Abstract
Background
Theoretical and empirical evidence suggests that impaired time perception and the neural circuitry contributing to internal timing mechanisms may contribute to severe psychiatric disorders, including mood disorders. The structures that are involved in subsecond timing, i.e., cerebellum and basal ganglia, have also been implicated in the pathophysiology of bipolar disorder. However, the timing of subsecond intervals has infrequently been studied in this population.
Methods
Paced finger-tapping tasks have been used to characterize internal timing processes in neuropsychiatric disorders. A total of 42 bipolar disorder patients (25 euthymic, 17 manic) and 42 age-matched healthy controls completed a finger-tapping task in which they tapped in time with a paced (500-ms intertap interval) auditory stimulus (synchronization), then continued tapping without auditory input while attempting to maintain the same pace (continuation). This procedure was followed using the dominant index finger, then with alternating thumbs.
Results
Bipolar disorder participants showed greater timing variability relative to controls regardless of pacing stimulus (synchronization versus continuation) or condition (dominant index finger versus alternating thumbs). Decomposition of timing variance into internal clock versus motor implementation components using the Wing–Kristofferson model showed higher clock variability in the bipolar disorder groups compared to controls, with no differences between groups on motor implementation variability.
Conclusion
These findings suggest that internal timing mechanisms are disrupted in bipolar disorder patients, independent of symptom status. Increased clock variability in bipolar disorder may be related to abnormalities in cerebellar function.
Keywords: bipolar disorder, cerebellum, depression, finger tapping, interval, mania, temporal, timing
Alterations in time perception and neural circuitry associated with internal timing have been found in several neuropsychiatric disorders that share phenomenological and genetic overlap with bipolar disorder, including schizophrenia (1, 2) and attention-deficit hyperactivity disorder (ADHD) (3). Precise knowledge of how time is encoded by the brain remains elusive, yet it has become apparent that a number of structures (e.g., the cerebellum, basal ganglia, orbitofrontal cortex, insula, parietal cortex, prefrontal cortex) and neurotransmitter systems (e.g., dopamine and glutamate) contribute in various ways to time perception. Many of these very same regions and systems have also been implicated in the pathophysiology of bipolar disorder (4). However, investigations of time perception in bipolar disorder for short durations, i.e., those in the second or millisecond range, are scarce. Only two reports, both from the same group (5, 6), have, to our knowledge, included bipolar disorder patients and compared them with a control group on time perception in the suprasecond range. However, in both cases, the sample was not restricted to bipolar disorder participants only, making results difficult to interpret. Bschor et al. (5) reported that depressed (meeting criteria for DSM-IV major depressive episode) and manic (DSM-IV manic) patients did not differ from controls on either a 7-second time production or an 8-second time-estimation task. In a subsequent study by the same group using the same criteria (6), depressed patients over-reproduced a 6-second interval, and no differences between patients and controls were observed in a 1-second time reproduction task. To our knowledge, no studies have examined explicit timing in bipolar disorder in the subsecond time domain, although bipolar disorder participants showed significant timing abnormalities on an implicit motor timing task, namely eyeblink conditioning (7).
It is generally accepted that the frontal cortex, basal ganglia, and cerebellum are integrally involved in time perception, with a consensus emerging that different timescales utilize different neural circuits (8–10). A recent meta-analysis of 41 functional neuroimaging studies of perceptual and motor timing by Weiner et al. (11) used a robust activation likelihood estimation algorithm and found strong support for the theory that subsecond and suprasecond durations depend on somewhat distinct neural networks, with the former more likely to recruit subcortical structures such as the basal ganglia and cerebellum and the latter more likely to activate cortical structures such as the supplementary motor area and prefrontal cortex. The finding that the cerebellum is activated predominantly during subsecond tasks is consistent with other suggestions that this structure may be critical for the encoding of subsecond time intervals (9, 12). Moreover, the Weiner et al. study found that activation of the cerebellum was consistent across motor and perceptual timing tasks, supporting the proposal that this structure serves as a timekeeper for brief durations (13).
In the present study, we examined explicit subsecond timing in bipolar disorder using a paced finger-tapping task with a 500-ms intertap interval using the synchronization–continuation paradigm. In this task, participants first tapped in time with a tone (synchronization). They then attempted to continue tapping in its absence while maintaining that pace (continuation). The 500-ms interval was chosen because it is short enough to minimize the engagement of brain regions involved in higher-order cognitive processes, including working memory. Subsecond intervals also prompt anticipatory taps; that is, taps are not in response to hearing the paced tone, but instead occur when participants expect it to occur (14). Therefore, at this interval duration, tapping pace can be expected to rely on an internal clock even during the synchronization portion of the task when external pacing tones are occurring (3).
The synchronization–continuation paradigm benefits from the existence of a widely known model of human timing developed by Wing and Kristofferson (15) that partitions the production of a sequence of finger taps during the continuation portion of the task into its fundamental components: (i) a central ‘clock’ timekeeper, and (ii) the delay between the neural command and the execution of the movement. This mathematical model uses the variance and autocovariance properties of the series of taps to determine whether the source of timing variability is due to the central timekeeper or the motor delay. In controls, Wing and Kristofferson (15) demonstrated that the central timekeeper variance increases as a function of the time interval being kept, while the variance in motor delay remains virtually constant across all intervals. By decomposing variability into clock and motor components, this model allows insight into the origins of any group differences in intrasubject timing variability.
We hypothesized that bipolar disorder patients would show greater intrasubject variability and shorter intertap intervals in comparison to controls, as well as higher central timekeeper variance as estimated by the clock component of the Wing–Kristofferson mathematical model. An additional goal of this study was to investigate whether deficits in finger-tapping performance could differentiate between acutely ill and clinically stable groups of bipolar disorder participants. We predicted that the manic bipolar disorder group would display increased temporal variability of finger tapping relative to control participants, and that euthymic bipolar disorder participants would fall between these groups. Moreover, we hypothesized that this variability would be most pronounced in the continuation condition because variability is generally expected to increase in the absence of a pacing stimulus, an effect that should be amplified in the manic group.
