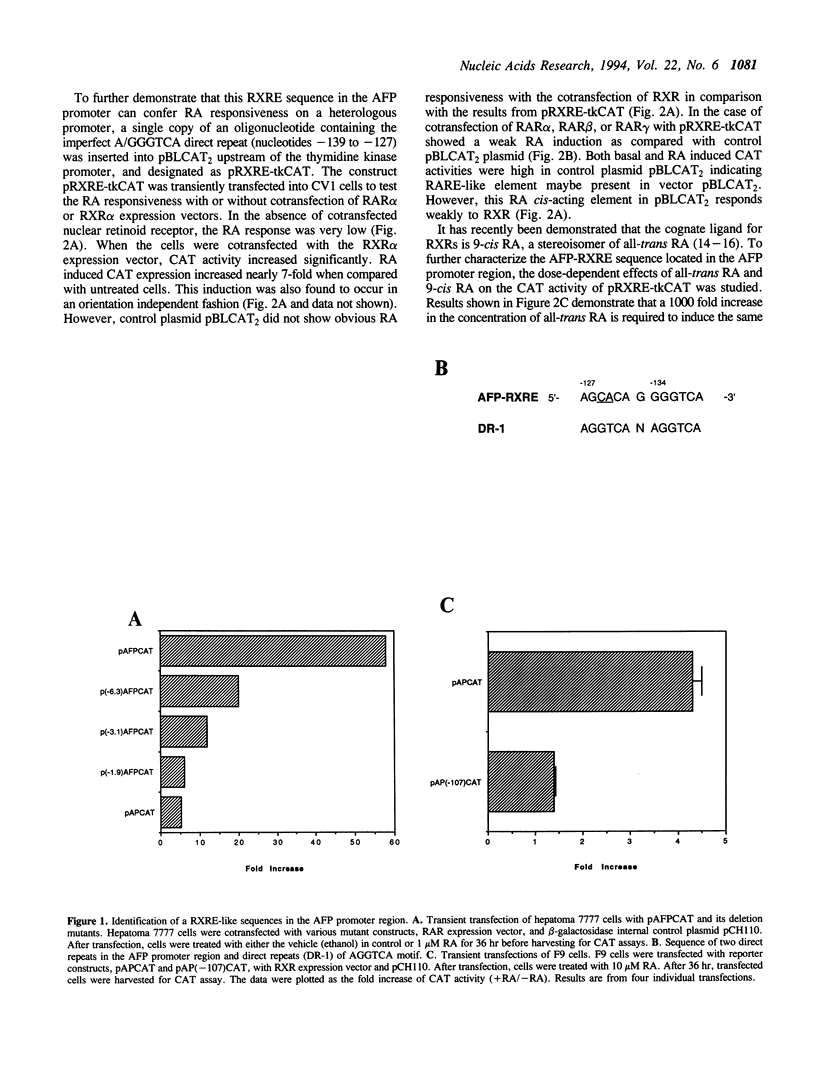
Abstract
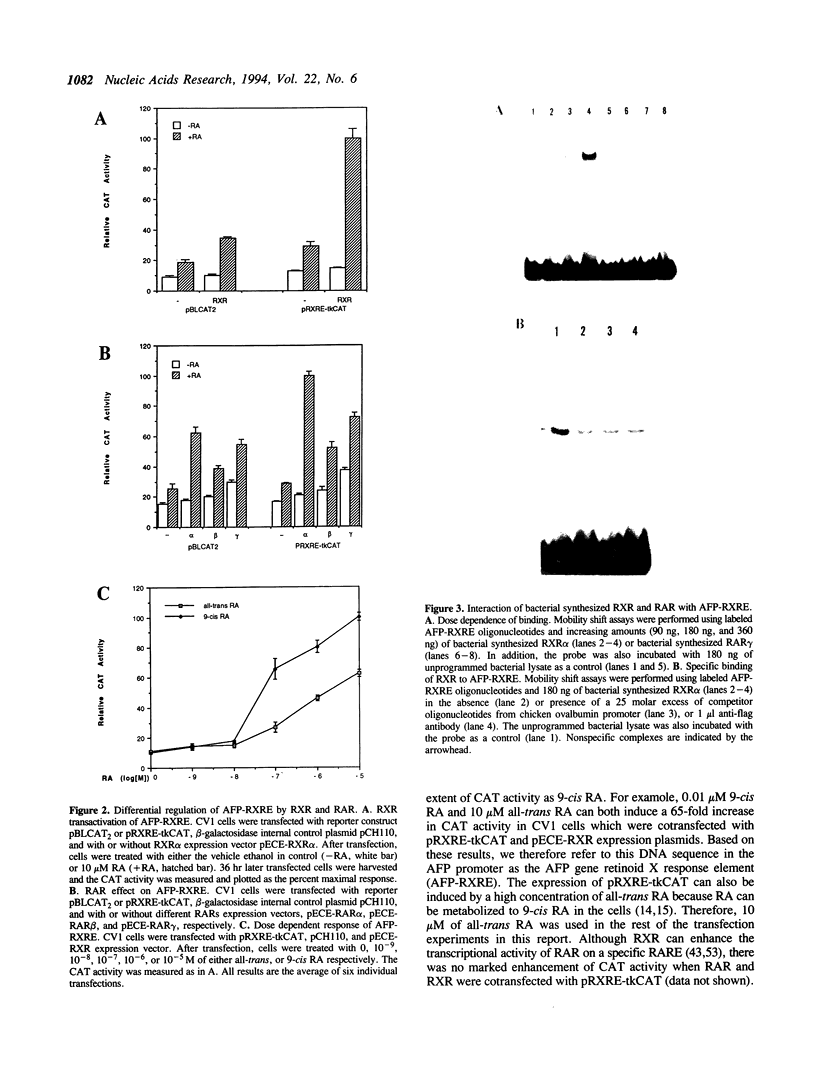
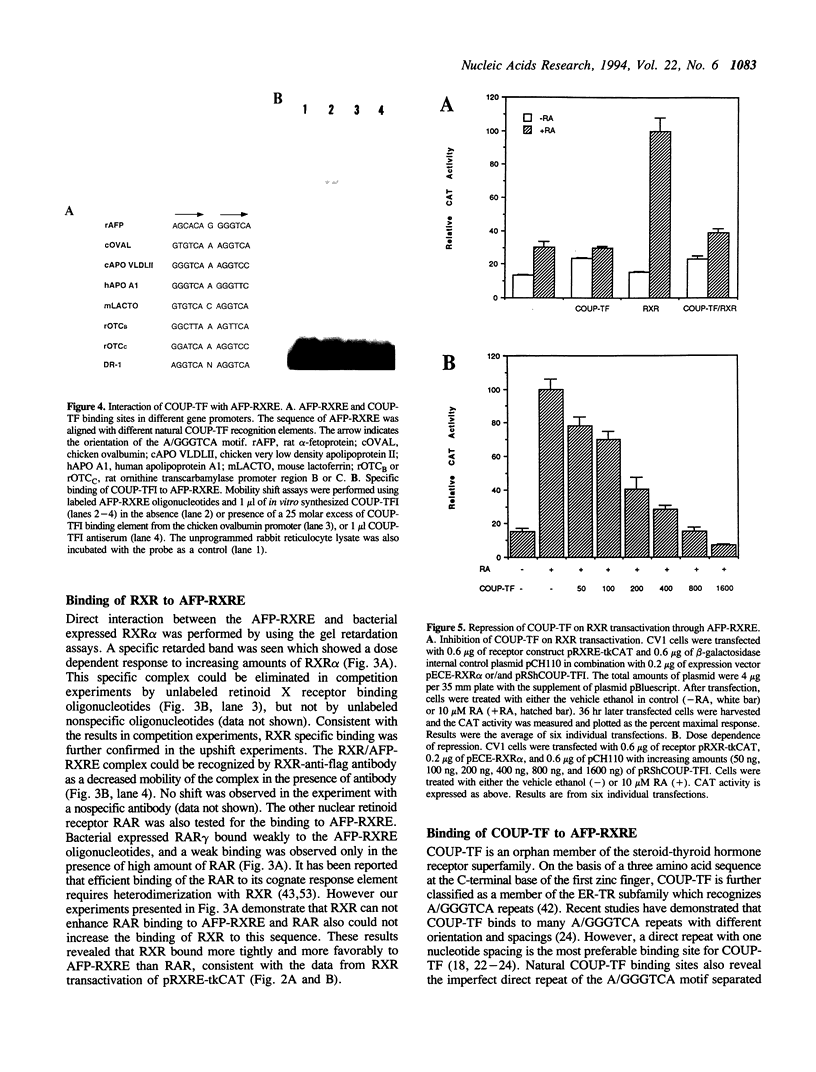
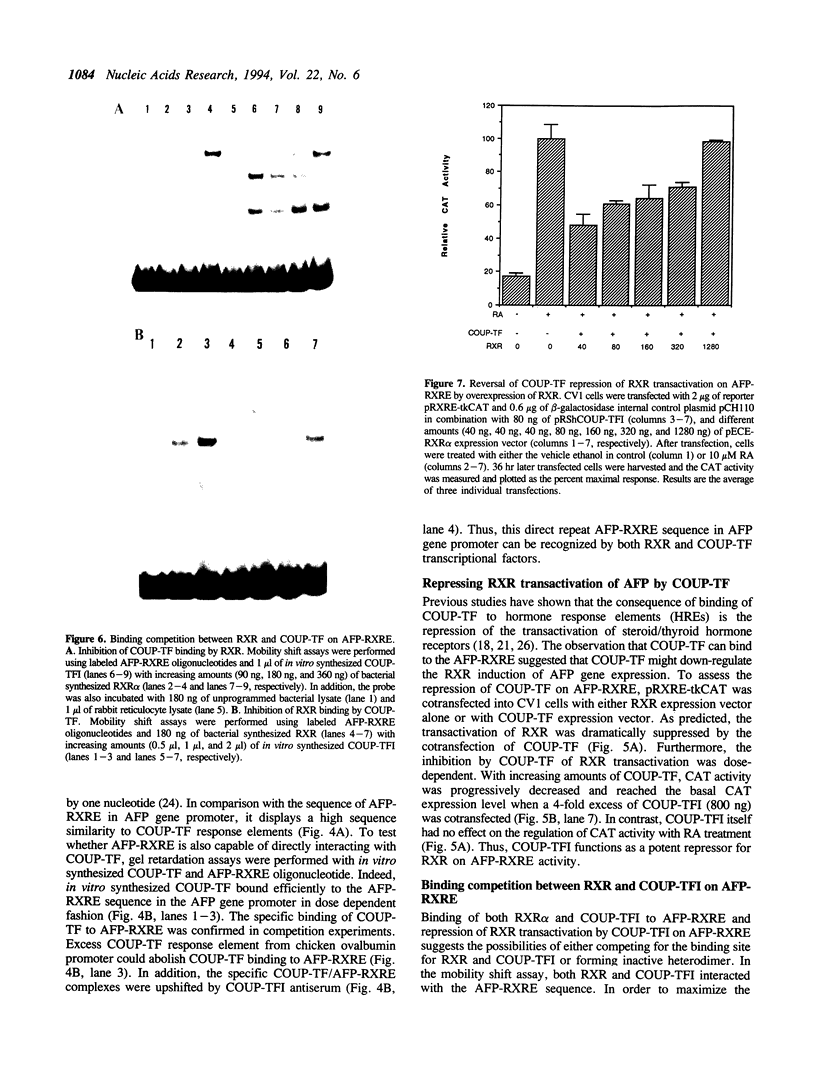
Retinoic acid (RA) is widely involved in the control of cell proliferation and differentiation, as well as embryo pattern formation. Transcription of the oncodevelopmental protein, alpha-fetoprotein (AFP), is stimulated by retinoic acid (RA) in neoplastic cells. To study RA regulation of AFP gene expression, the 5'-flanking region of AFP gene was cloned and analyzed. In the present study, transfection of deletion mutants and sequence analysis revealed a retinoid X receptor response element (AFP-RXRE) located at position -139 to -127 of the AFP promoter. Synthetic AFP-RXRE was ligated into a reporter construct with the heterologous promoter and chloramphenicol acetyltransferase (CAT). AFP-RXRE conferred a marked RA responsiveness in the cotransfection with retinoid X receptor (RXR), but not with retinoic acid receptors (RARs). Consistent with these data, only RXR bound to AFP-RXRE with high affinity in the mobility shift assays. Chicken ovalbumin upstream promoter transcription factor (COUP-TF), an orphan member of the steroid/thyroid hormone superfamily, also demonstrated specific binding activity to AFP-RXRE in vitro. In cotransfection assays, COUP-TF dramatically repressed the transactivation of RXR on AFP-RXRE. The mechanism of repression by COUP-TF may involve the mutual occupancy of the AFP-RXRE binding site between RXR and COUP-TF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelev G. I. