Our study results show a correlation between changes in total tissue hemoglobin at diffuse optical spectroscopic tomography and tumor angiogenesis and demonstrate how this noninvasive modality might allow individualization and optimization of neoadjuvant chemotherapy regimens.
Abstract
Purpose:
To investigate if changes in tumor angiogenesis associated with complete pathologic response (pCR) or partial pathologic response (pPR) to treatment can be demonstrated by using diffuse optical spectroscopic (DOS) tomography.
Materials and Methods:
All participants in this prospective, HIPAA-compliant, institutional review board–approved study provided written informed consent. Eleven women with invasive breast carcinoma were imaged with DOS tomography prior to, during, and at completion of neoadjuvant chemotherapeutic regimens. By using region of interest (ROI) analysis, the DOS measure of total tissue hemoglobin (HbT) was temporally correlated with quantitative measures of existing (CD31-expressing) and tumor-induced (CD105-expressing) vessels, in pretreatment and posttreatment tissue specimens, to assess change.
Results:
Quantified angiogenesis alone in pretreatment core biopsy specimens did not predict treatment response, but mean vessel density (MVD) and mean vessel area (MVA) of CD105-expressing vessels were significantly decreased in women with pCR (n = 7) (P < .001 and P = .003, respectively). MVA of CD105-expressing vessels was also significantly reduced at comparison of pre- and posttreatment residual tumor for women with pPR (n = 4) (P = .033). A longitudinal analysis showed significant decreases (P = .001) in mean HbT levels during neoadjuvant chemotherapy in breast abnormality ROIs for women with pCR but not women with pPR. For women with pCR, but not women with pPR, pretreatment MVD of CD105-expressing vessels correlated with pretreatment HbT (P ≤ .001).
Conclusion:
DOS tomographic examinations in women with breast cancer who are receiving neoadjuvant chemotherapy show a mean decrease in HbT with time in patients with pCR only. Observed pretreatment and posttreatment correlates with quantified angiogenesis markers confirm the likely biologic origin for this DOS signature and support its potential to predict angiogenic tissue response early in the treatment cycle.
© RSNA, 2011
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.11100699/-/DC1
Introduction
The ability to better track treatment response in patients with locally advanced breast cancers undergoing neoadjuvant chemotherapy would substantially improve individualized treatment management in this subset of patients (1). Results of pilot studies of newly developed breast imaging modalities (2–8) have demonstrated the capability of imaging biologic markers that reflect tissue function and hence should better predict response to therapy. Diffuse optical spectroscopic (DOS) tomography provides information about the intrinsic vascular and ultrastructural biophysical composition of breast tissue (9–17). The validation of DOS tomographic images and spectra requires both morphologic diagnoses from histopathologic examination and parametric analysis of tissue features, beyond the declared pathologic diagnosis (18). To assess whether DOS spectral imaging can predict treatment response, especially in the first few weeks of neoadjuvant chemotherapy, a robust correlation between key DOS spectral and underlying biologic measures needs to be demonstrated. Preexisting blood vessel density and size, identified with the pan-endothelial marker CD31, have already been correlated with DOS spectral signatures in a range of benign and malignant breast tumors (19,20), but the specific identification and quantification of new, tumor-induced vessels has not been correlated and would be invaluable in assessing treatment response. Additionally, the temporal change of these parameters in response to therapy has, to our knowledge, not been examined for correlation, yet this verification needs to be done to establish clearly the accuracy of tracking response to neoadjuvant chemotherapy.
CD105 (endoglin) is a transmembrane glycoprotein expressed on the surface of highly proliferating endothelial cells and a receptor for the transforming growth factor (TGF)-β superfamily, binding to both TGF-β1 and 3 isoforms with high affinity (21–23). Normal levels of CD105 antagonize the inhibitory effects of TGF-β1 on cell proliferation, migration, and capillary formation and thus contribute to angiogenesis (24). The upregulation of CD105 in solid tumor neovasculature may represent an endothelial cell–mounted defense against tumor-induced hypoxic stress (25–29). The reliably negative CD105 staining pattern in normal breast tissue, and its almost exclusive expression in neoplastic vessels, makes this antibody a useful marker of breast cancer neovasculature (21,25,26). The prognostic importance of CD105 expression in breast cancer has been demonstrated by the correlation of CD105 positive vessels with overall survival and risk of metastases in patients with node-negative cancer (30,31).
DOS tomography measures bulk, or large volume, total tissue hemoglobin (HbT) values through transmission measurements and is known to be sensitive to the smallest vasculature in tissue (13). Hence, HbT in tumors is thought to be a measure of the total vasculature and thus might be correlated with the volume of both preexisting and tumor-induced vasculature. Verifying the correlation between HbT and CD105- or CD31-expressing vessels would ensure that the measured quantity of HbT has a clear biologic interpretation. This pilot study investigates whether changes in tumor angiogenesis associated with complete pathologic response (pCR) or partial pathologic response (pPR) to treatment can be demonstrated by using DOS tomography.
Materials and Methods
Patient Recruitment
In this prospective study, which was compliant with the terms of the Health Insurance Portability and Accountability Act and was approved by the Trustees of Dartmouth College–Dartmouth-Hitchcock Medical Center Committee for the Protection of Human Subjects, written informed consent was obtained from the participants, who were compensated for their participation in the examination. Twenty-one patients had been enrolled in the study at the time of this report, with 16 completing all of their DOS imaging sessions. However, of this group, only 11 had complete pathologic and DOS imaging data within 5 days of the target dates established in the study protocol (Table 1) to allow comparison and testing for temporal correlation.
Table 1.
Summary of Clinical, Pathologic, and Treatment Outcome Data for the 11 Study Participants
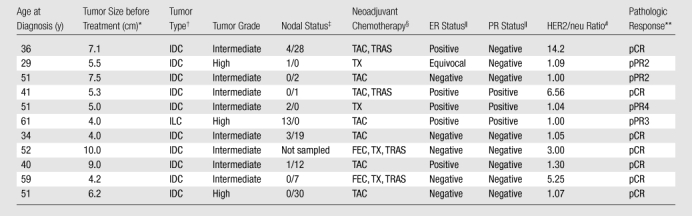
Maximum dimension at pretreatment magnetic resonance (MR) imaging.
IDC = infiltrating ductal carcinoma, ILC = infiltrating lobular carcinoma.
Number of axillary nodes positive for metastatic tumor/number of axillary nodes negative for metastatic tumor.
FEC = fluorouracil, epirubicin, and cyclophosphamide; TAC = docetaxel, doxorubicin, and cyclophosphamide; TRAS = trastuzumab; TX = paclitaxel or docetaxel.
According to the immunohistochemical (IHC) semiquantitative scoring system used, > 15% IHC staining = positive, 0% IHC staining = negative, and 1%–14% IHC staining = equivocal. ER = estrogen receptor, PR = progesterone receptor.
HER2/neu = human epidermal growth factor receptor 2. Gene analysis was performed with fluorescence in situ hybridization, with results compared against a normal control. A ratio < 2.0 = normal expression, a ratio between 2.0 and 4.0 = equivocal expression, and a ratio > 4.0 = gene overexpression.
pCR = complete pathologic response with either no residual carcinoma or no residual invasive tumor but ductal carcinoma in situ present; pPR = partial pathologic response, subclassified as pPR1 (near total treatment effect [<10% of tumor remaining]), pPR2 (evidence of response to therapy but with 10%–50% of tumor remaining), pPR3 (evidence of response to therapy but with between 50% and 90% of tumor remaining), and pPR4 (minimal evidence of response to therapy with > 90% of tumor remaining).
