Abstract
Estimates of individual patient risk, and epidemiologic studies assessing potential late effects, must use patient size–specific dose estimates—they cannot use only scanner output (volume CT dose index or dose-length product).
In 1981, Shope et al (1) published “A Method for Describing the Doses Delivered by Transmission X-ray Computed Tomography.” In that article, they introduced the computed tomography (CT) dose index (CTDI) as a metric to quantify the radiation output from a CT examination consisting of multiple contiguous CT scans (ie, multiple adjacent transverse rotations of the x-ray tube along the patient longitudinal axis). A new dosimetric method was required for CT because the irradiation geometry was quite different from that of other x-ray modalities in use at that time; namely, the x-ray tube irradiated only a narrow section of the anatomy while it made a full rotation around the patient and did so for multiple rotations along the length of the patient. The CTDI method sought to create an “index” to reflect the average dose to a cylindrical phantom in the central region of a series of scans. The word “index” was specifically included in CTDI’s name to distinguish the quantity from the radiation dose absorbed by a patient.
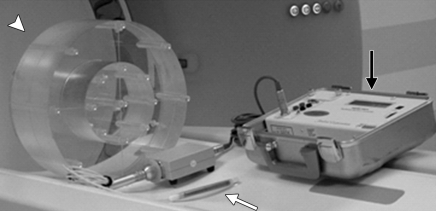
This method, which has been defined in detail elsewhere (2–6), was subsequently adopted by the Center for Devices in Radiological Health of the Food and Drug Administration and its definition included in the Code of Federal Regulations (6). These regulations specify the composition, diameter, and length of two polymethylmethacrylate (ie, acrylic, Lucite) cylindrical phantoms that are to be used for CTDI measurements. To quantify the scanner output for head CT examinations, a 16-cm-diameter phantom is to be used. To quantify scanner output for body examinations, a 32-cm-diameter phantom is to be used. These are referred to as the head and body CTDI phantoms, respectively. Both phantoms are 14–15 cm long (Fig 1).
Figure 1:
Equipment typically used to measure CTDI100 includes an integrating electrometer (black arrow), a 100-mm-long CTDI ionization chamber (white arrow), and a CTDI phantom made of polymethylmethacrylate (arrowhead). The phantom is placed with its long axis perpendicular to the plane of the transverse CT scan and the ion chamber placed in one of the holes through the phantom. CTDI100 is obtained by integrating the dose over 100 mm from a single transverse scan and dividing it by the nominal beam width. (Reprinted, with permission, from reference 7.)
The standardization of the CTDI phantoms marked a crucial step in quantifying the radiation output of a CT scanner in a consistent and reproducibly measured fashion. This is because the primary beam emitted from the scanner (originally a relatively thin fan beam, which with current technology has expanded to cone beams of up to 16 cm width along the patient longitudinal axis) produces a substantial amount of scattered radiation when it interacts with the patient. Hence, consistent radiation output measurements required consistent phantoms.
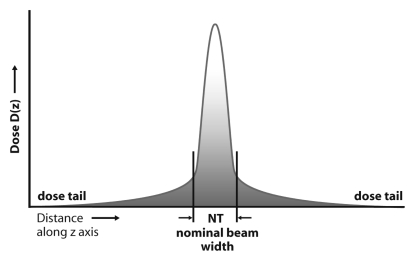
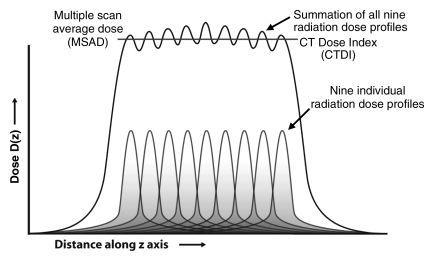
Early estimates of dose from a CT examination did not use the CTDI methodology and measured only the dose from a single scan acquisition. Specifically, only the peak radiation dose emitted by the scanner from a single tube rotation and at a single table position was measured, and this underestimated the dose delivered to a typical adult patient by a factor of two to three. The reason for this underestimation was that the measurement neglected the “tails” of the dose distribution caused by scattered radiation produced from scans at adjacent table positions (Fig 2a) (1,7). Because most clinical examinations involve multiple scans (ie, gantry rotations) as the patient is translated through the gantry, the dose distribution to the patient is the sum of the overlapped “single-scan” dose distributions (Fig 2b). For examinations with a sufficient number of scans, the average dose over the central scan width of the imaged anatomy will reach an equilibrium value, which is referred to as the multiple scan average dose (MSAD) (Fig 2b).
