats initially tested at 4 hours post injection (Figure 4A), confirming the pronociceptive effect of PPT. PPT at a higher dose (3 mg/kg) did not further increase the magnitude of the visceromotor response (data not shown).
Figure 4.
Selective ERα activation in OVx rats increases the visceromotor response to CRD. A: Systemically injected PPT increased the magnitude of the vmr 4 hours post injection compared to baseline (p<0.05). By 48 hours the response returned to baseline levels. * p<0.05 vs. baseline. B: The effects of systemically injected PPT on the magnitude of the visceromotor response were measured at several time points between 15 minutes and 4 hours post injection. There was an overall increase in the vmr by PPT (p<0.05) and the increase was significant at each time point examined (* p<0.05, ** p<0.01 vs. baseline). C: Intrathecally injected PPT facilitated the visceromotor response. * p<0.05 vs. pre-injection.
Intrathecal PPT increases the visceromotor response
In order to determine if PPT acts at a spinal site, PPT (50ng/5μl) or vehicle was injected intrathecally in OVx rats and the visceromotor response to 60 mmHg CRD was recorded 4 hours following injection. Spinal PPT significantly increased the magnitude of the visceromotor response (p<0.05, paired t-test, n = 10; Figure 4C). Vehicle injection had no effect (p=0.8, paired t-test, n=4).
Systemic and spinal PPT increase dorsal horn neuronal activity
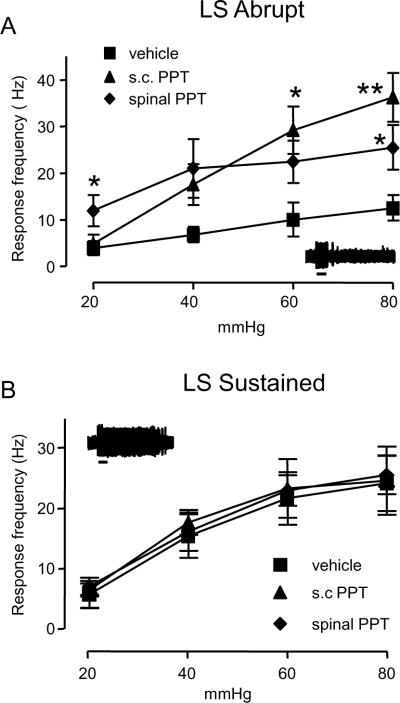
To further confirm that spinal activation of ERα mediates the pronociceptive component of estradiol on visceral nociceptive processing, spinal dorsal horn neuronal activity was examined using extracellular single unit recording. There was no difference in the response of Abrupt neurons to CRD after s.c. or spinal vehicle (two way RM ANOVA: i.t. vs. s.c., p=0.6) so the data were pooled for subsequent analyses. Between 4 and 7 hours following s.c. or spinal administration of PPT there was a significant increase in the response of Abrupt neurons compared to vehicle (two way RM ANOVA: treatment, p<0.05; pressure, p<0.001; interaction, p<0.001; n= 5–9; Figure 5A), but no difference in response between spinal or systemic administration. In contrast, PPT administered systemically or spinally did not alter the response of Sustained neurons (Figure 5B). This pattern of responses is similar to our previous report looking at the effect of E2 on the response of dorsal horn neurons to CRD [34], suggesting that activation of ERα mediates the pronociceptive effect of estradiol.
Figure 5.
ERα activation in OVx rats modulates the response of visceroceptive dorsal horn neurons. A: Subcutaneously (1mg/kg) and spinally (50 ng) administered PPT selectively facilitated the response of Abrupt neurons to CRD (two way RM ANOVA, p<0.05). * p<0.05, **p<0.01 vs. vehicle; n=5–9/group. B: PPT had no effect on the response of Sustained neurons (n=4–6/group). The insets show a typical response for each type of neuron. The small bar is the duration of the distention (20 sec).