Neoadjuvant Therapy
Enrolled patients received neoadjuvant treatment recommended by their medical oncologist (P.A.K.). The regimen generally used for women with locally advanced HER2/neu-negative breast cancer was docetaxel, doxorubicin, and cyclophosphamide, or TAC. Women with locally advanced HER2/neu-positive disease were treated with TAC or fluorouracil, epirubicin, and cyclophosphamide, or FEC, and trastuzumab. Antiangiogenic therapeutic agents, such as bevacizumab, were not used.
MR Imaging Methods
Appendix E1 (online) and the report of a prior study (32) provide details of the MR imaging examination, including the determination of tumor size and location, the optimal methods for DOS tomographic spectral analysis, and the extraction of standardized prognostic information.
DOS Tomographic Imaging Methods and Analysis
The DOS imaging system, a clinical prototype previously validated in both tissue phantoms and patient examinations (20,32–35), has already been described (Appendix E2 [online]). From the HbT images, a region of interest (ROI) was created (32) by using the study radiologist’s (S.P.P., with 18 years of experience in the interpretation of breast MR images) interpretation of pretreatment contrast material–enhanced MR images in both the ipsilateral and contralateral breasts. Average values of HbT inside and outside the tumor ROI, as well as average values in the contralateral breast, were determined from reconstructed images. To quantify DOS image changes, these values were normalized by the average value in the contralateral breast imaged before treatment to highlight their contrast over the course of therapy.
Imaging-Pathologic Correlation
All study participants underwent standard-of-care neoadjuvant chemotherapy and surgical treatment. For each patient, MR images of the diseased breast and DOS tomographic images of both breasts were acquired before the diagnostic core biopsy of tumor, 2–5 days before the start of neoadjuvant chemotherapy, throughout the treatment cycle, and 2–3 days prior to the final mastectomy surgery. The number of MR images obtained depended on clinical needs and on whether the subject also participated in a concurrent MR imaging study. The number of MR imaging sessions required during the treatment cycle varied from none to seven. At our institution, it is not the standard of care for a patient’s tumor to be repeatedly sampled for biopsy during neoadjuvant chemotherapy, so only the pre- and posttreatment average values of HbT were used for the imaging-pathologic correlation. The posttreatment mastectomy specimens were sectioned fresh in the sagittal plane, medial to lateral. Each tissue slice (on average, 1.0 cm thick) was examined grossly and was photographed for image correlation. Extensively sampled areas of treated tissue (no viable tumor left), residual tumor tissue (if present), and normal breast tissue were sampled and routinely processed for microscopic analysis according to standard laboratory protocols (Appendix E3 [online]). The two post–neoadjuvant chemotherapy clinical end points of pCR or pPR were classified according to Pinder et al (36) and are detailed in Table 1. In cases of pCR, quantitative measures for angiogenesis in the pretreatment tumor biopsy specimens were compared with those in posttreatment normal tissue and treated tissue (with no viable tumor left). In cases of pPR, quantitative measures for angiogenesis in the pretreatment tumor biopsy specimens were compared with those in posttreatment normal tissue, areas of treated tissue (with no viable tumor left), and areas of residual tumor. Expression of CD31 (1:40 [Dako, Carpinteria, Calif]) and CD105 (1:30 [Vector Laboratories, Burlingame, Calif]) was assessed immunohistochemically in the pretreatment tumor biopsy specimens and in representative tissue from the posttreatment final surgery, according to standard laboratory practice (Appendix E4 [online]).
Quantitation of Neoangiogensis
By using Image Pro Plus software (Media Cybernetics, Bethesda, Md) and automated stage-control bundled software, whole CD105- and CD31-immunostained slides from the pretreatment tumor biopsy specimens and from the distinct diagnostic categories of the posttreatment mastectomy specimens were digitally scanned at high resolution and montaged. The mean vessel density (MVD) was defined as the combined areas of CD31-positive or CD105-positive blood vessels, thresholded in pseudocolor in the diagnostic ROIs, as a percentage of the total area of the slide. The mean vessel area (MVA) was defined as the MVA of CD31-positive or CD105-positive blood vessels in each slide, thresholded in pseudocolor in the diagnostic ROIs. The diagnostic ROIs were selected by one author (W.A.W., a surgical pathologist with 15 years of expertise in breast pathologic examination and image analysis). The image thresholding and processing were preformed by another author (M.C.S., a technologist with 4 years of expertise in the Image Pro software). To discern if there was an overlap between the number of preexisting (CD31-positive) and tumor-induced (CD105-positive) vessels, the sums of CD31-positive and CD105-positive vessels, as well their ratios, were also analyzed. In mastectomy specimens in which multiple representative areas had been sampled in treated tissues (in cases of pCR) or treated tissues and residual tumor (in cases of pPR), the measures for MVA and MVD were averaged.
Statistical Analysis
Distributions for all imaging and pathologic measures were examined. After log transformation, these distributions appeared approximately normal. Wilcoxon nonparametric tests were used to compare the pathologic outcomes in the pretreatment tumor biopsies of those patients who subsequently were classified as having pCR or pPR. To account for intraindividual correlations, linear mixed models with random intercept terms were applied to conduct pairwise comparisons of the pathologic vascularity measures in pretreatment tumor biopsy samples and posttreatment normal tissue, treated tissue, and residual tumor. P values were adjusted by using Tukey method for multiple comparisons. A linear mixed-model analysis was also applied to assess the correlation of mean levels of HbT with time of neoadjuvant therapy (in months) in the patients with pCR and those with pPR separately; these levels were summarized in terms of slopes representing the time trend. Pearson correlation coefficients were computed among individual pathologic and imaging measures. All statistical analyses were performed with software (SAS, version 9.2; SAS Institute, Cary, NC), with a significance level of 5%.
Results
All of the breast cancer tumors except one were invasive ductal carcinomas. None of the tumors were low grade. Of the seven women with pCR, four had HER2/neu positive tumors and were treated with trastuzumab. The imaging, macroscopic, microscopic, and immunohistochemical findings in representative pCR and pPR cases are detailed in Figures 1 and 2.