Figure 2a:
(a) Radiation dose profile along a line perpendicular to the scan plane shows a peak dose level at the center of the primary beam and long dose tails caused by scattered radiation. NT = nominal beam width. (b) The radiation dose profiles from nine adjacent transverse CT scans along a line perpendicular to the transverse scans, when summed, produce the MSAD profile. The value of MSAD is the average value of this profile over one scan interval in the central portion of the profile. (Reprinted, with permission, from reference 7.)
Figure 2b:
(a) Radiation dose profile along a line perpendicular to the scan plane shows a peak dose level at the center of the primary beam and long dose tails caused by scattered radiation. NT = nominal beam width. (b) The radiation dose profiles from nine adjacent transverse CT scans along a line perpendicular to the transverse scans, when summed, produce the MSAD profile. The value of MSAD is the average value of this profile over one scan interval in the central portion of the profile. (Reprinted, with permission, from reference 7.)
In the early days of CT, direct measurement of the MSAD was a labor-intensive process. It required multiple scan acquisitions, which placed heavy loads on the x-ray tube. The long scan times necessitated use of dosimeters that could integrate dose accurately over several minutes, such as film or thermoluminescent dosimeters. Conversely, the introduction of CTDI by Shope et al (1) provided a much more practical method with which to estimate the MSAD and hence quantify the radiation output of a CT system. First, although the CTDI could be measured by using only a single rotation of the x-ray tube, it represented the dose from a series of scan acquisitions. Second, it facilitated the use of ionization chambers, making measurements faster and easier to acquire. Because the x-ray beam from a CT scanner was too narrow to completely cover the sensitive volume of existing ionization chambers, a 100-mm-long pencil ionization chamber was developed and the partial irradiation effect corrected on the basis of chamber length and nominal beam width (8).
The CTDI technique uses this long ionization chamber to integrate the primary and scattered radiation delivered with a single scan (ie, one gantry rotation) and normalizes it to the nominal beam width. This normalization cleverly incorporated a scanner’s dose efficiency. That is, if the radiation dose profile from a CT system was unnecessarily wide (ie, the primary beam was wider than the imaged section width), the CTDI would be higher than that from a system with a more narrow beam that better matched the width of the imaged section (9,10). In addition, Shope and colleagues (1) demonstrated that the CTDI could be easily scaled to reflect the common situation when the radiation beams were not contiguous (ie, when there were gaps or overlaps between consecutive rotations of the x-ray tube). Thus, CTDI-based metrics became the reference standard for measuring, comparing, and communicating the radiation output of a CT system (3).
In recent years, however, the strengths and weaknesses of the CTDI have been debated (11–15). Criticisms of the CTDI are based on two primary arguments: (a) the 100-mm-long pencil ionization chamber used to collect the dose may not be sufficiently long to measure all of the tails of the scattered dose distribution, and (b) the phantoms used for CTDI measurements are shorter than an adult torso and so do not produce as much scattered radiation as would occur in a typical adult. This means that the average dose (eg, MSAD) that would occur in the much longer “typical-sized” adult torso is underestimated with CTDI measurements in the 14-cm-long body CTDI phantom; the underestimation owing to the use of this phantom can be as much as 40% (11,13). Another important limitation of the CTDI concept is that it is not applicable for CT exposures where the patient remains stationary throughout the scan. Whether from wide cone-beam systems that image a large volume without table increment or CT perfusion examinations, the CTDI value presented on the scanner console is an overestimate of both the average dose within the scan volume and the dose to the skin (16–19).
These criticisms, however, are based on the belief that CTDI should estimate the patient dose, as opposed to quantifying the radiation output of CT systems. In fact, because patients and the wide range of clinical applications and scan protocols used to scan them vary so dramatically, there is no single phantom that can be used to accurately estimate the dose to all patients. Any dose metric designed to estimate patient dose for a “typical” adult will underestimate the actual absorbed dose for a pediatric patient or overestimate the actual absorbed dose for an obese patient.