Methods
Participants
A total of 42 (17 males, 25 females) bipolar disorder participants and 42 (20 males, 22 females) age-matched controls were included in this analysis.1 Diagnostic status was determined using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (16) sections for mood disorders, psychotic disorders, and substance abuse disorders, and chart review. Healthy controls were recruited through newspaper advertisements and fliers, and did not meet DSM criteria for any Axis I or Axis II disorder. Any participant who met criteria for substance dependency within three months prior to testing was excluded from the study. Trained research personnel performed diagnostic interviews and clinical ratings. Kappa inter-rater reliability in this laboratory setting has been 0.95 for mood disorders versus schizophrenia or other diagnoses. The study procedures were approved by the Indiana University-Purdue University Indianapolis Internal Review Board, and the study was conducted in accordance with the Declaration of Helsinki (Edinburgh amendments). Written informed consent was obtained from all participants.
The bipolar disorder group included individuals tested during manic (n = 17) and euthymic (n = 25) episodes. Acute symptom severity during the preceding week was assessed using the Young Mania Rating Scale (YMRS) (17) and Montgomery-Asberg Depression Rating Scale (MADRS) (18). Euthymic participants had a mean YMRS score of 5.7 (SD = 5.4) and an average MADRS score of 5.1 (SD = 5.0). For manic participants, YMRS scores averaged 27.9 (SD = 8.3), with mean MADRS scores of 7.4 (SD = 3.7). There were no significant correlations between YMRS or MADRS scores and any of the primary dependent variables in either the dominant index finger or alternating thumbs conditions.
Healthy controls were recruited and included in the study if their age fell within two years of a bipolar disorder participant’s in order to ensure that the groups were age-matched. This ensured that the mean age of bipolar disorder participants (41.0 years, SD = 11.5) did not differ from controls (40.9 years, SD = 11.5), t(82) = −0.05, p = ns. When bipolar disorder subtypes: manic (mean = 37.0 years, SD = 11.6) and euthymic (mean = 43.8 years, SD = 10.8), and controls were entered into a one-way ANOVA, there was not a significant difference between groups on age [F(2,81) = 1.84, p = ns], nor were Bonferonni-corrected follow-up comparisons significant. As assessed using the Edinburgh Handedness Inventory (19), handedness was not differently distributed across groups [χ2(2) = 1.37, p = ns). In the healthy control group, 9 of the 42 were left-handed; in the bipolar disorder group, 3 of 25 euthymic participants and 2 of 17 manic participants were left-handed. When handedness was used as an independent variable, with age and diagnostic group as covariates, there were no significant differences between left- and right-handed participants for any measure. Inclusion criteria were completion of grade school-level education, normal or corrected-to-normal hearing and vision, no history of cardiovascular or neurological disease, and no history of head injury that resulted in loss of consciousness.
A total of 8 individuals with bipolar disorder were unmedicated at the time of testing. Of the remaining 34 bipolar disorder participants, 25 were on antipsychotic drugs, 25 were on mood stabilizers, 9 were on antidepressants, and 1 participant was taking an anticholinergic medication. A chi-square test showed that medication status did not differ by bipolar disorder group assignment (p > 0.05).
Task procedure
The finger-tapping task consisted of two conditions, where participants first used their dominant index finger, followed by alternating thumbs, to press and release buttons on a response box. For the dominant index finger task, participants rested their dominant hand flat on a response box with their index finger positioned over a center push button. In the alternating thumbs condition, the response box was held in both hands, with the left and right thumbs placed over response buttons on each side. In each condition, the time that passed between subsequent depressions of the button was used to calculate the intertap interval.
Each trial began with a tone paced at 500-ms intertone intervals, and participants were instructed to press the response button at the same rate as the tone (synchronization tapping). After 12 taps, the tone was discontinued and participants were instructed to continue tapping at the same rate as the previously presented tone (continuation tapping). Trials ended following 30 continuation button presses. Intertap intervals that fell 250 ms above or below the 500-ms intertap interval during either the synchronization or continuation portion of the trial were counted as error taps. Successful completion of a total of 6 error-free trials, or when the maximum of 12 trials was reached, concluded the task.
Behavioral data and analysis
The first 6 error-free trials were included in the analysis. In cases where there were fewer than 6 error-free trials, if there were 2 or fewer errors that occurred in the synchronization portion of the task only, errors were removed and averages were taken from the remaining trials. To optimize results from the mathematical model applied to these data (described below) and to allow the same participants to contribute data for each dependent variable, participants who were unable to complete 6 error-free trials for the continuation portion were excluded from all analyses.
Mean intertap intervals were computed separately across trials for the synchronization and continuation portions of the task. Tapping variability was defined by the standard deviation of the intertap interval. In addition, the coefficients of variation were computed to derive a normalized measure of the dispersion of the intertap intervals between patients and nonpsychiatric participants. In order to maximize the power to assess whether tapping abnormalities in bipolar disorder are linked to core deficits of the disorder independent of clinical status, performance differences were assessed using 2 × 2 × 2 repeated-measure ANOVAs, with a between-subjects factor of group (bipolar disorder, nonpsychiatric control) and within-subjects factors of condition (dominant index finger, alternating thumbs) and pacing stimulus (synchronization/continuation). To analyze the effect of mood state on performance in bipolar disorder, 2 × 2 × 2 ANOVAs were conducted using only bipolar disorder participants with clinical status (euthymic, manic) as the between-subjects factor.
For all statistical analyses, post-hoc comparisons using the Bonferroni correction were conducted when main effects or interactions were significant (p < 0.05) or reached a trend level (p < 0.10). Results of the major dependent variables are reported with their corresponding effect sizes in the form of partial eta squared (ηp2), where values of ηp2 < 0.06 were considered small, effect sizes of 0.06 < ηp2 < 0.14 were considered moderate, and effect sizes of ηp2 > 0.14 were considered large (20).