Figure 1a:
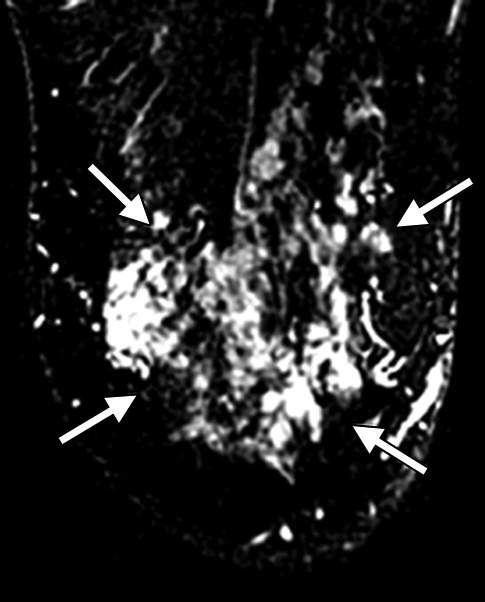
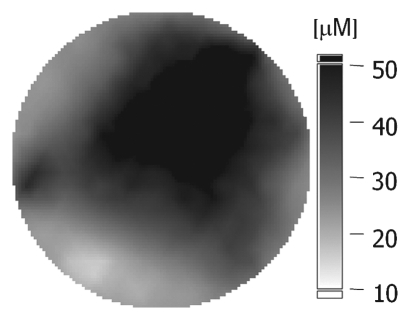
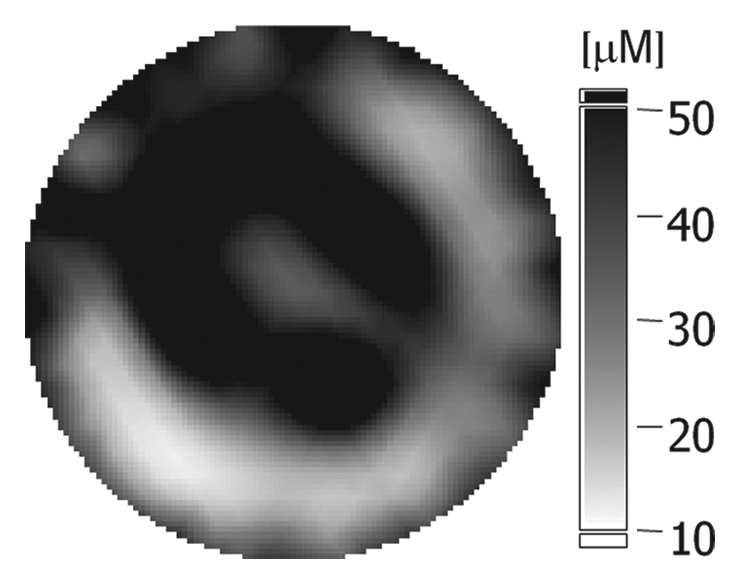
(a–c) Imaging and (d) pathologic findings before and after neoadjuvant chemotherapy in 52-year-old woman with pCR. (a) Prechemotherapy axial MR image shows extensive (10.00-cm) clumped enhancement in the upper half of the left breast (arrows) that approximated the distribution of calcifications seen at mammography. An enlarged (2.7-cm) globular left axillary lymph node, with loss of the fatty hilum, indicating metastatic involvement, was also identified at MR imaging but is not shown. (b) Prechemotherapy coronal DOS tomographic image of HbT level shows a maximum value of 45 μmol/L in the tumor ROI. (c) Postchemotherapy coronal DOS tomographic image shows that the HbT level had decreased to background levels in the tumor ROI; this correlated with resolution of both the regional clumped enhancement (primary breast carcinoma) and resolution of the axillary nodal metastasis. (d) Postchemotherapy immunohistochemical slide shows only rare tumor-induced CD105 (endoglin)-expressing blood vessels (arrow) in the area of the prior tumor. These findings correlated with resolution of both the regional clumped enhancement (primary breast carcinoma) and resolution of the axillary nodal metastasis. (Diaminobenzidine-detection substrate, hematoxylin counterstain; original magnification, ×200.) Histopathologic findings in the mastectomy specimen in this patient are shown in Figure E1 (online).
Figure 2a:
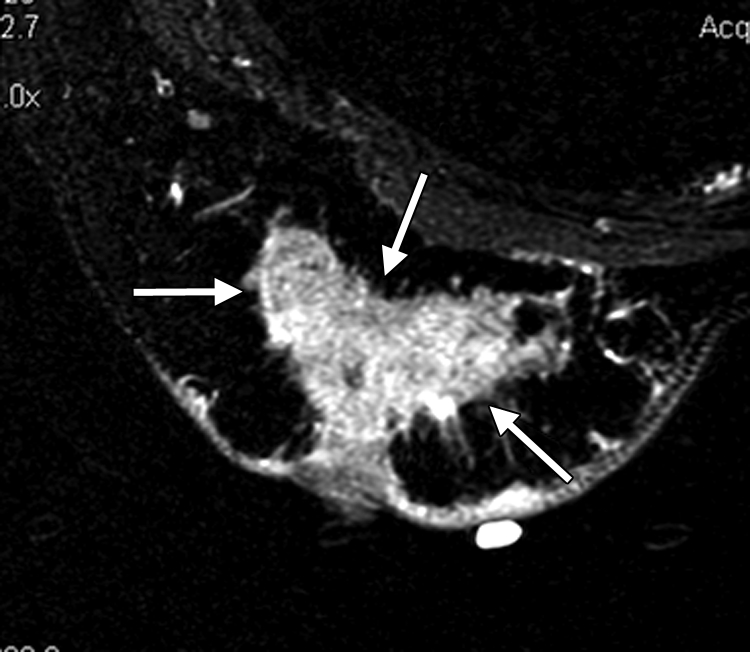
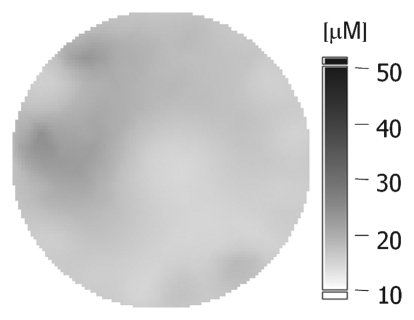
(a–c) Imaging and (d) pathologic findings before and after neoadjuvant chemotherapy in 51-year-old woman with pPR. (a) Prechemotherapy axial MR image shows an irregularly shaped enhancing mass with spiculated margins that measures 5.8 cm in its greatest transverse diameter (between arrows). (b) Prechemotherapy coronal DOS tomographic image of HbT level shows a maximum value of 50 μmol/L in the tumor ROI. (c) Postchemotherapy coronal DOS tomographic image shows that HbT remained at the same level in the tumor ROI and correlated with reduced but persistent malignant enhancement of the residual tumor. (d) Postchemotherapy immunohistochemical slide shows abundant tumor-induced CD105 (endoglin)-expressing blood vessels (arrows) in areas associated with residual tumor. (Diaminobenzidine-detection substrate, hematoxylin counterstain; original magnification, ×200.) Histopathologic findings in the mastectomy specimen in this patient are shown in Figure E2 (online).
Figure 1b:
(a–c) Imaging and (d) pathologic findings before and after neoadjuvant chemotherapy in 52-year-old woman with pCR. (a) Prechemotherapy axial MR image shows extensive (10.00-cm) clumped enhancement in the upper half of the left breast (arrows) that approximated the distribution of calcifications seen at mammography. An enlarged (2.7-cm) globular left axillary lymph node, with loss of the fatty hilum, indicating metastatic involvement, was also identified at MR imaging but is not shown. (b) Prechemotherapy coronal DOS tomographic image of HbT level shows a maximum value of 45 μmol/L in the tumor ROI. (c) Postchemotherapy coronal DOS tomographic image shows that the HbT level had decreased to background levels in the tumor ROI; this correlated with resolution of both the regional clumped enhancement (primary breast carcinoma) and resolution of the axillary nodal metastasis. (d) Postchemotherapy immunohistochemical slide shows only rare tumor-induced CD105 (endoglin)-expressing blood vessels (arrow) in the area of the prior tumor. These findings correlated with resolution of both the regional clumped enhancement (primary breast carcinoma) and resolution of the axillary nodal metastasis. (Diaminobenzidine-detection substrate, hematoxylin counterstain; original magnification, ×200.) Histopathologic findings in the mastectomy specimen in this patient are shown in Figure E1 (online).