Instead, because the volume CTDI (CTDIvol) (3,5,20) is displayed on the scanner console before the initiation of a scan (to allow the operator to confirm that the proper scanner output is programmed) and recorded as part of the patient’s examination information, many users incorrectly assume that it is the dose to that particular patient. The CTDI values are included in either a screen-captured “patient dose report” or a structured Digital Imaging and Communications in Medicine dose report, which reinforces the incorrect belief that CTDI is a measure of patient dose. In fact, the actual dose to any given patient is directly dependent on the size and shape of the patient (19,21–24). The CTDIvol is a standardized measure of the radiation output of a CT system, measured in a cylindrical acrylic phantom, that enables users to gauge the amount of emitted radiation and compare the radiation output between different scan protocols or scanners. Complex calculations are required to map scanner output to patient dose, taking into account the patient’s size, irradiated organs, body composition, and scan range (19,21–25).
A simple analogy is the following: The operation of a car’s engine is reflected by a tachometer. It reports revolutions per minute of the engine’s crankshaft. Although the literal translation for the term “tachometer” from the Greek is “speed measurer,” it does not, in fact, measure the speed (velocity) of a car. The speedometer, which reports the distance (miles or kilometers) that the car will travel in 1 hour at its current velocity, must be calibrated for the specific tire diameter on the vehicle. By changing the size of the tires from those for which the vehicle was calibrated, one will reduce the accuracy of the speedometer. Increasing the tire’s diameter allows a greater distance to be traveled per revolution of the crankshaft, and, thus, a higher speed is achieved for a given number of revolutions per minute. Likewise, for a given CT scanner output (ie, tachometer, engine output), changing the diameter of the patient (ie, tire diameter) will change the dose absorbed by that patient (vehicle velocity).
Although the need to take patient size into account when estimating patient dose has been well established, the widespread misinterpretation of CTDI as a measure of patient dose continues.
Equipped with accurate knowledge of scanner output and estimates of patient size (eg, from the CT radiograph), scan region (eg, thorax or abdomen), and scan length, estimates of patient-size–specific dose may be determined with an accuracy of approximately 10% (19,22–27). Thus, as long as scanner output continues to be measured and reported by using a standardized, highly reproducible, and pragmatic measurement technique, such as the CTDIvol method, patient dose can be accurately estimated. It is imperative, however, that the community be aware that the CTDI is not patient dose.
CTDIvol provides a very useful way to compare the doses delivered by various scan protocols or to achieve a specific level of image quality for a specific size patient. With use of technique charts and diagnostic reference levels, CTDIvol can be used to prescribe the right dose for a specific patient size and diagnostic task. However, CTDIvol cannot be used as a surrogate for patient dose, either in epidemiologic assessments of potential late effects or for potential deterministic effects (eg, skin injury) (17,18). Neither CTDIvol nor its derivative, dose-length product (DLP, which is the product of CTDIvol and the irradiated scan length), should be used to estimate effective dose or potential cancer risk for any individual patient. The published “k factors” used to convert DLP to effective dose all assume a standard-sized patient (28–31). The “standard” patient used for adult k factors is relatively thin by today’s standards (nominal body mass of 70 kg) (28–32). Similarly, the k factors for newborns and 1-, 5-, 10-, and 15-year-old children refer to a generic child of that age, even though the dimensions assigned to an age do not always correlate well with individual patient sizes (33). For both children and adults, the idealized patient model is a hermaphrodite; that is, it has the sexual organs of both sexes. Thus, the patient models used to estimate dose by using DLP do not represent a real patient.
Examples of the inappropriate use of CTDIvol, DLP, and effective dose include the widely publicized reports of large variations in “doses” from CT examinations (34). The problem with such reports, however, is the lack of correction for patient size. For example, in Monte Carlo simulations of absorbed patient dose that take into account patient size, it has been shown that the effective dose increases much more slowly than does the CTDIvol or DLP. To achieve similar image quality, the scanner output (CTDIvol) should be increased by about a factor of two as patient size changes from a typical adult abdomen (lateral dimension, 35–40 cm) to an obese adult abdomen (lateral width, 45–50 cm) (35–37). Even though the scanner output increases by a factor of two, the dose to many of the radiosensitive internal organs used in the calculation of effective dose does not increase by the same amount owing to the attenuation of the additional adipose tissue. Rather, the factor of two increase in the CTDIvol, combined with the larger patient size, results in a net increase in effective dose of only approximately 20%–30% (23,24,38).