Wing–Kristofferson mathematical model
The Wing–Kristofferson model (15, 21) was developed as a means of delineating the contributions of the central and peripheral nervous components to variability in timing of inter-response intervals. Based on the work of Stevens (22), the Wing–Kristofferson model provides an account of the timing variability using the continuation portion of the finger-tapping task described above. One of the key observations made by Stevens (22) was that the inter-response intervals of the continuation finger taps (without the metronome) followed a zigzag pattern, i.e., shorter inter-response interval followed by a longer inter-response interval, followed by a shorter, etc. Based on this finding, Wing and Kristofferson (15) hypothesized that the continuation responses were the reflection of a central timekeeper that emitted pulses to initiate the motor response. The central timekeeper itself is imprecise due to temporal ‘noise’ that results in random variation in the timing of the central motor command. A second independent source of variance is the motor delay that is the time between the initiation and occurrence of the response.
The Wing–Kristofferson model thus takes the following form when j > 1:
| [1] |
where Ij is the jth intertap interval, while Cj and Dj represent the central timekeeper and motor implementation delays, respectively. The Wing–Kristofferson model functions on the assumption that the clock intervals and motor implementation delays are independent random variables. As a result, the covariance between C and D will be zero, and the covariance of cov (Cj, Cj–k) and cov (Dj, Dj–k) will be zero for all j and k, with the exception of k = 0. Thus, when k = 0:
| [2] |
Since the data collected from the participant only provides the intertap intervals, or I, Equation 2 must be rearranged to provide the clock and motor variances of var(C) and var(D), where:
| [3] |
Hence, the variance of the central timekeeper is the sum of the variance of the inter-response intervals and 2 × the lag-1 autocovariance. The variance of the motor delay is then:
| [4] |
The variance of the motor delay can thus be computed as the negative of the lag-1 autocovariance of the inter-response intervals.
Implementation and analysis
Because of its sequence dependency, i.e., its use of autocovariance, the Wing–Kristofferson model is less tolerant to extreme values within a trial block. Therefore, as in the analyses of intertap interval and variability, estimates of clock and motor variance were computed only for participants with at least 6 error-free trials on both the dominant index finger and alternating thumbs continuation tapping tasks.
One limitation of the Wing–Kristofferson mathematical model is that it can sometimes produce negative values, which is theoretically impossible and therefore untenable. In the current sample, this occurred on 24% of blocks of interval sequences, which is consistent with previous studies that have reported occurrences of 10–30%, depending on the population studied (13, 23–25). Due to the impossibility of a negative variance, such values were set to zero in the present study, which is the traditional approach in models with more than one variance parameter (26), including the Wing–Kristofferson model (21). The average variance across the 6 blocks in each condition was computed, then this value was entered into separate 2 × 2 repeated-measures ANOVAs with diagnosis as the between-subjects variable and condition (dominant index finger, alternating thumbs) as the within-subjects variable for central timekeeper (clock) and motor implementation delay (motor) variance. These tests were conducted using diagnosis (bipolar disorder, nonpsychiatric controls) as a between-subjects factor. In addition, analyses of differences in performance due to clinical status were conducted using the bipolar disorder group only, with mood state (euthymic, manic) as a between-subjects factor.
Results
Medication analysis
To evaluate possible medication effects on tapping performance, participants with bipolar disorder were collapsed into a single group with medication status as the independent variable. Participants were divided into three groups: (i) those on antipsychotic medication (typical or atypical) were assigned to the antipsychotic group (n = 25), (ii) those who were on other psychotropic drugs but were not taking antipsychotic medication were assigned to the other psychotropic category (n = 9), and (iii) those who were not currently taking medication were included in the unmedicated group (n = 8). Repeated-measures ANOVAs were then conducted for all primary dependent variables using mood state as a covariate (manic, euthymic). No significant differences for medication status were found for any primary dependent variables using this approach. Finally, bipolar disorder participants were coded as on or off for the following medication categories: atypical antipsychotic drug use (on = 22), lithium use (on = 8), and a test of medicated (any psychotropic medication including antipsychotics) versus unmedicated participants (on = 34). Separate ANOVAs were conducted for each category and for each dependent variable. No significant differences between groups were observed using these categorizations.
Intertap interval pacing and variability
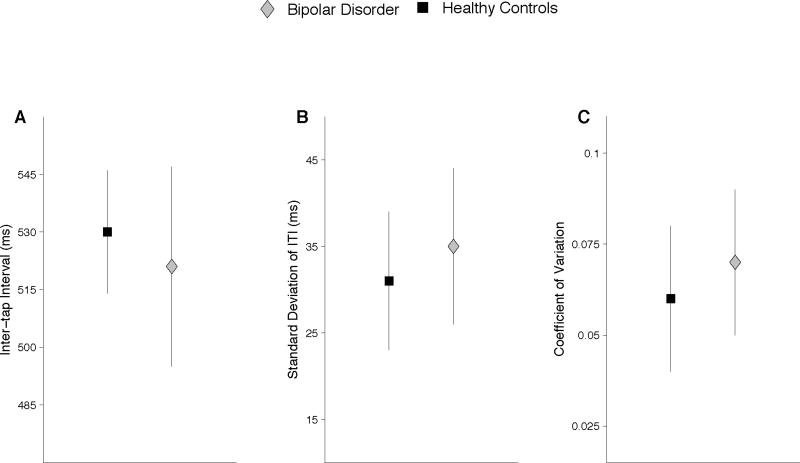
Tables 1 and 2 show means and standard deviations for the intertap interval, standard deviation of the intertap interval, and the coefficient of variation for the dominant index finger and alternating thumbs conditions, respectively. These results are depicted graphically in Figure 1, panels A–C.
Table 1.