Figure 1c:
(a–c) Imaging and (d) pathologic findings before and after neoadjuvant chemotherapy in 52-year-old woman with pCR. (a) Prechemotherapy axial MR image shows extensive (10.00-cm) clumped enhancement in the upper half of the left breast (arrows) that approximated the distribution of calcifications seen at mammography. An enlarged (2.7-cm) globular left axillary lymph node, with loss of the fatty hilum, indicating metastatic involvement, was also identified at MR imaging but is not shown. (b) Prechemotherapy coronal DOS tomographic image of HbT level shows a maximum value of 45 μmol/L in the tumor ROI. (c) Postchemotherapy coronal DOS tomographic image shows that the HbT level had decreased to background levels in the tumor ROI; this correlated with resolution of both the regional clumped enhancement (primary breast carcinoma) and resolution of the axillary nodal metastasis. (d) Postchemotherapy immunohistochemical slide shows only rare tumor-induced CD105 (endoglin)-expressing blood vessels (arrow) in the area of the prior tumor. These findings correlated with resolution of both the regional clumped enhancement (primary breast carcinoma) and resolution of the axillary nodal metastasis. (Diaminobenzidine-detection substrate, hematoxylin counterstain; original magnification, ×200.) Histopathologic findings in the mastectomy specimen in this patient are shown in Figure E1 (online).
Figure 1d:
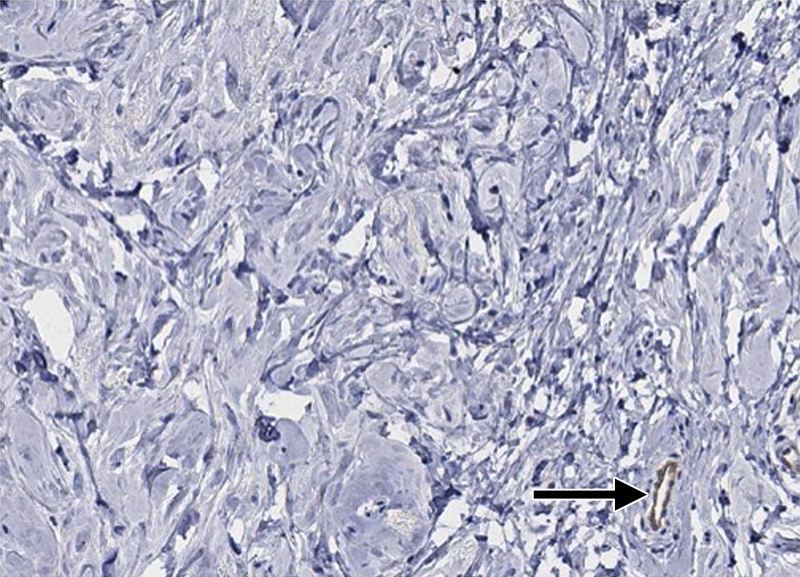
(a–c) Imaging and (d) pathologic findings before and after neoadjuvant chemotherapy in 52-year-old woman with pCR. (a) Prechemotherapy axial MR image shows extensive (10.00-cm) clumped enhancement in the upper half of the left breast (arrows) that approximated the distribution of calcifications seen at mammography. An enlarged (2.7-cm) globular left axillary lymph node, with loss of the fatty hilum, indicating metastatic involvement, was also identified at MR imaging but is not shown. (b) Prechemotherapy coronal DOS tomographic image of HbT level shows a maximum value of 45 μmol/L in the tumor ROI. (c) Postchemotherapy coronal DOS tomographic image shows that the HbT level had decreased to background levels in the tumor ROI; this correlated with resolution of both the regional clumped enhancement (primary breast carcinoma) and resolution of the axillary nodal metastasis. (d) Postchemotherapy immunohistochemical slide shows only rare tumor-induced CD105 (endoglin)-expressing blood vessels (arrow) in the area of the prior tumor. These findings correlated with resolution of both the regional clumped enhancement (primary breast carcinoma) and resolution of the axillary nodal metastasis. (Diaminobenzidine-detection substrate, hematoxylin counterstain; original magnification, ×200.) Histopathologic findings in the mastectomy specimen in this patient are shown in Figure E1 (online).
Figure 2b:
(a–c) Imaging and (d) pathologic findings before and after neoadjuvant chemotherapy in 51-year-old woman with pPR. (a) Prechemotherapy axial MR image shows an irregularly shaped enhancing mass with spiculated margins that measures 5.8 cm in its greatest transverse diameter (between arrows). (b) Prechemotherapy coronal DOS tomographic image of HbT level shows a maximum value of 50 μmol/L in the tumor ROI. (c) Postchemotherapy coronal DOS tomographic image shows that HbT remained at the same level in the tumor ROI and correlated with reduced but persistent malignant enhancement of the residual tumor. (d) Postchemotherapy immunohistochemical slide shows abundant tumor-induced CD105 (endoglin)-expressing blood vessels (arrows) in areas associated with residual tumor. (Diaminobenzidine-detection substrate, hematoxylin counterstain; original magnification, ×200.) Histopathologic findings in the mastectomy specimen in this patient are shown in Figure E2 (online).
Figure 2c:
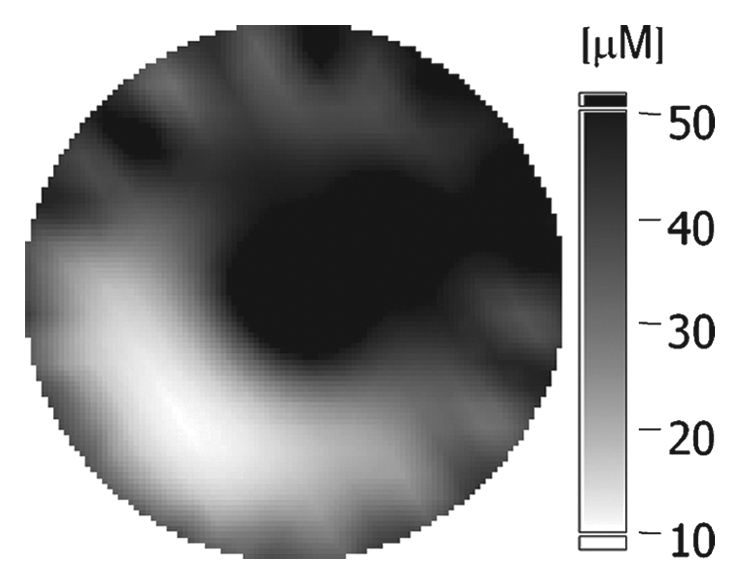
(a–c) Imaging and (d) pathologic findings before and after neoadjuvant chemotherapy in 51-year-old woman with pPR. (a) Prechemotherapy axial MR image shows an irregularly shaped enhancing mass with spiculated margins that measures 5.8 cm in its greatest transverse diameter (between arrows). (b) Prechemotherapy coronal DOS tomographic image of HbT level shows a maximum value of 50 μmol/L in the tumor ROI. (c) Postchemotherapy coronal DOS tomographic image shows that HbT remained at the same level in the tumor ROI and correlated with reduced but persistent malignant enhancement of the residual tumor. (d) Postchemotherapy immunohistochemical slide shows abundant tumor-induced CD105 (endoglin)-expressing blood vessels (arrows) in areas associated with residual tumor. (Diaminobenzidine-detection substrate, hematoxylin counterstain; original magnification, ×200.) Histopathologic findings in the mastectomy specimen in this patient are shown in Figure E2 (online).