An important implication of the need to take patient size into account, both when estimating patient dose and when prescribing the correct scanner output settings, is that considerable variation in CTDI-based dose metrics can, and should, be expected. Facilities that adjust their CT technique appropriately for patient size, whether with use of manual technique charts or automatic exposure control (35,39–56), will prescribe a wide range of scanner output (CTDI) values. This is a good outcome, reflecting the facility’s conscientiousness in “right-sizing” the dose settings on the basis of specific patient body habitus. Furthermore, variability in the image quality criteria for various diagnostic tasks and clinical applications introduces variability in the scanner output settings that one should prescribe, even for patients of the same size. For example, scanner output should vary markedly between CT colonography and CT enterography, even for the same patient. Thus, radiation management in CT requires choosing the correct settings for scanner output, not only for patient size but also for the imaging task.
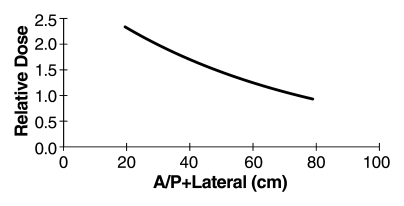
In conclusion, it is imperative that measures of the radiation output of a CT system can be easily and practically measured in a consistent and robust fashion. CTDIvol meets these criteria. It is defined both in the United States and in international regulatory communities, and the equipment used to measure it is ubiquitously available worldwide. The CTDIvol tells the medical physicist precisely how the machine was operated, and it can be used, in conjunction with information regarding patient size and the scanned anatomy, to estimate patient dose (19,23–25). Dose estimates can be for organ dose, skin dose, or a mean dose in the center of the scan volume (17,19,23–25,57). The CTDI values are not, however, patient dose estimates. CTDI is the tachometer in a CT scanner—not the speedometer. Estimates of individual patient risk, and epidemiologic studies assessing potential late effects, must use patient size–specific dose estimates—they cannot use only scanner output (CTDIvol or DLP). Rather, use of the known exponential relationship between patient size and absorbed dose will allow patient size–specific dose estimates to be made from scanner output values (19,23,24) (Fig 3).
Figure 3:
Graph shows relative dose (mean patient dose per 1 mGy of scanner output, CTDIvol) for an abdominal CT scan and different patient sizes (here represented by the sum of anteroposterior [A/P] and lateral dimensions). Over the range of patient sizes from a newborn to a large adult, relative dose is exponentially related to patient size. For a patient with an anteroposterior dimension of 30 cm and a lateral dimension of 40 cm, the anteroposterior + lateral value would be 70 cm and the mean patient dose in the center of the scan range would be approximately equivalent to the CTDIvol value reported on the console. For a neonate having both anteroposterior and lateral dimensions of 10 cm, the anteroposterior + lateral value would be 20 cm and the mean patient dose in the center of an abdomen scan would be about 2.3 times the displayed CTDI value, for body CTDIvol measurements made by using a 32-cm phantom. CTDIvol measurements made on the basis of 16-cm phantoms would require a different scale factor.
Disclosures of Potential Conflicts of Interest: C.H.M. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution received a grant or grants are pending from Siemens Medical Solutions. Other relationships: none to disclose. S.L. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. L.Y. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. D.D.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received payment for lectures including service on speakers bureaus from MTMI. Other relationships: none to disclose. J.M.B. Financial activities related to the present article: holds a patent on a method for computing dose at CT. Financial activities not related to the present article: receives money for various consulting activities; received money for expert testimony; institution received a grant or grants are pending from several vendors; receives royalties from Williams & Wilkins. Other relationships: none to disclose. M.F.M.G. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: received a grant or grants are pending from Siemens Medical Solutions; institution received a grant or grants are pending from Siemens Medical Solutions; received payment for development of educational presentations from MTMI; received money for travel/accommodations/meeting expenses unrelated to activities listed from Siemens Medical Solutions; institution received money for travel/accommodations/meeting expenses unrelated to activities listed from Siemens Medical Solutions. Other relationships: none to disclose.
Received September 8, 2010; revision requested October 25; revision received December 16; final version accepted December 27.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: This research was supported in part by the National Institutes of Health (grants EB004898, EB002138, and DK59933).