Means and standard deviations (in parentheses) of average intertap interval (ITI), as well as the two measures of intertap interval variability (standard deviation and coefficient of variation) for the dominant index finger
| ITI Mean | ITI SD | Coefficient of variation | ||||
|---|---|---|---|---|---|---|
| S | C | S | C | S | C | |
| Healthy controls | 538 (9) | 523 (18) | 32 (10) | 24 (6) | 0.06 (0.02) | 0.05 (0.01) |
| Bipolar disorder | 536 (11) | 519 (29) | 35 (9) | 27 (7) | 0.07 (0.02) | 0.05 (0.01) |
| Euthymic | 536 (12) | 517 (27) | 33 (10) | 27 (7) | 0.06 (0.02) | 0.05 (0.01) |
| Manic | 538 (10) | 521 (32) | 37 (7) | 28 (7) | 0.07 (0.01) | 0.05 (0.01) |
S = synchronization; C = continuation.
Table 2.
Means and standard deviations (in parentheses) of average intertap interval (ITI), as well as the two measures of intertap interval variability (standard deviation and coefficient of variation) for alternating thumbs
| ITI Mean | ITI SD | Coefficient of variation | ||||
|---|---|---|---|---|---|---|
| S | C | S | C | S | C | |
| Healthy controls | 537 (12) | 521 (25) | 37 (11) | 31 (8) | 0.07 (0.02) | 0.06 (0.02) |
| Bipolar disorder | 527 (23) | 503 (42) | 41 (12) | 36 (9) | 0.08 (0.03) | 0.07 (0.02) |
| Euthymic | 526 (20) | 498 (40) | 43 (14) | 37 (10) | 0.08 (0.03) | 0.08 (0.02) |
| Manic | 529 (28) | 509 (44) | 40 (11) | 36 (7) | 0.08 (0.02) | 0.07 (0.02) |
S = synchronization; C = continuation.
Fig. 1.
Tapping performance for healthy controls and bipolar disorder groups in the dominant index finger and alternating thumbs conditions. Panels A–C show the mean intertap interval, standard deviation of the intertap interval, and the coefficient of variation. The bipolar disorder group had a faster tapping rate and increased variability relative to controls.
Intertap interval
The bipolar disorder group tapped at a faster rate than controls, with average intertap intervals of 521 ms versus 530 ms, respectively, which resulted in a main effect of group, F(1,82) = 4.58, p < 0.05 (ηp2 = 0.05). There was a significant main effect of condition, F(1,82) = 13.95, p < 0.001 (ηp2 = 0.14), in which participants tapped faster in the alternating thumbs condition than in the dominant index finger condition. Bipolar disorder participants tapped significantly faster than controls (p < 0.01) in the alternating thumbs condition, resulting in a condition-by-diagnosis interaction, F(1,82) = 7.80, p < 0.01 (ηp2 = 0.09). Finally, a main effect of pacing stimulus indicated that participants tapped significantly faster in the continuation compared to the synchronization condition, F(1,82) = 59.22, p < 0.001 (ηp2 = 0.42). The interaction between pacing stimulus and condition was significant, F(1,82) = 4.52, p < 0.05 (ηp2 = 0.05). While participants tapped faster in the continuation relative to the synchronization conditions, this acceleration was more pronounced in the alternating thumbs condition.
Standard deviation
A main effect of diagnosis was found for the standard deviation of intertap intervals, F(1,82) = 6.68, p < 0.05 (ηp2 = 0.07), with the bipolar disorder group showing increased variability compared to controls. Neither tapping condition nor pacing stimulus interacted significantly with diagnosis. Overall, participants demonstrated increased variability in the alternating thumbs tasks, resulting in a main effect of condition, F(1,82) = 114.24, p < 0.0001 (ηp2 = 0.58). Finally, a main effect of pacing stimulus was evident, F(1,82) = 48.62, p < 0.001 (ηp2 = 0.37), with higher standard deviations on synchronization compared to continuation tapping.
Coefficient of variation
Participants with bipolar disorder had higher variability compared to controls as measured by the coefficient of variation, resulting in a main effect of group, F(1,82) = 8.37, p < 0.01 (ηp2 = 0.09). There were no interactions between diagnostic group and any other variables. However, there was a main effect of condition, F(1,82) = 112.58, p < 0.001 (ηp2 = 0.58), due to significantly higher coefficients of variation on the alternating thumbs compared to the dominant index finger tasks. There was also a main effect of pacing stimulus due to significantly higher coefficients of variation in the synchronization condition, F(1,82) = 29.04, p < 0.001 (ηp2 = 0.26). Finally, there was an interaction between condition and pacing stimulus, F(1,82) = 5.74, p < 0.05 (ηp2 = 0.07). Variability was higher in both tapping conditions in the synchronization portion of the task, but the coefficients of variation showed a larger decrease in the dominant index finger condition than the alternating thumbs condition during the continuation portion of the task.
Bipolar disorder mood state analysis
Comparison of mood states within the bipolar disorder group produced no significant differences between manic and euthymic groups on mean intertap interval, standard deviation, or coefficient of variation. An interaction between group and condition was found for both standard deviation [F(1,39) = 7.19, p < 0.05 (ηp2 = 0.16)] and coefficient of variation [F(1,39) = 6.54, p < 0.05 (ηp2 = 0.14)], with the euthymic group displaying decreased variability in the dominant index finger condition and higher variability in the alternating thumbs condition compared to the manic group. Group differences were not significant in either condition in post-hoc tests.
Wing–Kristofferson results
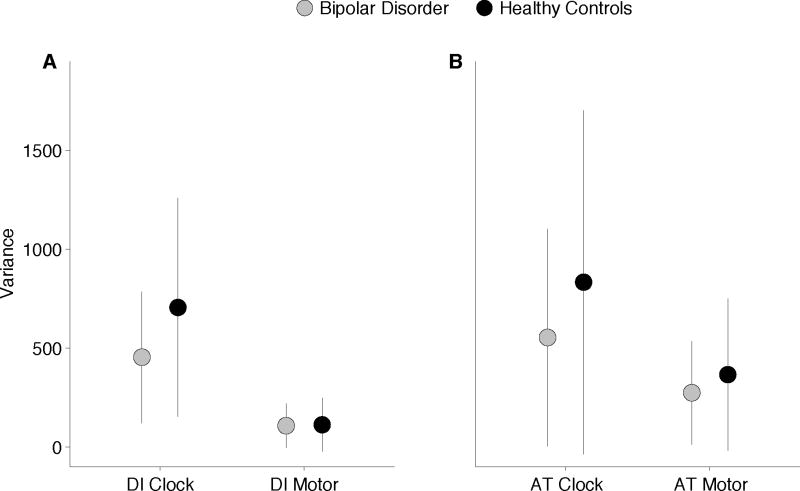
Means and standard deviations for clock and motor variability for each group in each condition can be found in Table 3. Overall means and standard deviations are depicted graphically in Figure 2.