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beekman J. M., Wijnholds J., Schippers I. J., Pot W., Gruber M., Ab G. Regulatory elements and DNA-binding proteins mediating transcription from the chicken very-low-density apolipoprotein II gene. Nucleic Acids Res. 1991 Oct 11;19(19):5371–5377. doi: 10.1093/nar/19.19.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrodin T. J., Marks M. S., Ozato K., Linney E., Lazar M. A. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein. Mol Endocrinol. 1992 Sep;6(9):1468–1478. doi: 10.1210/mend.6.9.1331778. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Chen C. A., Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988 Jul-Aug;6(7):632–638. [PubMed] [Google Scholar]
- Chou J. Y., Wan Y. J. Regulation of expression of the rat alpha-fetoprotein gene. Oxf Surv Eukaryot Genes. 1989;6:1–31. [PubMed] [Google Scholar]
- Cooney A. J., Leng X., Tsai S. Y., O'Malley B. W., Tsai M. J. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem. 1993 Feb 25;268(6):4152–4160. [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992 Sep;12(9):4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor binds to a negative regulatory region in the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1991 Jun;65(6):2853–2860. doi: 10.1128/jvi.65.6.2853-2860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca L. M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991 Nov;5(14):2924–2933. [PubMed] [Google Scholar]