References
- 1.Shope TB, Gagne RM, Johnson GC. A method for describing the doses delivered by transmission x-ray computed tomography. Med Phys 1981;8(4):488–495 [DOI] [PubMed] [Google Scholar]
- 2.American Association of Physicists in Medicine Standardized methods for measuring diagnostic x-ray exposures. New York, NY: American Association of Physicists in Medicine, 1990 [Google Scholar]
- 3.American Association of Physicists in Medicine The measurement, reporting and management of radiation dose in CT. Report 96. AAPM Task Group 23 of the Diagnostic Imaging Council CT Committee. College Park, Md: American Association of Physicists in Medicine, 2008 [Google Scholar]
- 4.European Commission European guidelines on quality criteria for computed tomography. EUR 16262 EN. Luxembourg: European Commission, 2000 [Google Scholar]
- 5.International Electrotechnical Commission Medical electrical equipment. Part 2-44: Particular requirements for the safety of x-ray equipment for computed tomography. IEC publication no. 60601-2-44 Ed. 2.1 Geneva, Switzerland: International Electrotechnical Commission Central Office, 2002 [Google Scholar]
- 6.Performance standards for ionizing radiation-emitting products: diagnostic x-ray systems and their major components. 21 CFR §1020.30 (2009). [PubMed] [Google Scholar]
- 7.Bauhs JA, Vrieze TJ, Primak AN, Bruesewitz MR, McCollough CH. CT dosimetry: comparison of measurement techniques and devices. RadioGraphics 2008;28(1):245–253 [DOI] [PubMed] [Google Scholar]
- 8.Jucius RA, Kambic GX. Radiation dosimetry in computed tomography: application of optical instrumentation in medicine (part VI). Proc Soc Photo Opt Instrum Eng 1977;127:286–295 [Google Scholar]
- 9.McCollough CH, Zink FE. Performance evaluation of a multi-slice CT system. Med Phys 1999;26(11):2223–2230 [DOI] [PubMed] [Google Scholar]
- 10.Zink FE, McCollough CH. The measurement of radiation dose profiles for electron-beam computed tomography using film dosimetry. Med Phys 1994;21(8):1287–1291 [DOI] [PubMed] [Google Scholar]
- 11.Boone JM. The trouble with CTD100. Med Phys 2007;34(4):1364–1371 [DOI] [PubMed] [Google Scholar]
- 12.Dixon RL. A new look at CT dose measurement: beyond CTDI. Med Phys 2003;30(6):1272–1280 [DOI] [PubMed] [Google Scholar]
- 13.Dixon RL. Restructuring CT dosimetry: a realistic strategy for the future—requiem for the pencil chamber. Med Phys 2006;33(10):3973–3976 [DOI] [PubMed] [Google Scholar]
- 14.McCollough CH. It is time to retire the computed tomography dose index (CTDI) for CT quality assurance and dose optimization: against the proposition. Med Phys 2006;33(5):1190–1191 [DOI] [PubMed] [Google Scholar]
- 15.Mori S, Endo M, Nishizawa K, et al. Enlarged longitudinal dose profiles in cone-beam CT and the need for modified dosimetry. Med Phys 2005;32(4):1061–1069 [DOI] [PubMed] [Google Scholar]
- 16.Dixon RL, Boone JM. Cone beam CT dosimetry: a unified and self-consistent approach including all scan modalities—with or without phantom motion. Med Phys 2010;37(6):2703–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leng S, Vrieze T, Yu L, McCollough C. SU‐GG‐I‐38: a direct skin dose calculation method in CT scans without table motion: influence of patient size and beam collimation. Med Phys 2010;37(6):3110 [Google Scholar]
- 18.Rong X, Cody D. How accurate is estimating CT skin dose based on CTDI? Med Phys 2010;37(6):3463 [Google Scholar]
- 19.McCollough CH, Christner JA, Rueda V, Ramirez Giraldo J, Vrieze TJ, Leng S. Estimating patient-specific dose from scanner output (CTDIvol): yes we can! [abstr]. In: Radiological Society of North America scientific assembly and annual meeting program Oak Brook, Ill: Radiological Society of North America, 2010; 380 [Google Scholar]
- 20.McNitt-Gray MF. AAPM/RSNA physics tutorial for residents: topics in CT—radiation dose in CT. RadioGraphics 2002;22(6):1541–1553 [DOI] [PubMed] [Google Scholar]
- 21.Siegel MJ, Schmidt B, Bradley D, Suess C, Hildebolt C. Radiation dose and image quality in pediatric CT: effect of technical factors and phantom size and shape. Radiology 2004;233(2):515–522 [DOI] [PubMed] [Google Scholar]