Table 3.
Means and standard deviations (in parentheses) of clock and motor implementation variance estimated from the Wing–Kristofferson model
| Dominant index finger | Alternating thumbs | |
|---|---|---|
| Clock variance | ||
| Healthy controls | 453 (332) | 552 (548) |
| Bipolar disorder | 705 (552) | 832 (868) |
| Euthymic | 727 (644) | 973 (1012) |
| Manic | 673 (399) | 624 (736) |
| Motor variance | ||
| Healthy controls | 107 (112) | 273 (262) |
| Bipolar disorder | 111 (136) | 365 (385) |
| Euthymic | 103 (140) | 341 (397) |
| Manic | 104 (128) | 353 (364) |
Fig. 2.
Wing–Kristofferson estimates of (A) clock and (B) motor variance. Clock, but not motor variance, was significantly higher in the bipolar disorder group relative to controls.
Analysis of clock variability revealed a main effect of diagnosis, F(1,82) = 5.24, p < 0.05 (ηp2 = 0.06), with the bipolar disorder group showing significantly increased variability relative to controls. In addition, there was a main effect of condition, F(1,88) = 4.22, p < 0.05 (ηp2 = 0.05), due to significantly higher variability overall in the alternating thumbs condition. No other significant main effects or interactions were observed.
Motor implementation variance, in contrast, did not significantly differ between groups, F(1,82) = 1.14, p = ns, and no significant interactions were observed. There was a significant main effect of condition, F(1,82) = 46.92, p < 0.001 (ηp2 = 0.36), with increased motor implementation variance in the alternating thumbs task relative to the dominant index finger.
Bipolar disorder mood state analysis
There was an interaction between group and tapping condition on clock variability, F(1,39) = 4.32, p < 0.05 (ηp2 = 0.10), when only the bipolar disorder group was included and mood state (euthymic, manic) was used as the between-subjects factor. Group differences were not significant in either condition in post-hoc comparisons. There were no other differences on either clock or motor variability.
Discussion
The primary goal of this study was to investigate whether timing deficits exist in bipolar disorder as measured by a paced finger-tapping task. In addition, we examined the potential clinical relevance of timing variability by comparing euthymic and manic bipolar disorder participants. The findings indicate that bipolar disorder participants tapped at a faster rate than controls. In addition, tapping variability was significantly higher in bipolar disorder participants as measured by both the standard deviation and coefficient of variation of the intertap interval. Group differences on the coefficient of variation measure indicate that greater variability in the bipolar disorder group was not due to differences in mean tapping rate. Notably, the bipolar disorder group had significantly higher clock variability than controls, but there was no difference between groups on motor implementation variability, suggesting that impaired interval timing in bipolar disorder is due to central timekeeper abnormalities. Interestingly, the manic and euthymic groups did not significantly differ from each other on any of the tapping variables.
Across all of the participants, the alternating thumbs task produced faster intertap intervals and higher variability compared to the dominant index finger task. Intertap intervals were also faster in the absence of an external pacing stimulus in comparison to the synchronization condition. The lack of an interaction between pacing stimulus and group, however, suggests that the increased interval timing variability observed in the bipolar disorder patients persisted regardless of whether or not the participants were provided with an external pacing stimulus. An additional finding that was somewhat surprising was the increased variability in the synchronization tapping task relative to continuation tapping. It is generally expected that variability will increase in the absence of an external pacing stimulus due to the greater demand on the internal timekeeping system (27), but in this case it appears that it may have interfered with sensorimotor timing.
Results from analysis of the finger-tapping data using the Wing–Kristofferson method revealed that clock variability was higher in the bipolar disorder group compared to controls. The mean clock variability for euthymic and manic bipolar disorder participants was nearly identical, suggesting that increased timekeeper variance may be a characteristic of bipolar disorder independent of mood state. In general, these results were consistent with those of Ivry et al. (28), who observed increased central timekeeper variance but no deficits in motor implementation variance in patients with lesions in the lateral hemispheres of the cerebellum, as well as with recent reports in adult ADHD (3).
Dopamine serves a neuromodulatory role in temporal processing, in particular the nigrostriatal pathway (29, 30). Both animal and human studies indicate that dopamine agonists accelerate clock speed, resulting in an overestimation (and underproduction) of temporal intervals, whereas antagonism of dopamine receptors slows clock speed and is associated with underestimation (and overproduction) of temporal intervals (31–35). In addition, examination of interval timing in the milliseconds range in Parkinson’s disease suggests that decreased brain dopamine levels may be associated with increased timing variability (36).
Dopaminergic dysregulation has been implicated in bipolar disorder, with mania generally associated with increased dopamine transmission and depression with a decrease in dopaminergic activity. Specifically, the mesolimbic and mesocortical dopamine systems are predominantly associated with bipolar disorder and with the emergence of mood episodes, although there is also evidence to suggest alterations in the nigrostriatal dopamine system (37). Taken together, all else being equal, it could be expected that mania would increase variability in finger tapping compared to euthymia. The fact that no differences were observed between manic and euthymic groups may indicate a central timekeeper deficit that represents a core feature of the disorder, existing independently of clinical status.