Figure 2d:
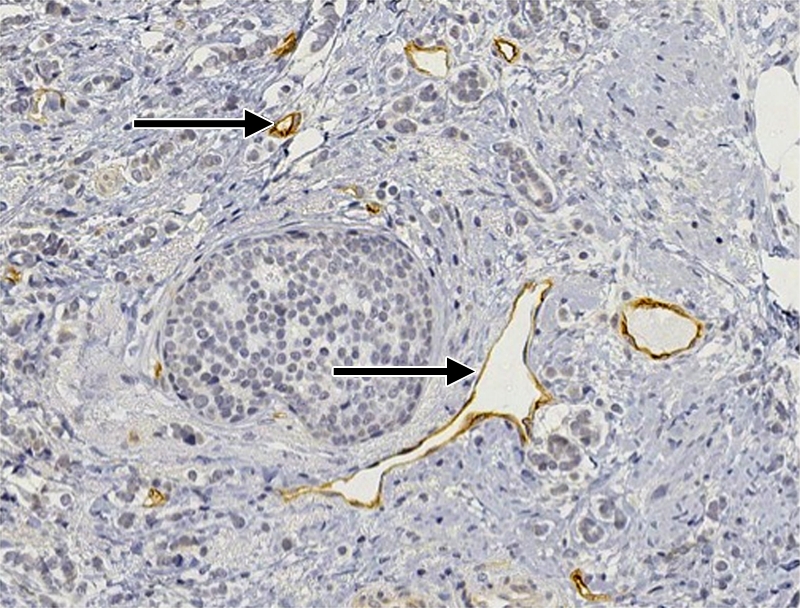
(a–c) Imaging and (d) pathologic findings before and after neoadjuvant chemotherapy in 51-year-old woman with pPR. (a) Prechemotherapy axial MR image shows an irregularly shaped enhancing mass with spiculated margins that measures 5.8 cm in its greatest transverse diameter (between arrows). (b) Prechemotherapy coronal DOS tomographic image of HbT level shows a maximum value of 50 μmol/L in the tumor ROI. (c) Postchemotherapy coronal DOS tomographic image shows that HbT remained at the same level in the tumor ROI and correlated with reduced but persistent malignant enhancement of the residual tumor. (d) Postchemotherapy immunohistochemical slide shows abundant tumor-induced CD105 (endoglin)-expressing blood vessels (arrows) in areas associated with residual tumor. (Diaminobenzidine-detection substrate, hematoxylin counterstain; original magnification, ×200.) Histopathologic findings in the mastectomy specimen in this patient are shown in Figure E2 (online).
Vascularity Measures in Core Biopsy Tumor Specimens in Patients Subsequently Classified as Having Either pCR or pPR
There was no significant difference in MVD (P = .73) or MVA (P = .3) in the pretreatment diagnostic core biopsy specimens of tumors that might be used to predict subsequent clinical outcome after neoadjuvant chemotherapy.
Vascularity Measures in Pretreatment Diagnostic Core Biopsy Tumor Specimens and Posttreatment Mastectomy Tissue
There was a significant difference in the MVD of preexisting CD31-expressing vessels between pretreatment core tumor and posttreatment normal tissue in both women with pCR (decrease from 1.47% to 0.40%, P = .013) and women with pPR (decrease from 1.56% to 0.22%, P = .004). The MVD of preexisting CD31-expressing vessels before and after treatment was not significantly different in either the pCR or the pPR patient group (P = .206 and P = .855, respectively). The MVA of preexisting CD31-expressing vessels before and after treatment was also not significantly different in either the pCR or the pPR patient group (P = .568 and P = .559, respectively).
For tumor-induced CD105 (endoglin)-expressing vessels, there were significant differences in MVD and MVA when we compared all pretreatment and posttreatment tissues in both women with pPR and women with pCR (Table 2), except between pretreatment tumor and posttreatment residual tumor in women with pPR. The MVD of CD105-expressing vessels decreased significantly (P < .001) from 2.51% (95% confidence interval [CI]: 0.81%, 7.8%) to 0.04% (95% CI: 0.02%, 0.1%) when we compared pretreatment core tumor and posttreatment tissue in women with pCR but not when we compared pretreatment tumor and posttreatment residual tumor in women with pPR (P = .071). The MVA of CD105-expressing vessels decreased significantly from 40.65 μm2 (95% CI: 28.83, 57.3 μm2) to 20.45 μm2 (95% CI: 16.32, 25.63 μm2) in patients with pCR (P = .003) and from 39.3 μm2 (95% CI: 29.54, 52.28 μm2) to 25.11 μm2 (95% CI: 19.64, 32.11 μm2) in areas of residual tumor in patients with pPR (P = .033). A summation and ratio of preexisting and tumor-induced vessel densities (the MVD of CD31-expressing vessels plus the MVD of CD105-expressing vessels and the MVD of CD105-expressing vessels divided by the MVD of CD31-expressing vessels) also showed significant differences between pretreatment and posttreatment tissue in women with pCR (adjusted P values < .001). In women with pPR, there was no significant relationship between the sum of the MVDs of CD31-expressing vessels and CD105-expressing vessels when we compared the pretreatment tumor biopsy specimens and the areas of residual tumor.
Table 2.
P Values for Significant Results of Pairwise Comparison of Quantitative Measures of Tumor-induced and Preexisting Vessels in Pretreatment Core Biopsy Specimens versus Posttreatment Mastectomy Specimens
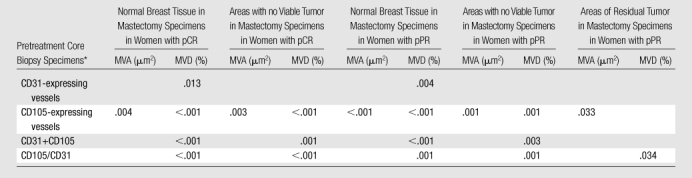
Note.—Residual tumor after neoadjuvant chemotherapy = microscopic presence of residual, viable tumor cells in areas of the final resection specimen in cases of pPR. No viable tumor cells after neoadjuvant chemotherapy = microscopic absence of viable tumor cells throughout the resection specimen in cases of pCR or the microscopic absence of viable tumor cells in some areas of the resection specimen in cases of pPR. Normal breast tissue = normal tissue adjacent to the known tumor site in the mastectomy specimens.
CD31+CD105 = value in CD31-expressing vessels plus value in CD105-expressing vessels; CD105/CD31 = ratio of value in CD31-expressing vessels to value in CD105-expressing vessels.
Time Effect of Months of Treatment and DOS Tomographic HbT Levels
A linear mixed-model analysis demonstrated a significant (P = .001) decrease in mean levels of HbT during treatment (in months) in breast abnormality ROIs in women with pCR (n = 7). The mean value of HbT decreased from 37.1 μmol/L at a rate of 3.77 μmol/L per month. The fitted line for women with pPR (n = 4) showed a nonsignificant decline in HbT in breast abnormality ROIs with time (slope, −0.21; P = .91).
Correlation of Pathologic Vascularity Measures with DOS Tomographic HbT Levels according to Degree of Clinical Response
For women with pCR (n = 7), there was a significant correlation between the MVD of CD105-expressing vessels in the pretreatment tumor biopsy specimens and pretreatment HbT levels (P ≤ .001); there was no such correlation for women with pPR (P = .11). For women with pPR (n = 4), there was a significant correlation between the MVD of CD31-expressing vessels in the posttreatment tumor biopsy specimens and pretreatment HbT levels (P = .01). There was no significant relationship between measures of HbT and the sums or ratios of the MVDs of CD105-expressing vessels and CD31-expressing vessels in either women with pCR or women with pPR.
Discussion
Individualized treatment management for patients with locally advanced breast cancers undergoing neoadjuvant chemotherapy is an important clinical goal (1). Newly developed breast imaging modalities have been shown to better correlate with response to therapy in patients with locally advanced breast cancers undergoing neoadjuvant chemotherapy than current clinical standards (2–8). DOS tomographic analysis provides two-dimensional information about the intrinsic and ultrastructural biophysical composition of breast tissue (9–17). Our study sought to validate the DOS imaging measure of HbT with quantitative measures of preexisting and tumor-induced vessel formation before and after neoadjuvant chemotherapy.