ERα activation facilitates visceral nociceptive processing via ERK activation
PPT increases spinal ERK activation
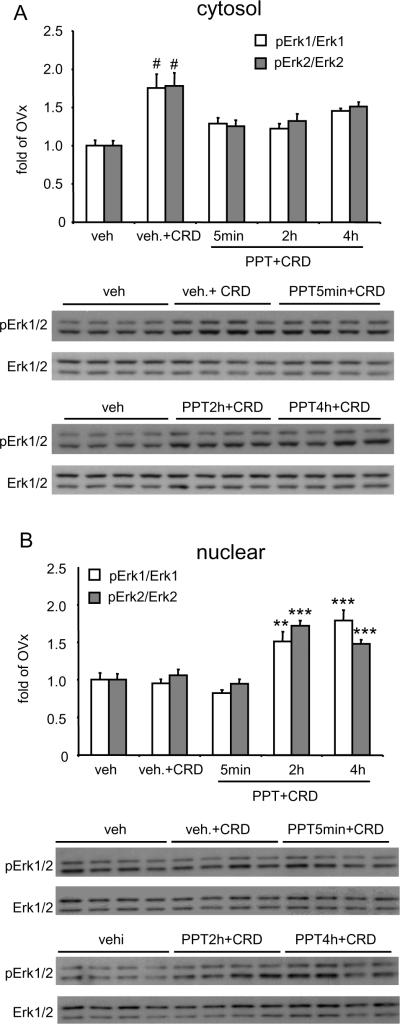
To test whether ERα activation induces activation of ERK in the spinal cord, 1 mg/kg PPT or vehicle was administered subcutaneously in OVx rats. The rats were distended at a noxious pressure (80mmHg) for 30 minutes (30 sec on; 90 sec off) starting 5 minutes, 2 or 4 hours following PPT injection. Control rats were not distended but were injected with vehicle for PPT. Western blot data showed a parallel change in the cytosolic pERK1/ERK1 and pERK2/ERK2 ratio in all groups following CRD (Figure 6A). CRD increased ERK phosphorylation in the cytosol in vehicle-injected OVx rats compared with control rats (One Way ANOVA, pERK1 p<0.001, pERK2 p<0.005, n=4/group). However, in PPT treated rats there was no change in cytosolic ERK phosphorylation. In contrast, the nuclear pERK/ERK ratio did not change in vehicle treated rats following distention, but CRD-induced pERK expression was significantly elevated in PPT injected rats 2 and 4 hours post injection (One Way ANOVA, pERK1 p<0.001, pERK2 p<0.001, n= 4/group; Figure 6B), suggesting a translocation of pERK from cytoplasm to the nucleus.
Figure 6.
ERα activation in OVx rats induced changes in spinal ERK expression measured with Western blots. A: CRD increased pERK expression in vehicle- treated rats in the cytosolic fraction (ERK1 p<0.001; ERK2 p<0.005). There was no effect of CRD in PPT treated rats. # p<0.005 vs. veh, PPT 5 minutes, PPT 2 hour. B: CRD increased pERK expression in the nuclear fraction in PPT treated rats (ERK1 p<0.001; ERK2 p<0.001). There was no effect of CRD in vehicle treated rats. ** p< 0.005, *** p<0.001 vs. veh, veh + CRD, 5 min PPT.
Inhibition of spinal ERK activation by PD98059 decreases the visceromotor response
To determine whether ERK activation contributed to the PPT-induced increase in the visceromotor response, OVx rats were injected s.c. with PPT or its vehicle (veh-PPT). Four hours later rats were injected intrathecally with the MEK inhibitor PD 98059 (1 μg/5 μl) (n=8) or its vehicle (PD-veh; n=6) and the visceromotor response to CRD (80mmHg) was recorded. In PPT treated rats, PD 98059 significantly decreased the magnitude of the visceromotor response compared to the PD-veh (Two way RM ANOVA, treatment, p<0.001; n=6–8/group; Figure 7A). In PPT vehicle treated rats PD 98059 had no effect on the visceromotor response compared to PD-veh (Two way RM ANOVA, p=0.4; n=5–6/group; Figure 7B).