Medication use represents a significant difficulty in determining underlying mechanisms associated with bipolar disorder. The foregoing discussion begs the question of how psychotropic medications may have affected the current results. A number of commonly used treatments for bipolar disorder interact with dopamine systems, notably antipsychotic drugs which antagonize dopamine receptors. Such antagonism would be expected to decrease clock speed and therefore tapping rate; therefore, it seems unlikely that these particular drugs can account for the observed differences in tapping rate given that bipolar disorder participants tapped faster than controls in the current study. However, the finding that L-DOPA normalized timing variability in Parkinson’s disease patients in a finger-tapping task with intertap intervals in the milliseconds range (37) suggests that, conversely, dopamine antagonism may increase timing variability. Given that the bipolar disorder group showed a large and consistent increase in timing variability, possible medication effects cannot be completely ruled out. However, it is worth noting that although haloperidol, which primarily antagonizes the dopamine D2 receptor, has been reported to disrupt discrimination of durations in the milliseconds range, atypical antipsychotic drugs, which exhibit a broader pharmacological profile, do not appear to affect subsecond time perception (35). The majority of bipolar disorder participants in this study who were taking antipsychotic drugs were on atypical medications.
A significant obstacle to dissociating the effects of illness vis-à-vis medication effects is exemplified in this study, specifically that unmedicated participants are often symptomatic. Here, 6 of 8 unmedicated bipolar disorder participants were in a manic state, thus confounding the interpretation of medication effects with mood state. Moreover, individuals with the most severe course of illness often have the most extensive medication use and medication histories. In our sample, more than half were on at least two psychotropic medications. Nevertheless, comparison of participants based on categories of medication use provides some evidence that the observed effects are not due the effects of psychotropic drugs. Both typical and atypical antipsychotic drugs (38–40) and lithium (41) have been reported to affect time reproduction in suprasecond intervals. However, consistent with earlier reports that atypical antipsychotic drugs do not affect timing in the millisecond range (35), the present study did not uncover any differences between patients prescribed atypical antipsychotics compared to those who were not. Moreover, the finding that unmedicated participants performed similarly to those on psychotropic medications is in accordance with the finding that there were no statistically significant differences in post-hoc tests comparing manic and euthymic participants. Given that all but two unmedicated bipolar disorder participants were manic, this suggests that neither mood state nor medication use (at least current use) substantively affected performance and points to a more fundamental impairment in internal timekeeping.
A review of the neuroimaging literature on timing by Lewis and Miall (12) reported that across interval durations spanning subsecond to suprasecond durations, regardless of whether the task was a motor or perceptual task, the supplementary motor area and cerebellum were the two brain areas that showed consistent activation. More recent reviews of neuroimaging literature have concluded that the cerebellum is especially critical for the perception of temporal intervals below one sec (9, 42). Particularly strong evidence for this assertion comes from repetitive transcranial magnetic stimulation (rTMS) studies in which stimulation of the cerebellum selectively interferes with performance at intervals spanning hundreds of milliseconds (around 500 ms), which has recently been demonstrated in time reproduction tasks including paced finger tapping (43, 44) and time estimation (45), as well as in a time perception task (46). Moreover, neuroimaging studies have reported activation in cerebellum during both synchronization and continuation tapping at subsecond intervals (26, 47, 48). Taken together with our recent finding that cerebellar-dependent eyeblink conditioning is impaired in bipolar disorder (7), it is possible that the observed increase in clock variability is due to disruptions in cerebellar function. Indeed, structural abnormalities of the cerebellum have been reported in imaging studies of patients with mood disorders, including bipolar disorder (49–57) and unipolar depression (58, 59).
In light of the present findings of abnormalities in the paced finger-tapping task in bipolar disorder, strong evidence of cerebellar involvement in paced finger tapping suggests that the cerebellum may be impaired in the disorder. These behavioral findings are consistent with previous reports of neurochemical and cellular anomalies in the cerebella of bipolar disorder patients. For example, reductions of approximately 50% in glutamic acid decarboxylase 67 (GAD 67) and reelin have been reported in the cerebella of bipolar disorder patients (60–62). Specifically, these studies reported that GAD 67 was reduced in Purkinje cells, suggesting impairment of these principal neurons of the cerebellum, and this deficiency was accompanied by significant reductions in reelin messenger RNA in cerebellar granule cells. Reelin is an important secretory glycoprotein that guides neurons and glia during embryonic brain development, ensuring normal brain lamination, and, in the adult brain, may mediate neuroplasticity. Another study found that Purkinje cell density was decreased by 20% in bipolar disorder, and this deficiency was unrelated to antipsychotic drug exposure or substance abuse (63). Such anomalies could have profound effects on cerebellar output, which occurs solely through Purkinje cell modulation of deep nuclei.
Overall, impaired performance on the repetitive tapping task employed in this study suggests that internal timing mechanisms are disturbed in bipolar disorder. While more recent evidence, outlined above, supports the notion that finger-tapping tasks at the 500-ms intertap interval employed in this study rely on cerebellar timing mechanisms, it is important to note that brain areas besides the cerebellum are involved in paced finger-tapping tasks. For example, the striatum has been consistently identified as part of the neural circuitry supporting internal timing. While it seems clear that both are involved, and indeed the cerebellum projects to the striatum (64), there may be differential contributions of the cerebellum and basal ganglia, with the former more consistently implicated in subsecond temporal intervals and the latter contributing to estimation of suprasecond intervals, which may require cognitive operations such as working memory and attention (9).
To our knowledge, this is the first study to assess timekeeping performance on this task in mood disorders, although paced finger tapping has been used to assess the integrity of internal timing mechanisms in a variety of neurological and neuropsychiatric disorders, including cerebellar lesions (28), Parkinson’s disease (64), ADHD (3), and schizophrenia (1, 2). The present findings are important because it may be that abnormalities in internal timing systems manifest themselves in behavioral abnormalities associated with psychiatric symptoms. For example, Andreasen (65) postulated that dysfunctional cerebellar modulation of neural networks implicated in higher cognition, especially the cortico-cerebello-thalamic-cortical circuit, may result in disturbed temporal coordination of thoughts and lead to ‘cognitive dysmetria’, analogous to the motor dysmetria that results from cerebellar lesions. Likewise, symptoms strikingly similar to bipolar disorder, including mania, depression, rapid cycling, impulsivity, and contextually inappropriate behavior, have been observed following cerebellar lesions (66). Even excluding a cerebellar explanation or one directly linked to timing per se, deficits in internal timing point to disruptions in distinct brain regions and circuits implicated in timing functions and may point to novel therapeutic targets that deserve further investigation.