CD105 (endoglin), a transmembrane glycoprotein expressed predominantly on the surface of highly proliferating endothelial cells, contributes to angiogenesis by antagonizing the inhibitory effects of TGF-β1 on cell proliferation, migration, and capillary formation and may represent an endothelial cell–mounted defense against tumor-induced hypoxic stress (24–29). The reliably negative CD105 staining pattern in normal breast tissue and its almost exclusive expression in neoplastic vessels make this antibody a particularly useful marker of breast cancer neovasculature (21,25,26). Other studies have already demonstrated that CD105 provides greater diagnostic and prognostic efficacy in measuring tumor angiogenesis than pan–endothelial cell markers such as CD31 and CD34 because the latter are not expressed in activated endothelial cells with high specificity (28,37–40). Tumor-induced vessels in breast cancer, which stain intensely for monoclonal anti-CD105 antibodies, have shown only weak or absent staining for anti-CD31 antibodies (39).
Our study confirmed significant correlations between the MVD and MVA of CD105-expressing vessels in the pretreatment tumor biopsy specimens and posttreatment mastectomy specimens for both women with pCR and women with pPR (P < .03). We found no correlation between the CD31-positive vessels in the pretreatment tumor biopsy specimens and treated or residual tumor in the posttreatment mastectomy specimens, confirming the findings of prior studies that the specificity of tumor-induced endothelial cell expression of CD31 is much less than that of CD105 (31,39). The predominant expression of CD105 in angiogenesis, and its significant reduction with treatment response, was also confirmed in the analysis of the sums and ratios of preexisting and tumor-induced vessel densities (ie, the MVD of CD31-expressing vessels plus that of CD105-expressing vessels and the MVD of CD105-expressing vessels divided by the MVD of CD31-expressing vessels). These ratios showed significant differences between pretreatment tumor tissue and posttreatment normal and treated tissue in women with pCR (P < .001). In women with pPR, similar results were identified, but there was no significant relationship in MVD of CD31-expressing vessels plus that of CD105-expressing vessels when we compared the pretreatment tumor biopsy specimens and the areas of residual tumor.
The prognostic importance of CD105 expression in breast cancer has been demonstrated by the significant correlation of CD105-positive vessels with overall survival and the risk of metastases in the subset of patients with node-negative cancer (28,30,31). In contrast, CD31 is not an independent prognostic indicator in patients with node-negative cancer (31,41,42). CD105 has also been shown to predict both overall and disease-free survival, independent of patient age and menopausal status and tumor grade, nodal involvement, size, and estrogen and progesterone receptor status (28). There are no significant differences in tumor vascularity, as assessed with CD105 expression, in different molecular subtypes of breast cancer (43). Prognostic importance appears to depend on the number of CD105-positive vessels rather than the percentage of CD105-positive surface (30). For this reason, we decided to quantify not only the percentage of CD105-positive vessels (ie, MVD) but also the MVA. Absolute vessel number can be misleading given the random orientation of blood vessels and nonstandardized tissue slicing. Prior studies (19,20) have already demonstrated that while the MVD of CD31-expressing vessels increases significantly across the four diagnostic categories of normal breast tissue, fibrocystic disease, fibroadenomas, and invasive breast tumors, the MVA for invasive tumors is higher than that for normal breast tissue and fibrocystic disease but significantly smaller than that for benign fibroadenomas. In our study, preexisting CD31-positive vessels were significantly different in the pretreatment tumor biopsy specimens and the morphologically normal tissue in the posttreatment mastectomy specimens for both women with pCR (P = .013) and women with pPR (P = .004), but only for measures of MVD, not MVA. The MVD of CD105-expressing vessels exactly reflected the MVA of CD105-expressing vessels in pretreatment and posttreatment tissue for both women with pCR and women with pPR.
The potential prognostic utility of CD105 expression in predicting the response of patients with breast cancer to chemotherapy has been investigated, to our knowledge, in only one prior study (44), in which women with clinical response to neoadjuvant chemotherapy were found to have lower pretreatment CD105-positive vessel counts than clinical nonresponders. However, the correlation with histopathologic response failed to achieve statistical significance, as did the other 17 statistical analyses performed in that study. Our study found that there was no significant difference in the MVD and the MVA of CD105-expressing vessels in the pretreatment tumor biopsy specimens in patients subsequently classified as having pCR and those subsequently classified as having pPR, indicating that angiogenesis measures in the pretreatment tumor biopsies could not be used to predict subsequent clinical outcome after neoadjuvant chemotherapy.
Monitoring of breast cancer treatment response to neoadjuvant chemotherapy with diffuse optical spectroscopy (9) or tomography (11,12,32,45) has demonstrated encouraging initial results in single-trial studies. Zhu and colleagues (11), who evaluated vessel density changes in 16 subjects in response to neoadjuvant chemotherapy while monitoring them with near-infrared imaging, observed a moderately significant (P = .056) correlation between the quantitative MVD measures of existing vessels (CD31-positive vessels) in residual tumor specimens and HbT level, but no statistical significance was found between responder and nonresponder groups. In our study, we provide a clear biologic interpretation between DOS-measured bulk tissue HbT values and tumor-induced (CD105-expressing) vessels in pretreatment and posttreatment tissue specimens.
At our institution, it is not the standard of care for a patient’s tumor to be repeatedly sampled for biopsy during neoadjuvant chemotherapy, although research trials are now established that allow this (8). Because no biopsies were performed during treatment, only the pre- and posttreatment average values of HbT could be used in the imaging-pathologic correlations. HbT values during treatment in a linear mixed-model analysis demonstrated a significant (P = .001) decrease in breast abnormality ROIs among women with pCR (n = 7). No significant time effect for any DOS image outcome was observed for the women with pPR (n = 4). In patients with pCR but not in patients with pPR, there were significant correlations between both the pretreatment CD105 measures and the pretreatment HbT measures (P ≤ .001). For women with pPR (n = 4), there was a significant correlation between MVD of CD31-expressing vessels in the pretreatment tumor biopsy specimens and the pretreatment HbT measures (P = .01).
A pCR after neoadjuvant chemotherapy corresponds to a survival rate of more than 90% at 10 years, compared with a survival rate of 60% or less at 10 years for pPR (44). Given this difference in clinical outcome, it is surprising that a universally accepted and reproducible classification system for pCR and pPR does not exist. There are numerous pathologic classifications to detail complete (pCR) or partial (pPR) response to neoadjuvant chemotherapy (46–50). These classifications vary according to the laboratory handling and sampling of the specimens, definitions of partial response, reported reproducibility, links with clinical outcome data, the relationship to other prognostic factors, and the inclusion of treatment response in axillary lymph nodes. In our report, we have used the specimen sampling and reporting approaches of Pinder and colleagues (36), including their definitions for pCR, pPR, and nodal involvement. We have shown that there are significant correlations between both pretreatment and posttreatment CD105 expression and HbT measures in patients with pCR and a significant decrease in mean levels of HbT during neoadjuvant chemotherapy. No such correlations were seen in patients with pPR. Other studies of imaging treatment response with MR spectroscopy or diffusion (32) are revealing different measures of tumor pathophysiology. A direct comparison between the measured data and their relationship to pathologic changes will be important as the modalities show their clinical value.