Figure 7.
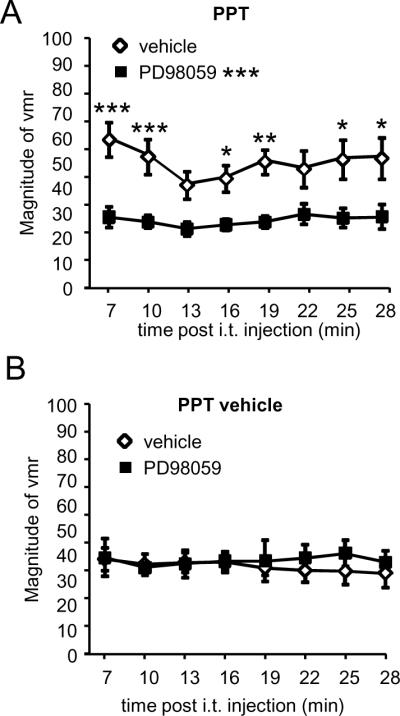
Intrathecal injection of the MEK inhibitor PD 98059 (1 μg) blocked the facilitation of the visceromotor response induced by PPT. A: In PPT-treated rats, the visceromotor response following i.t. PD 98059 was significantly less compared to i.t. vehicle (p<0.001). *, **, *** p<0.05, 0.01, 0.001 vs. vehicle at that time point. B: In rats injected with the vehicle for PPT, i.t. PD 98059 had no effect on the magnitude of the visceromotor response compared to i.t. vehicle.
Discussion
The present study demonstrates that estradiol facilitates colorectal distention-induced visceral nociceptive processing at the level of the spinal cord, at least partially through activation of spinal ERα. An increase in ERK phosphorylation in the spinal cord may contribute to E2-induced pronociception. This facilitatory effect of E2 is observed at 4 and 48 hours, while selective agonist activation of ERα-mediated facilitation of visceral nociception occurs slightly faster. Colonic afferents appear not to contribute to the E2-induced facilitation.
Spinal Estrogen receptors modulate nociceptive activity
We have previously reported that E2 systemically administrated to OVx rats facilitated the visceromotor response to CRD, but the site of action was not clear [34]. The present study suggests this was not mediated by colonic afferents since the response of colonic primary afferent fibers to CRD did not differ between ovariectomized and intact female rats and retrogradely labeled colonic afferents did not label immunocytochemically for ERα. This observation was surprising since ERα is expressed in afferents innervating the urinary bladder [6] and E2 increased activity in afferents innervating the uterine-cervix [47] as well as unidentified DRG neurons in vitro [16,53]. However, we cannot rule out the possibility that ERs modulate transmitter release at the primary afferent terminal [71] as this would not be apparent in the primary afferent recordings since the colonic afferents were decentralized.
A spinal site of action of E2 was supported by the present observation that intrathecally injected E2 facilitated the visceromotor response similar to systemically administered E2, and intrathecal injection of the anti-estrogen ICI-182,780 [30] attenuated the visceromotor response to CRD in intact female rats. However, it took several days for the ER antagonist to take effect. Estrogen induces transcriptional activation directly through nuclear receptor activation or indirectly following rapid membrane receptor mediated events (for review, see [17,45,84]). In a normal cycling female rat, it could take several days for a loss of E2/ER interaction and subsequent downstream effects to be manifest as a decrease in pain sensitivity. This concept is supported by the observation that multiple injections of ICI attenuated uretal calculosis crises in intact female rats [2]. Although it seems to conflict with the immediate attenuation in thermal sensitivity in Japanese quail following i.t. administration of an aromatase inhibitor [22], comparable results of an aromatase inhibitor have not been reported in rodents and the mechanisms through which estrogen modulates nociceptive processing remain unclear. Indeed, our observation that PPT had a relatively rapid onset of action in facilitating the visceromotor response to CRD suggests membrane ERs could have been activated [12,56,84]. However, ERK phosphorylation was not elevated within the first 30 minutes, indicating other intracellular mechanism(s) might be involved. The increase in the visceromotor response at four hours post PPT clearly involves ERK activation, since the MAPK inhibitor blocked PPT-induced visceral pronociception.