- Deutsch H. F. Chemistry and biology of alpha-fetoprotein. Adv Cancer Res. 1991;56:253–312. doi: 10.1016/s0065-230x(08)60483-2. [DOI] [PubMed] [Google Scholar]
- Dong J. M., Li F., Chiu J. F. Induction of F9 cell differentiation by transient exposure to retinoic acid. Biochem Biophys Res Commun. 1990 Jul 16;170(1):147–152. doi: 10.1016/0006-291x(90)91252-n. [DOI] [PubMed] [Google Scholar]
- Dong J. M., Nordloh P. W., Chiu J. F. The mechanism of the bidirectional regulation of the rat alpha-fetoprotein gene by glucocorticoid hormone. Mol Cell Endocrinol. 1989 Sep;66(1):109–114. doi: 10.1016/0303-7207(89)90054-3. [DOI] [PubMed] [Google Scholar]
- Drouin J., Sun Y. L., Nemer M. Glucocorticoid repression of pro-opiomelanocortin gene transcription. J Steroid Biochem. 1989;34(1-6):63–69. doi: 10.1016/0022-4731(89)90066-6. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Gitlin D., Boesman M. Sites of serum alpha-fetoprotein synthesis in the human and in the rat. J Clin Invest. 1967 Jun;46(6):1010–1016. doi: 10.1172/JCI105590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., DiRenzo J., Kurokawa R., Han Z. H. Regulation of gene expression by retinoic acid receptors. DNA Cell Biol. 1991 Nov;10(9):623–638. doi: 10.1089/dna.1991.10.623. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Hwung Y. P., Crowe D. T., Wang L. H., Tsai S. Y., Tsai M. J. The COUP transcription factor binds to an upstream promoter element of the rat insulin II gene. Mol Cell Biol. 1988 May;8(5):2070–2077. doi: 10.1128/mcb.8.5.