There were several limitations in this study. To date, the sample size of patients undergoing neoadjuvant chemotherapy who consented to the imaging study and for whom complete imaging and pathologic data were available was small (n = 11). This limitation supports the need for multi-institutional studies, with standardized imaging technology and pathologic evaluation, for this group of patients. Population bias was related to the imaging, during treatment, of only patients in whom neoadjuvant chemotherapy had been recommended. Our study showed that pretreatment angiogenesis assessment (in the diagnostic core biopsy samples) did not predict treatment outcome after neoadjuvant chemotherapy, but this finding cannot be extrapolated to the far more numerous patients today who receive chemotherapy after definitive surgery. The digitization and analysis of histologic slices, to quantitatively evaluate vessel size and density, are not routinely used in the discipline of surgical pathology today but may be in the future. The technical limitations of DOS tomographic imaging include the accuracy of the fiber array positioning during the longitudinal series of imaging examinations and how the heterogeneity of tumor response affects the interpretation of the imaging results. The positioning accuracy is near 1 cm, and repeatability of estimation of HbT values has a standard deviation that is near 10% (32). Another practical but important limitation is the imaging timeline. Ideally, every patient would be imaged the same day after a specific treatment cycle or surgery. In reality, the imaging dates in this study were quite variable. The error induced by this variability is unknown, but data to date indicate that the largest changes occur within the first few days after the start of initial chemotherapy.
In conclusion, our study results show a correlation between changes in HbT at DOS tomography and tumor angiogenesis and demonstrate how this noninvasive modality might allow individualization and optimization of neoadjuvant chemotherapy regimens.
Advances in Knowledge.
Diffuse optical spectroscopic (DOS) tomography examinations in women with breast cancer receiving neoadjuvant chemotherapy showed a significant decrease in mean levels of total tissue hemoglobin (HbT) (from 37.1 μmol/L at a rate of 3.77 μmol/L per month) during therapy in patients with a complete pathologic response (pCR) but not in those with a partial pathologic response (pPR).
Mean vessel density (MVD) of tumor-induced CD105 (endoglin)-expressing vessels decreased significantly from 2.51% to 0.04% in patients with pCR but not in areas of residual tumor in patients with pPR; the MVD of preexisting CD31-expressing vessels before and after treatment was not significantly different in either group.
Mean vessel area (MVA) of tumor-induced CD105-expressing vessels decreased significantly from 40.65 μm2 to 20.45 μm2 in patients with pCR and from 39.3 μm2 to 25.11 μm2 in areas of residual tumor in patients with pPR; MVA of preexisting CD31-expressing vessels before and after treatment was not significantly different in either group.
MVD of tumor-induced CD105-expressing vessels in pretreatment tumor biopsy specimens correlated significantly with pretreatment DOS tomographic HbT measures for patients with pCR but not for those with pPR.
Implication for Patient Care.
The correlation between bulk (or large volume) HbT values, as measured by using DOS tomography, and tumor-induced angiogenesis, as measured by the quantification of CD105 (endoglin)-expressing vessels, provides a biologic interpretation for this spectral signature, which could lead to superior individualized patient treatment.
Disclosures of Potential Conflicts of Interest: M.G.P. No potential conflicts of interest to disclose. W.A.W. No potential conflicts of interest to disclose. M.C.S. No potential conflicts of interest to disclose. H.M.F. No potential conflicts of interest to disclose. S.J. No potential conflicts of interest to disclose. Z.L. No potential conflicts of interest to disclose. T.D.T. No potential conflicts of interest to disclose. S.P.P. No potential conflicts of interest to disclose. P.A.K. No potential conflicts of interest to disclose. B.W.P. No potential conflicts of interest to disclose. K.D.P. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: has patents or patents pending for some aspects of the DOS imaging technology used in the study, with interests shared between the investigator and institution that have the potential for third-party payments; no third-party payments have been received to date. Other relationships: none to disclose.
Acknowledgments
Special thanks go to Rebecca O’Meara, MT, of the Pathology Translational Research Laboratory, Department of Pathology, Dartmouth-Hitchcock Medical Center, Lebanon, NH, for performing all of the immunohistochemical studies.
Received May 5, 2010; revision requested June 16; revision received September 10; accepted October 6; final version accepted December 20.
Funding: This research was supported by the National Institutes of Health (grants PO1 CA80139, U54, CA105480, and K25 CA106863).
Abbreviations:
- CI
- confidence interval
- DOS
- diffuse optical spectroscopy
- HbT
- total tissue hemoglobin
- HER2/neu
- human epidermal growth factor receptor 2
- MVA
- mean vessel area
- MVD
- mean vessel density
- pCR
- complete pathologic response
- pPR
- partial pathologic response
- ROI
- region of interest
- TGF
- transforming growth factor
References
- 1.Esserman L. Neoadjuvant chemotherapy for primary breast cancer: lessons learned and opportunities to optimize therapy. Ann Surg Oncol 2004;11(1 Suppl):3S–8S [DOI] [PubMed] [Google Scholar]
- 2.Partridge SC, Gibbs JE, Lu Y, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005;184(6):1774–1781 [DOI] [PubMed] [Google Scholar]
- 3.Manton DJ, Chaturvedi A, Hubbard A, et al. Neoadjuvant chemotherapy in breast cancer: early response prediction with quantitative MR imaging and spectroscopy. Br J Cancer 2006;94(3):427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunnwald LK, Gralow JR, Ellis GK, et al. Residual tumor uptake of [99mTc]-sestamibi after neoadjuvant chemotherapy for locally advanced breast carcinoma predicts survival. Cancer 2005;103(4):680–688 [DOI] [PubMed] [Google Scholar]
- 5.Meisamy S, Bolan PJ, Baker EH, et al. Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy—a pilot study at 4 T. Radiology 2004;233(2):424–431 [DOI] [PubMed] [Google Scholar]
- 6.Tripathy D, Jiang L, Rao N, et al. Blood oxygen level dependent (BOLD) contrast MRI and breast cancer chemotherapy response. In: Annual Meeting of American Society of Clinical Oncology; Proceedings Part I. 2006 [Google Scholar]
- 7.Rousseau C, Devillers A, Sagan C, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol 2006;24(34):5366–5372 [DOI] [PubMed] [Google Scholar]
- 8.Hylton NM, Blume J, Bernreuter W, et al. MRI assessment of breast cancer response to neoadjuvant chemotherapy: preliminary findings of the American College of Radiology Imaging Network (ACRIN) Trial 6657 [abstr]. In: Radiological Society of North America Scientific Assembly and Annual Meeting Program. Oak Brook, Ill: Radiological Society of North America, 2008; 267 [Google Scholar]
- 9.Cerussi A, Hsiang D, Shah N, et al. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc Natl Acad Sci U S A 2007;104(10):4014–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan S, Pogue BW, Jiang S, et al. In vivo hemoglobin and water concentrations, oxygen saturation, and scattering estimates from near-infrared breast tomography using spectral reconstruction. Acad Radiol 2006;13(2):195–202 [DOI] [PubMed] [Google Scholar]