Alternatively, ICI is an agonist at the putative estrogen receptor GPR30 (GPER) [50,60] and these effects could have countered the antagonist action at ERα/β in the short term.
Both ERα and ERβ are expressed in spinal cord neurons in rodents and primates [4,54,58,74,81,83]. In primates, the lumbosacral spinal cord and the caudal spinal trigeminal nucleus had significantly more ERα immunoreactive neurons than other spinal segments [83]. If this differential segmental distribution is maintained in humans, it might help explain why the severity of a few pain syndromes such as migraine headaches, temporomandibular disorders and irritable bowel syndrome fluctuate with the estrous cycle.
In contrast to the differential segmental distribution in primates, estrogen receptors in rodents are evenly distributed along the rostrocaudal length of the spinal cord [54,81]. However, very subtle or no changes in acute somatic nociception were observed during the estrous cycle although there are profound changes in visceral nociceptive processing [14,15,29,36,63,68,76]. One potential explanation for the discrepancy is that the noxious intensity of acute somatic stimuli (mechanical, thermal) are relatively brief and may not activate cellular processes that are modulated by estrogens. In contrast, acute visceral stimuli are applied for tens of seconds and may activate different cellular processes. Indeed, acute visceral, but not somatic, stimuli activate NMDA receptor signaling [37,80] and NMDA receptor activity is modulated by E2 [53,74,88]. In addition, acute visceral, but not somatic stimuli, increase ERK activation, the extent of which is modulated by E2 [24,31,42].
The specific role of the classical estrogen receptors (ERα, ERβ) in nociceptive processing is unclear. Depending on experimental parameters E2 is reported to have pro- or antinociceptive effects. Our data support a pronociceptive effect of estrogen that is mediated by spinal ERα since administration of the ERα agonist PPT mimicked the effect of E2 by increasing the magnitude of the visceromotor response to acute distention. In addition, similar to the effects of systemic E2 [34,64], systemic and spinal PPT facilitated the response of visceroceptive Abrupt spinal dorsal horn neurons to colonic stimuli, but had no effect on Sustained neurons, providing further support that spinal ERα is involved in E2-induced facilitation of visceral nociception.
The role of ERβ in visceral pain processing is yet to be clarified since the ERβ agonist DPN gives inconsistent results (unpublished observations). However, during the interphase of the formalin test ERβ knockout mice had less nociceptive behaviors than wildtype, suggesting ERβ dampened inhibitory mechanisms [72]. In contrast, the ERβ agonist ERb-041 was antihyperalgesic in several pain models [26,44,59].
Signaling pathways
The spinal MAPK pathway is a key signaling pathway in the spinal processing of noxious stimuli. Nociceptive stimuli applied to somatic tissue elicits ERK phosphorylation in the spinal dorsal horn [31,32,39]. Acute inflammation and distention of the colon induced ERK activation in the spinal cord in male rats [24,89]. In the present study, we demonstrated increased CRD-induced spinal pERK expression in the nucleus of dorsal horn neurons in the presence of E2. In addition, we have shown a rapid increase in CRD-induced cytoplasmic pERK in OVx rats in the absence of ERα activation.
ERK activity is extensively modulated by E2. E2 increased masseter muscle inflammation-induced pERK in trigeminal ganglion cells exacerbating hyperalgesia and allodynia, and a MAPK inhibitor attenuated spinomedullary dorsal horn neuronal activity evoked by ATP in the normal and inflamed TMJ [49,75]. Significantly more ERK2 phosphorylation was detected in the hippocampus of E2-treated rats than OVx rats [9]. In addition, normally cycling rats in proestrus had more pERK than diestrus rats [10] and pERK expression was positively correlated with the plasma E2 concentration [9,10,61]. The present finding that E2 increased ERK2 phosphorylation extends E2 modulation of ERK to the spinal cord following visceral stimulation.