Future studies should investigate additional timing tasks in bipolar disorder. For example, time reproduction and estimation tasks at intervals ranging from hundreds of milliseconds to seconds would be informative regarding the nature of timing deficits in bipolar disorder and the effects of mood state. Combining these behavioral tasks with neuroimaging could provide important information regarding which brain regions are specifically impaired in the disorder. Finally, it will also be important to extend the present findings by including a larger sample of bipolar disorder participants in a manic episode, as well as in depressed and mixed mood states, to provide further information regarding the contribution of clinical status to timing abnormalities in bipolar disorder.
Acknowledgments
This research was supported by a 2007 NARSAD Young Investigator Grant awarded to ARB and National Institute of Mental Health grant R01 MH074983 to WPH. We are grateful to Colleen Merrill, Ashley Steffen, and Sam Kaiser for their assistance in collecting finger-tapping data. We also thank the clinical research team at Larue D. Carter Memorial Hospital and the Indiana University Neuroscience Clinical Research Center for their support. Finally, many thanks to Nicholas Port for his assistance and advice on the figures included in this work.
Footnotes
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
A boxplot method of outlier identification (SPSS statistical package) was used to classify extreme data values separately for each analysis. Extreme outliers were defined as data values > 6 quartiles from the upper or lower ends of the interquartile range. Following age matching, there were 45 participants in each group, but 2 bipolar disorder patients and 1 control were removed from the analysis due to classification as extreme outliers in the dominant index finger or alternating thumbs conditions on either the mean intertap interval or the standard deviation measurement. In each case, the age-matched participant for each outlier was also removed. All demographic and statistical information is reported for the remaining 42 participants in each group.
References
- 1.Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn. 2009;70:181–190. doi: 10.1016/j.bandc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71:345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valera EM, Spencer RM, Zeffiro TA, et al. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 5.Bschor T, Ising M, Bauer M, et al. Time experience and time judgment in major depression, mania and healthy subjects. A controlled study of 93 subjects. Acta Psychiatr Scand. 2004;109:222–229. doi: 10.1046/j.0001-690x.2003.00244.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahlberg R, Kienast T, Bschor T, Adli M. Evaluation of time memory in acutely depressed patients, manic patients, and healthy controls using a time reproduction task. Eur Psychiatry. 2008;23:430–433. doi: 10.1016/j.eurpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Bolbecker AR, Mehta C, Johannesen JK, et al. Eyeblink conditioning anomalies in bipolar disorder suggest cerebellar dysfunction. Bipolar Disord. 2009;11:19–32. doi: 10.1111/j.1399-5618.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- 8.Lewis PA, Miall RC. The precision of temporal judgement: milliseconds, many minutes, and beyond. Philos Trans R Soc Lond B Biol Sci. 2009;364:1897–1905. doi: 10.1098/rstb.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philos Trans R Soc Lond B Biol Sci. 2009;364:1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittmann M. The inner experience of time. Philos Trans R Soc Lond B Biol Sci. 2009;364:1955–1967. doi: 10.1098/rstb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiener M, Turkeltaub P, Coslett HB. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- 12.Lewis PA, Miall RC. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003;41:1583–1592. doi: 10.1016/s0028-3932(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 13.Ivry RB, Keele SW. Timing of functions of the cerebellum. J Cogn Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 14.Mates J, Radil T, Muller R, Poppel E. Temporal integration in sensorimotor synchronization. J Cogn Neurosci. 1994;6:332–340. doi: 10.1162/jocn.1994.6.4.332. [DOI] [PubMed] [Google Scholar]
- 15.Wing AM, Kristofferson AB. Response delays and the timing of discrete motor responses. Percept Psychophys. 1973;14:5–12. [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) Washington, DC: Psychiatry Press; 1994. [Google Scholar]
- 17.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 197(9):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Eta-squared and partial eta-squared in communication science. Human Comm Res. 1973;28:473–490. [Google Scholar]
- 21.Wing AM, Kristofferson AB. The timing of inter-response intervals. Percept Psychophys. 1973;13:455–460. [Google Scholar]
- 22.Stevens LT. On the time sense. Mind. 1886;11:393–404. [Google Scholar]
- 23.Lundy-Ekman L, Ivry R, Keele S, Woollacott M. Timing and force control deficits in clumsy children. J Cogn Neurosci. 1991;3:367–376. doi: 10.1162/jocn.1991.3.4.367. [DOI] [PubMed] [Google Scholar]
- 24.Williams HG, Woollacott MH, Ivry R. Timing and motor control in clumsy children. J Mot Behav. 1992;24:165–172. doi: 10.1080/00222895.1992.9941612. [DOI] [PubMed] [Google Scholar]
- 25.Geuze RH, Kalverboer AF. Tapping a rhythm: A problem of timing for children who are clumsy and dyslexic? Adapt Phys Activ Q. 1994;11:202–213. [Google Scholar]
- 26.Kooistra L, Snijders TA, Schellekens JM, Kalverboer AF, Geuze RH. Timing variability in children with early-treated congenital hypothyroidism. Acta Psychol. 1997;96:61–73. doi: 10.1016/s0001-6918(96)00047-9. [DOI] [PubMed] [Google Scholar]
- 27.Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- 29.Meck WH. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2006;1109:93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 30.Meck WH. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 2006;1108:157–167. doi: 10.1016/j.brainres.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Frederick DL, Allen JD. Effects of selective dopamine D1- and D2-agonists and antagonists on timing performance in rats. Pharmacol Biochem Behav. 1996;53:759–764. doi: 10.1016/0091-3057(95)02103-5. [DOI] [PubMed] [Google Scholar]