- 22.DeMarco JJ, Cagnon CH, Cody DD, et al. Estimating radiation doses from multidetector CT using Monte Carlo simulations: effects of different size voxelized patient models on magnitudes of organ and effective dose. Phys Med Biol 2007;52(9):2583–2597 [DOI] [PubMed] [Google Scholar]
- 23.Turner A, Zankl M, Demarco JJ, Angel E, Zhang D, McNitt-Gray MF. A method to estimate organ doses from multidetector row CT abdominal exams from patient sized corrected CT dose index (CTDI) values: a Monte Carlo study [abstr]. In: Radiological Society of North America scientific assembly and annual meeting program Oak Brook, Ill: Radiological Society of North America, 2009; 472 [Google Scholar]
- 24.Turner A, Zhang D, Khatonabadi M, et al. The feasibility of patient size–corrected, scanner-independent organ dose estimates for abdominal CT exams. Med Phys (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner AC, Zankl M, DeMarco JJ, et al. The feasibility of a scanner-independent technique to estimate organ dose from MDCT scans: using CTDIvol to account for differences between scanners. Med Phys 2010;37(4):1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng S, Vrieze T, Yu L, McCollough C. A direct skin dose calculation method in CT scans without table motion: influence of patient size and beam collimation. Presented at the 52nd Annual Meeting of the American Association of Physicists in Medicine, Philadelphia, Pa, July 18–22, 2010 [Google Scholar]
- 27.Leng S, Vrieze TJ, Yu L, McCollough CH. Skin dose estimation from CT perfusion studies: influence of patient size, beam collimation and scanner type [abstr]. In: Radiological Society of North America scientific assembly and annual meeting program Oak Brook, Ill: Radiological Society of North America, 2010; 423 [Google Scholar]
- 28.Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol 2010;194(4):881–889 [DOI] [PubMed] [Google Scholar]
- 29.McCollough CH, Christner JA, Kofler JM. How effective is effective dose as a predictor of radiation risk? AJR Am J Roentgenol 2010;194(4):890–896 [DOI] [PubMed] [Google Scholar]
- 30.Shrimpton P. Assessment of patient dose in CT. Appendix C, European guidelines for multislice computed tomography. Contract number FIGM-CT2000-20078-CT-TIP. Funded by the European Commission. 2004 [Google Scholar]
- 31.Shrimpton P, Hillier M, Lewis M, Dunn M. Doses from computed tomography (CT) examinations in the UK—2003 review. Chilton, England: National Radiological Protection Board, 2005 [Google Scholar]
- 32.Cristy M, Eckerman K. Specific absorbed fractions of energy at various ages from internal photon sources. Parts I-VII (ORNL/TM-8381). Oak Ridge, Tenn: Oak Ridge National Laboratory, 1987; 1–74 [Google Scholar]
- 33.Kleinman PL, Strauss KJ, Zurakowski D, Buckley KS, Taylor GA. Patient size measured on CT images as a function of age at a tertiary care children’s hospital. AJR Am J Roentgenol 2010;194(6):1611–1619 [DOI] [PubMed] [Google Scholar]
- 34.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169(22):2078–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalra MK, Maher MM, Toth TL, et al. Techniques and applications of automatic tube current modulation for CT. Radiology 2004;233(3):649–657 [DOI] [PubMed] [Google Scholar]
- 36.Kalra MK, Maher MM, D’Souza RV, et al. Detection of urinary tract stones at low-radiation-dose CT with z-axis automatic tube current modulation: phantom and clinical studies. Radiology 2005;235(2):523–529 [DOI] [PubMed] [Google Scholar]
- 37.Wilting JE, Zwartkruis A, van Leeuwen MS, Timmer J, Kamphuis AG, Feldberg M. A rational approach to dose reduction in CT: individualized scan protocols. Eur Radiol 2001;11(12):2627–2632 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt B, Kalender WA. A fast voxel-based Monte Carlo method for scanner- and patient-specific dose calculations in computed tomography. Physica Medica 2002;18(2):43–53 [Google Scholar]