Activation of ERK and its role in synaptic plasticity is complex. ERK phosphorylation is initiated by the activation of many receptors. One pathway involves NMDA receptor activation-Ca2+ influx-PKA activation-ERK phosphorylation [77,85]. Glutamate is one of the major neurotransmitters released from primary afferents upon stimulation. Spinal NMDA receptors are activated by noxious and non-noxious visceral and/or somatic stimuli [19,25,37,65,80] and a noxious stimulus-induced increase in spinal ERK was attenuated by NMDA receptor antagonists [31,38,40]. We previously reported E2 increased expression of spinal NMDA receptors, which could partially account for increased NMDA receptor activity [74] and enhanced ERK phosphorylation in E2-treated rats in the present study. On the other hand, NMDA receptors could be a downstream target for pERK. PKA-ERK activation induced phosphorylation of NMDA receptor subunits in the spinal cord and a MAPK inhibitor attenuated NMDA-evoked currents in the amygdala of arthritic rats [23,70]. Spinal PKA inhibitors attenuated NR1 phosphorylation decreasing the visceromotor response to CRD [74]. Our observation that a MAPK inhibitor also blocked the distention-evoked visceromotor response in PPT-treated rats provides further evidence that the MAPK signaling pathway is involved in estrogen receptor-mediated enhancement of visceral sensitivity.
ERK translocation
E2-induced rapid activation of cell signaling pathways could result in changes in the nucleus that regulate gene expression, cell proliferation and cell death. The MAPK cascade is required for E2-mediated neuroprotection. However, not all MAPK activation has neuroprotective effects. The differential distribution of pERK in the cell body seems to be a predictive factor in whether a neuroprotective outcome would occur. For example, E2 and progesterone rapidly and transiently activated nuclear ERK in primary cultured hippocampal neurons and attenuated a glutamate induced [Ca2+]i rise[57]. In contrast, ERK activated by the synthetic progestin medroxyprogesterone acetate (MPA) remained cytosolic with no nuclear signal and E2-induced neuroprotection was blunted [57].
In the present study, in OVx rats we observed colorectal distention induced cytosolic ERK activation with no detectable changes in nuclear pERK and no effect of a MAPK inhibitor on the visceromotor response. In contrast, nuclear pERK increased in PPT-treated rats following colorectal distention at later time points. This paralleled the increase in the visceromotor response which was attenuated by the MAPK inhibitor. These data suggest transcriptional changes induced by pERK translocation to the nucleus likely contributes to E2 induced visceral pronociception.
In conclusion, the present study demonstrates that E2 increases the processing of colorectal nociception by activation of spinal ERα and modulation of spinal ERK activity. These findings further our understanding of the mechanism underlying E2 modulation of visceral pain and provide a basis for therapeutic management of E2 related pain syndromes.
Summary.
Estradiol exacerbates visceral pain in ovariectomized rats. Selective activation of the estrogen receptor alpha isoform in the spinal cord underlies this pronociceptive effect.
Acknowledgements
Supported by NIH R01 NS 37424.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no conflicts of interest.