- 32.Maricq AV, Roberts S, Church RM. Methamphetamine and time estimation. J Exp Psychol Anim Behav Process. 1981;7:18–30. doi: 10.1037//0097-7403.7.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- 34.Cevik MO. Effects of methamphetamine on duration discrimination. Behav Neurosci. 2003;117:774–784. doi: 10.1037/0735-7044.117.4.774. [DOI] [PubMed] [Google Scholar]
- 35.Rammsayer TH. Are there dissociable roles of the mesostriatal and mesolimbocortical dopamine systems on temporal information processing in humans? Neuropsychobiology. 1997;35:36–45. doi: 10.1159/000119328. [DOI] [PubMed] [Google Scholar]
- 36.Merchant H, Luciana M, Hooper C, Majestic S, Tuite P. Interval timing and Parkinson’s disease: heterogeneity in temporal performance. Exp Brain Res. 2008;184:233–248. doi: 10.1007/s00221-007-1097-7. [DOI] [PubMed] [Google Scholar]
- 37.Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar Disord. 2009;11:787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald CJ, Meck WH. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacol. 2005;182:232–244. doi: 10.1007/s00213-005-0074-8. [DOI] [PubMed] [Google Scholar]
- 39.Rammsayer TH. On dopaminergic modulation of temporal information processing. Biol Psychol. 1993;36:209–222. doi: 10.1016/0301-0511(93)90018-4. [DOI] [PubMed] [Google Scholar]
- 40.Rammsayer TH. Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psychol B. 1999;52:273–286. doi: 10.1080/713932708. [DOI] [PubMed] [Google Scholar]
- 41.Elsass P, Mellerup ET, Rafaelsen OJ, Theilgaard A. Lithium effects on time estimation and mood in manic-melancholic patients. A study of diurnal variations. Acta Psychiatr Scand. 1979;60:263–271. doi: 10.1111/j.1600-0447.1979.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 42.Penney TB, Vaitilingam L. Imaging time. In: Grondin S, editor. Psychology of Time. Bingley: Emerald; 2008. pp. 261–294. [Google Scholar]
- 43.Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol. 2007;98:145–152. doi: 10.1152/jn.01088.2006. [DOI] [PubMed] [Google Scholar]
- 44.Theoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306:29–32. doi: 10.1016/s0304-3940(01)01860-2. [DOI] [PubMed] [Google Scholar]
- 45.Koch G, Oliveri M, Torriero S, Salerno S, Lo Gerfo E, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp Brain Res. 2007;179:291–299. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- 46.Lee KH, Egleston PN, Brown WH, Gregory AN, Barker AT, Woodruff PW. The role of the cerebellum in subsecond time perception: evidence from repetitive transcranial magnetic stimulation. J Cogn Neurosci. 2007;19:147–157. doi: 10.1162/jocn.2007.19.1.147. [DOI] [PubMed] [Google Scholar]
- 47.Riecker A, Wildgruber D, Mathiak K, Grodd W, Ackermann H. Parametric analysis of rate-dependent hemodynamic response functions of cortical and subcortical brain structures during auditorily cued finger tapping: a fMRI study. Neuroimage. 2003;18:731–739. doi: 10.1016/s1053-8119(03)00003-x. [DOI] [PubMed] [Google Scholar]
- 48.Muller JL, Roder C, Schuierer G, Klein HE. Subcortical overactivation in untreated schizophrenic patients: a functional magnetic resonance image finger-tapping study. Psychiatry Clin Neurosci. 2002;56:77–84. doi: 10.1046/j.1440-1819.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 49.Monkul ES, Hatch JP, Sassi RB, et al. MRI study of the cerebellum in young bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:613–619. doi: 10.1016/j.pnpbp.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moorhead TW, McKirdy J, Sussmann JE, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Mills NP, DelBello MP, Adler CM, Strakowski SM. MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am J Psychiatry. 2005;162:1530–1532. doi: 10.1176/appi.ajp.162.8.1530. [DOI] [PubMed] [Google Scholar]
- 52.DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacol. 1999;21:63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 53.Lippmann S, Manshadi M, Baldwin H, Drasin G, Rice J, Alrajeh S. Cerebellar vermis dimensions on computerized tomographic scans of schizophrenic and bipolar patients. Am J Psychiatry. 1982;139:667–668. doi: 10.1176/ajp.139.5.667. [DOI] [PubMed] [Google Scholar]
- 54.Nasrallah HA, Jacoby CG, McCalley-Whitters M. Cerebellar atrophy in schizophrenia and mania. Lancet. 1981;1:1102. doi: 10.1016/s0140-6736(81)92266-2. [DOI] [PubMed] [Google Scholar]
- 55.Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. J Clin Psychiatry. 1982;43:439–441. [PubMed] [Google Scholar]
- 56.Yadalam KG, Jain AK, Simpson GM. Mania in two sisters with similar cerebellar disturbance. Am J Psychiatry. 1985;142:1067–1069. doi: 10.1176/ajp.142.9.1067. [DOI] [PubMed] [Google Scholar]
- 57.Cutting JC. Chronic mania in childhood: case report of a possible association with a radiological picture of cerebellar disease. Psychol Med. 1976;6:635–642. doi: 10.1017/s0033291700018286. [DOI] [PubMed] [Google Scholar]
- 58.Shah SA, Doraiswamy PM, Husain MM, et al. Posterior fossa abnormalities in major depression: a controlled magnetic resonance imaging study. Acta Psychiatr Scand. 1992;85:474–479. doi: 10.1111/j.1600-0447.1992.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 59.Escalona PR, Early B, McDonald WM, et al. Reduction of cerebellar volume in major depression: a controlled MRI study. Depression. 1993;1:156–158. [Google Scholar]
- 60.Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 61.Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 62.Maloku E, Covelo IR, Hanbauer I, et al. Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci U S A. 2010;107:4407–4411. doi: 10.1073/pnas.0914483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 64.O’Boyle DJ, Freeman JS, Cody FW. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain. 1996;119:51–70. doi: 10.1093/brain/119.1.51. [DOI] [PubMed] [Google Scholar]
- 65.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 66.Lauterbach EC. Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biol Psychiatry. 1996;40:726–730. doi: 10.1016/0006-3223(96)82516-9. [DOI] [PubMed] [Google Scholar]