- 39.Söderberg M, Gunnarsson M. Automatic exposure control in computed tomography: an evaluation of systems from different manufacturers. Acta Radiol 2010;51(6):625–634 [DOI] [PubMed] [Google Scholar]
- 40.van Straten M, Deak P, Shrimpton PC, Kalender WA. The effect of angular and longitudinal tube current modulations on the estimation of organ and effective doses in x-ray computed tomography. Med Phys 2009;36(11):4881–4889 [DOI] [PubMed] [Google Scholar]
- 41.Angel E, Yaghmai N, Jude CM, et al. Monte Carlo simulations to assess the effects of tube current modulation on breast dose for multidetector CT. Phys Med Biol 2009;54(3):497–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angel E, Yaghmai N, Jude CM, et al. Dose to radiosensitive organs during routine chest CT: effects of tube current modulation. AJR Am J Roentgenol 2009;193(5):1340–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deak P, van Straten M, Shrimpton PC, Zankl M, Kalender WA. Validation of a Monte Carlo tool for patient-specific dose simulations in multi-slice computed tomography. Eur Radiol 2008;18(4):759–772 [DOI] [PubMed] [Google Scholar]
- 44.Rizzo S, Kalra M, Schmidt B, et al. Comparison of angular and combined automatic tube current modulation techniques with constant tube current CT of the abdomen and pelvis. AJR Am J Roentgenol 2006;186(3):673–679 [DOI] [PubMed] [Google Scholar]
- 45.McCollough CH, Bruesewitz MR, Kofler JM., Jr CT dose reduction and dose management tools: overview of available options. RadioGraphics 2006;26(2):503–512 [DOI] [PubMed] [Google Scholar]
- 46.Graser A, Wintersperger BJ, Suess C, Reiser MF, Becker CR. Dose reduction and image quality in MDCT colonography using tube current modulation. AJR Am J Roentgenol 2006;187(3):695–701 [DOI] [PubMed] [Google Scholar]
- 47.Mulkens TH, Bellinck P, Baeyaert M, et al. Use of an automatic exposure control mechanism for dose optimization in multi-detector row CT examinations: clinical evaluation. Radiology 2005;237(1):213–223 [DOI] [PubMed] [Google Scholar]
- 48.Kalra MK, Rizzo S, Maher MM, et al. Chest CT performed with z-axis modulation: scanning protocol and radiation dose. Radiology 2005;237(1):303–308 [DOI] [PubMed] [Google Scholar]
- 49.Kalra MK, Maher MM, Toth TL, Kamath RS, Halpern EF, Saini S. Comparison of Z-axis automatic tube current modulation technique with fixed tube current CT scanning of abdomen and pelvis. Radiology 2004;232(2):347–353 [DOI] [PubMed] [Google Scholar]
- 50.Kalra MK, Maher MM, Kamath RS, et al. Sixteen–detector row CT of abdomen and pelvis: study for optimization of Z-axis modulation technique performed in 153 patients. Radiology 2004;233(1):241–249 [DOI] [PubMed] [Google Scholar]
- 51.Jakobs TF, Wintersperger BJ, Herzog P, et al. Ultra-low-dose coronary artery calcium screening using multislice CT with retrospective ECG gating. Eur Radiol 2003;13(8):1923–1930 [DOI] [PubMed] [Google Scholar]
- 52.Jakobs TF, Becker CR, Ohnesorge B, et al. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol 2002;12(5):1081–1086 [DOI] [PubMed] [Google Scholar]
- 53.Greess H, Nömayr A, Wolf H, et al. Dose reduction in CT examination of children by an attenuation-based on-line modulation of tube current (CARE Dose). Eur Radiol 2002;12(6):1571–1576 [DOI] [PubMed] [Google Scholar]
- 54.Greess H, Wolf H, Baum U, et al. Dose reduction in computed tomography by attenuation-based on-line modulation of tube current: evaluation of six anatomical regions. Eur Radiol 2000;10(2):391–394 [DOI] [PubMed] [Google Scholar]
- 55.Kalender WA, Wolf H, Suess C. Dose reduction in CT by anatomically adapted tube current modulation. II. Phantom measurements. Med Phys 1999;26(11):2248–2253 [DOI] [PubMed] [Google Scholar]
- 56.Gies M, Kalender WA, Wolf H, Suess C. Dose reduction in CT by anatomically adapted tube current modulation. I. Simulation studies. Med Phys 1999;26(11):2235–2247 [DOI] [PubMed] [Google Scholar]
- 57.Boone JM. Dose spread functions in computed tomography: a Monte Carlo study. Med Phys 2009;36(10):4547–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]