Reference List
- 1.Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, Trentalance A, Muramatsu M, Marino M. Survival versus apoptotic 17beta-estradiol effect: role of ER alpha and ER beta activated non-genomic signaling. J.Cell Physiol. 2005;203:193–201. doi: 10.1002/jcp.20219. [DOI] [PubMed] [Google Scholar]
- 2.Affaitati G, Ceccarelli I, Fiorenzani P, Rossi C, Pace MC, Passavanti MB, Aurilio C, Sorda G, Danielli B, Giamberardino MA, Aloisi AM. Sex differences in the analgesic effects of ICI 182,780 and Flutamide on ureteral calculosis in rats. Horm.Behav. 2010 doi: 10.1016/j.yhbeh.2010.09.008. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 3.Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81:193–199. doi: 10.1159/000087002. [DOI] [PubMed] [Google Scholar]
- 4.Amandusson A, Blomqvist A. Estrogen receptor-[alpha] expression in nociceptive-responsive neurons in the medullary dorsal horn of the female rat. European Journal of Pain. 2010;14:245–248. doi: 10.1016/j.ejpain.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J.Neurochem. 2004;91:462–470. doi: 10.1111/j.1471-4159.2004.02734.x. [DOI] [PubMed] [Google Scholar]
- 6.Bennett HL, Gustafsson JA, Keast JR. Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton.Neurosci. 2003;105:90–100. doi: 10.1016/S1566-0702(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 7.Bereiter DA. Sex differences in brainstem neural activation after injury to the TMJ region. Cells Tissues.Organs. 2001;169:226–237. doi: 10.1159/000047886. [DOI] [PubMed] [Google Scholar]
- 8.Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005;117:58–67. doi: 10.1016/j.pain.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Bi R, Foy MR, Thompson RF, Baudry M. Effects of estrogen, age, and calpain on MAP kinase and NMDA receptors in female rat brain. Neurobiol.Aging. 2003;24:977–983. doi: 10.1016/s0197-4580(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 10.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. PNAS. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol.Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 12.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol Activates Group I and II Metabotropic Glutamate Receptor Signaling, Leading to Opposing Influences on cAMP Response Element-Binding Protein. J.Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradshaw HB, Berkley KJ. Estrous changes in responses of rat gracile nucleus neurons to stimulation of skin and pelvic viscera. J.Neurosci. 2000;20:7722–7727. doi: 10.1523/JNEUROSCI.20-20-07722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–197. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 15.Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm.Behav. 2003;44:123–131. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 16.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca(2+) signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 17.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dechering K, Boersma C, Mosselman S. Estrogen receptors alpha and beta: two receptors of a kind? Curr.Med.Chem. 2000;7:561–576. doi: 10.2174/0929867003375010. [DOI] [PubMed] [Google Scholar]
- 19.Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. TINS. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 20.Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, Oprea TI, Dun NJ. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J.Neurosci.Res. 2009;87:1610–1619. doi: 10.1002/jnr.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evrard HC. Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. Am.J.Physiol Regul.Integr.Comp Physiol. 2006;291:R291–R299. doi: 10.1152/ajpregu.00930.2005. [DOI] [PubMed] [Google Scholar]
- 22.Evrard HC, Balthazart J. Rapid Regulation of Pain by Estrogens Synthesized in Spinal Dorsal Horn Neurons. J.Neurosci. 2004;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol.Pain. 2008;4:26. doi: 10.1186/1744-8069-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galan A, Cervero F, Laird JM. Extracellular signaling-regulated kinase-1 and -2 (ERK 1/2) mediate referred hyperalgesia in a murine model of visceral pain. Brain Res.Mol Brain Res. 2003;116:126–134. doi: 10.1016/s0169-328x(03)00284-5. [DOI] [PubMed] [Google Scholar]
- 25.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol.Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardell LR, Hyldtoft L, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Brann MR, Olsson R, Piu F. Differential modulation of inflammatory pain by a selective estrogen receptor beta agonist. Eur.J.Pharmacol. 2008;592:158–159. doi: 10.1016/j.ejphar.2008.06.107. [DOI] [PubMed] [Google Scholar]