- 11.Zhu Q, Tannenbaum S, Hegde P, Kane M, Xu C, Kurtzman SH. Noninvasive monitoring of breast cancer during neoadjuvant chemotherapy using optical tomography with ultrasound localization. Neoplasia 2008;10(10):1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soliman H, Gunasekara A, Rycroft M, et al. Functional imaging using diffuse optical spectroscopy of neoadjuvant chemotherapy response in women with locally advanced breast cancer. Clin Cancer Res 2010;16(9):2605–2614 [DOI] [PubMed] [Google Scholar]
- 13.Franceschini MA, Moesta KT, Fantini S, et al. Frequency-domain techniques enhance optical mammography: initial clinical results. Proc Natl Acad Sci U S A 1997;94(12):6468–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tromberg BJ, Shah N, Lanning R, et al. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2000;2(1-2):26–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Pogue BW, Jiang S, et al. Image reconstruction of effective Mie scattering parameters of breast tissue in vivo with near-infrared tomography. J Biomed Opt 2006;11(4):041106. [DOI] [PubMed] [Google Scholar]
- 16.Bartek M, Wang X, Wells W, Paulsen KD, Pogue BW. Estimation of subcellular particle size histograms with electron microscopy for prediction of optical scattering in breast tissue. J Biomed Opt 2006;11(6):064007. [DOI] [PubMed] [Google Scholar]
- 17.Wells WA, Wang X, Daghlian CP, Paulsen KD, Pogue BW. Phase contrast microscopy analysis of breast tissue: differences in benign vs. malignant epithelium and stroma. Anal Quant Cytol Histol 2009;31(4):197–207 [PMC free article] [PubMed] [Google Scholar]
- 18.Wells WA, Barker PE, MacAulay C, Novelli M, Levenson RM, Crawford JM. Validation of novel optical imaging technologies: the pathologists’ view. J Biomed Opt 2007;12(5):051801. [DOI] [PubMed] [Google Scholar]
- 19.Wells WA, Daghlian CP, Tosteson TD, et al. Analysis of the microvasculature and tissue type ratios in normal vs. benign and malignant breast tissue. Anal Quant Cytol Histol 2004;26(3):166–174 [PubMed] [Google Scholar]
- 20.Poplack SP, Tosteson TD, Wells WA, et al. Electromagnetic breast imaging: results of a pilot study in women with abnormal mammograms. Radiology 2007;243(2):350–359 [DOI] [PubMed] [Google Scholar]
- 21.Gougos A, Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol 1988;141(6):1925–1933 [PubMed] [Google Scholar]
- 22.Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J 2003;17(9):984–992 [DOI] [PubMed] [Google Scholar]
- 23.Cheifetz S, Bellón T, Calés C, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem 1992;267(27):19027–19030 [PubMed] [Google Scholar]
- 24.Li CG, Hampson IN, Hampson L, Kumar P, Bernabeu C, Kumar S. CD105 antagonizes the inhibitory signaling of transforming growth factor beta1 on human vascular endothelial cells. FASEB J 2000;14(1):55–64 [DOI] [PubMed] [Google Scholar]
- 25.Burrows FJ, Derbyshire EJ, Tazzari PL, et al. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res 1995;1(12):1623–1634 [PubMed] [Google Scholar]
- 26.Kumar P, Wang JM, Bernabeu C. CD 105 and angiogenesis. J Pathol 1996;178(4):363–366 [DOI] [PubMed] [Google Scholar]
- 27.Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Over-expression of endoglin (CD105): a marker of breast carcinoma-induced neo-vascularization. Anticancer Res 1998;18(5A):3621–3628 [PubMed] [Google Scholar]
- 28.Kumar S, Ghellal A, Li C, et al. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res 1999;59(4):856–861 [PubMed] [Google Scholar]
- 29.Sánchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabéu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem 2002;277(46):43799–43808 [DOI] [PubMed] [Google Scholar]
- 30.Dales JP, Garcia S, Bonnier P, et al. CD105 expression is a marker of high metastatic risk and poor outcome in breast carcinomas: correlations between immunohistochemical analysis and long-term follow-up in a series of 929 patients. Am J Clin Pathol 2003;119(3):374–380 [DOI] [PubMed] [Google Scholar]
- 31.Dales JP, Garcia S, Andrac L, et al. Prognostic significance of angiogenesis evaluated by CD105 expression compared to CD31 in 905 breast carcinomas: correlation with long-term patient outcome. Int J Oncol 2004;24(5):1197–1204 [PubMed] [Google Scholar]
- 32.Jiang S, Pogue BW, Carpenter CM, et al. Evaluation of breast tumor response to neoadjuvant chemotherapy with tomographic diffuse optical spectroscopy: case studies of tumor region-of-interest changes. Radiology 2009;252(2):551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasan S, Pogue BW, Jiang S, et al. Interpreting hemoglobin and water concentration, oxygen saturation, and scattering measured in vivo by near-infrared breast tomography. Proc Natl Acad Sci U S A 2003;100(21):12349–12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang S, Pogue BW, McBride TO, Doyley MM, Poplack SP, Paulsen KD. Near-infrared breast tomography calibration with optoelastic tissue simulating phantoms. J Electron Imaging 2003;12(4):613–620 [Google Scholar]
- 35.Pogue BW, Jiang S, Dehghani H, et al. Characterization of hemoglobin, water, and NIR scattering in breast tissue: analysis of intersubject variability and menstrual cycle changes. J Biomed Opt 2004;9(3):541–552 [DOI] [PubMed] [Google Scholar]
- 36.Pinder SE, Provenzano E, Earl H, Ellis IO. Laboratory handling and histology reporting of breast specimens from patients who have received neoadjuvant chemotherapy. Histopathology 2007;50(4):409–417 [DOI] [PubMed] [Google Scholar]
- 37.Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res 1997;3(7):1031–1044 [PubMed] [Google Scholar]
- 38.Wang JM, Kumar S, Pye D, van Agthoven AJ, Krupinski J, Hunter RD. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer 1993;54(3):363–370 [DOI] [PubMed] [Google Scholar]
- 39.Wang JM, Kumar S, Pye D, Haboubi N, al-Nakib L. Breast carcinoma: comparative study of tumor vasculature using two endothelial cell markers. J Natl Cancer Inst 1994;86(5):386–388 [DOI] [PubMed] [Google Scholar]
- 40.Fonsatti E, Del Vecchio L, Altomonte M, et al. Endoglin: an accessory component of the TGF-beta-binding receptor-complex with diagnostic, prognostic, and bioimmunotherapeutic potential in human malignancies. J Cell Physiol 2001;188(1):1–7 [DOI] [PubMed] [Google Scholar]
- 41.Charpin C, Garcia S, Bouvier C, et al. CD31/PECAM automated and quantitative immunocytochemical assays in breast carcinomas: correlation with patient follow-up. Am J Clin Pathol 1997;107(5):534–541 [DOI] [PubMed] [Google Scholar]
- 42.Charpin C, Devictor B, Bergeret D, et al. CD31 quantitative immunocytochemical assays in breast carcinomas: correlation with current prognostic factors. Am J Clin Pathol 1995;103(4):443–448 [DOI] [PubMed] [Google Scholar]
- 43.Lopes N, Sousa B, Vieira D, Milanezi F, Schmitt F. Vessel density assessed by endoglin expression in breast carcinomas with different expression profiles. Histopathology 2009;55(5):594–599 [DOI] [PubMed] [Google Scholar]
- 44.Beresford MJ, Harris AL, Ah-See M, Daley F, Padhani AR, Makris A. The relationship of the neo-angiogenic marker, endoglin, with response to neoadjuvant chemotherapy in breast cancer. Br J Cancer 2006;95(12):1683–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choe R, Corlu A, Lee K, et al. Diffuse optical tomography of breast cancer during neoadjuvant chemotherapy: a case study with comparison to MRI. Med Phys 2005;32(4):1128–1139 [DOI] [PubMed] [Google Scholar]
- 46.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47(1):207–214 [DOI] [PubMed] [Google Scholar]
- 47.Carey LA, Metzger R, Dees EC, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst 2005;97(15):1137–1142 [DOI] [PubMed] [Google Scholar]
- 48.Chevallier B, Roche H, Olivier JP, Chollet P, Hurteloup P. Inflammatory breast cancer: pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol 1993;16(3):223–228 [PubMed] [Google Scholar]
- 49.Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg 1995;180(3):297–306 [PubMed] [Google Scholar]
- 50.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001;(30):96–102 [DOI] [PubMed] [Google Scholar]