- 27.Geraldes P, Sirois MG, Bernatchez PN, Tanguay JF. Estrogen regulation of endothelial and smooth muscle cell migration and proliferation: role of p38 and p42/44 mitogen-activated protein kinase. Arterioscler.Thromb.Vasc.Biol. 2002;22:1585–1590. doi: 10.1161/01.atv.0000035393.11854.6a. [DOI] [PubMed] [Google Scholar]
- 28.Guillemin I, Becker M, Ociepka K, Friauf E, Nothwang HG. A subcellular prefractionation protocol for minute amounts of mammalian cell cultures and tissue. Proteomics. 2005;5:35–45. doi: 10.1002/pmic.200400892. [DOI] [PubMed] [Google Scholar]
- 29.Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distention in male and female rats anesthetized with halothane. Br.J.Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- 30.Howell A, Osborne CK, Morris C, Wakeling AE. ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer. 2000;89:817–825. doi: 10.1002/1097-0142(20000815)89:4<817::aid-cncr14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat.Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 32.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J.Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res.Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Y, Murphy AZ, Traub RJ. Estrogen Modulates the Visceromotor Reflex and Responses of Spinal Dorsal Horn Neurons to Colorectal Stimulation in the Rat. J.Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji Y, Tang B, Traub RJ. Estrogen increases and progesterone decreases behavioral and neuronal responses to colorectal distention following colonic inflammation in the rat. Pain. 2005;117:433–442. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji Y, Traub RJ. Spinal NMDA receptors contribute to neuronal processing of acute noxious and nonnoxious colorectal stimulation in the rat. J.Neurophysiol. 2001;86:1783–1791. doi: 10.1152/jn.2001.86.4.1783. [DOI] [PubMed] [Google Scholar]
- 38.Karim F, Hu HJ, Adwanikar H, Kaplan D, Gereau RW. Impaired inflammatory pain and thermal hyperalgesia in mice expressing neuron-specific dominant negative mitogen activated protein kinase kinase (MEK) Mol.Pain. 2006;2:2. doi: 10.1186/1744-8069-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karim F, Wang CC, Gereau RW. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J.Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der MC, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J.Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res. 1996;742:352–354. doi: 10.1016/s0006-8993(96)01108-0. [DOI] [PubMed] [Google Scholar]
- 42.Klinger MB, Sacks S, Cervero F. A role for extracellular signal-regulated kinases 1 and 2 (ERK 1/2) in the maintenance of persistent mechanical hyperalgesia in ovariectomized mice. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 43.Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 44.Leventhal L, Brandt MR, Cummons TA, Piesla MJ, Rogers KE, Harris HA. An estrogen receptor-beta agonist is active in models of inflammatory and chemical-induced pain. Eur.J Pharmacol. 2006;553:146–148. doi: 10.1016/j.ejphar.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol.Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol.Metab. 2009 doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B, Eisenach JC, Tong C. Chronic estrogen sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. J Neurophysiol. 2005;93:2167–2173. doi: 10.1152/jn.01012.2004. [DOI] [PubMed] [Google Scholar]
- 48.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 49.Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia. 2009 doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J.Endocrinol. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 51.Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol Replacement in Ovariectomized Rats Is Antihyperalgesic in the Formalin Test. J.Pain. 2006 doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol.Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 53.McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148:1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J.Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 55.Mermelstein PG. Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. J.Neuroendocrinol. 2009;21:257–262. doi: 10.1111/j.1365-2826.2009.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc.Natl.Acad.Sci.U.S.A. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- 59.Piu F, Cheevers C, Hyldtoft L, Gardell LR, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Schiffer HH, Donello JE, Olsson R, Gil DW, Brann MR. Broad modulation of neuropathic pain states by a selective estrogen receptor beta agonist. Eur.J.Pharmacol. 2008;590:423–429. doi: 10.1016/j.ejphar.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 60.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Molecular and Cellular Endocrinology. 2007;265–266:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raval AP, Saul I, Dave KR, DeFazio RA, Perez-Pinzon MA, Bramlett H. Pretreatment with a single estradiol-17beta bolus activates cyclic-AMP response element binding protein and protects CA1 neurons against global cerebral ischemia. Neuroscience. 2009;160:307–318. doi: 10.1016/j.neuroscience.2009.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol.Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 63.Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–356. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- 64.Robbins MT, Mebane H, Ball CL, Shaffer AD, Ness TJ. Effect of estrogen on bladder nociception in rats. J.Urol. 2010;183:1201–1205. doi: 10.1016/j.juro.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salter MW, Kalia LV. SRC KINASES: A HUB FOR NMDA RECEPTOR REGULATION. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 66.Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: A model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 67.Sanoja R, Cervero F. Estrogen modulation of ovariectomy-induced hyperalgesia in adult mice. European Journal of Pain. 2008;12:573–581. doi: 10.1016/j.ejpain.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- 69.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J.Comp.Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 70.Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci. 2004;20:1769–1778. doi: 10.1111/j.1460-9568.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- 71.Smejkalova T, Woolley CS. Estradiol Acutely Potentiates Hippocampal Excitatory Synaptic Transmission through a Presynaptic Mechanism. J.Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spooner MF, Robichaud P, Carrier JC, Marchand S. Endogenous pain modulation during the formalin test in estrogen receptor beta knockout mice. Neuroscience. 2007;150:675–680. doi: 10.1016/j.neuroscience.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 73.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:540–549. doi: 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tashiro A, Okamoto K, Bereiter DA. Chronic inflammation and estradiol interact through MAPK activation to affect TMJ nociceptive processing by trigeminal caudalis neurons. Neuroscience. 2009;164:1813–1820. doi: 10.1016/j.neuroscience.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terner JM, Lomas LM, Picker MJ. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005;6:372–383. doi: 10.1016/j.jpain.2005.01.354. [DOI] [PubMed] [Google Scholar]
- 77.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat.Rev.Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 78.Traub RJ. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. NeuroReport. 2000;11:2113–2116. doi: 10.1097/00001756-200007140-00011. [DOI] [PubMed] [Google Scholar]
- 79.Traub RJ, Herdegen T, Gebhart GF. Differential expression of c-Fos and c-Jun in two regions of the rat spinal cord following noxious colorectal distention. Neurosci.Lett. 1993;160:121–125. doi: 10.1016/0304-3940(93)90394-z. [DOI] [PubMed] [Google Scholar]
- 80.Traub RJ, Zhai QZ, Ji Y, Kovalenko M. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention-induced Fos expression and the visceromotor reflex. Neurosci. 2002;113:205–211. doi: 10.1016/s0306-4522(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 81.VanderHorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J.Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- 82.VanderHorst VG, Terasawa E, Ralston HJ., III Estrogen receptor-alpha immunoreactive neurons in the ventrolateral periaqueductal gray receive monosynaptic input from the lumbosacral cord in the rhesus monkey. J.Comp Neurol. 2002;443:27–42. doi: 10.1002/cne.10098. [DOI] [PubMed] [Google Scholar]
- 83.VanderHorst VGJM, Terasawa E, Ralston HJ., III Estrogen receptor-[alpha] immunoreactive neurons in the brainstem and spinal cord of the female rhesus monkey: Species-specific characteristics. Neuroscience. 2009;158:798–810. doi: 10.1016/j.neuroscience.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol.Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 86.Wang G, Tang B, Traub RJ. Differential processing of noxious colonic input by thoracolumbar and lumbosacral dorsal horn neurons in the rat. J.Neurophysiol. 2005;94:3788–3794. doi: 10.1152/jn.00230.2005. [DOI] [PubMed] [Google Scholar]
- 87.Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J.Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang XJ, Li Z, Chung EK, Zhang HQ, Xu HX, Sung JJ, Bian ZX. Activation of extracellular signal-regulated protein kinase is associated with colorectal distension-induced spinal and supraspinal neuronal response and neonatal maternal separation-induced visceral hyperalgesia in rats. J.Mol.Neurosci. 2009;37:274–287. doi: 10.1007/s12031-008-9134-y. [DOI] [PubMed] [Google Scholar]
- 90.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]