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki Y., Toyoshima K., Yamamoto T. Ear3/COUP-TF binds most tightly to a response element with tandem repeat separated by one nucleotide. Biochem Biophys Res Commun. 1992 Mar 16;183(2):492–498. doi: 10.1016/0006-291x(92)90509-j. [DOI] [PubMed] [Google Scholar]
- Kimura A., Nishiyori A., Murakami T., Tsukamoto T., Hata S., Osumi T., Okamura R., Mori M., Takiguchi M. Chicken ovalbumin upstream promoter-transcription factor (COUP-TF) represses transcription from the promoter of the gene for ornithine transcarbamylase in a manner antagonistic to hepatocyte nuclear factor-4 (HNF-4). J Biol Chem. 1993 May 25;268(15):11125–11133. [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992 Oct;17(10):427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang N., Teng C. T. COUP-TF acts as a competitive repressor for estrogen receptor-mediated activation of the mouse lactoferrin gene. Mol Cell Biol. 1993 Mar;13(3):1836–1846. doi: 10.1128/mcb.13.3.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982 Feb 25;295(5851):672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Mietus-Snyder M., Sladek F. M., Ginsburg G. S., Kuo C. F., Ladias J. A., Darnell J. E., Jr, Karathanasis S. K. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol Cell Biol. 1992 Apr;12(4):1708–1718. doi: 10.1128/mcb.12.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima N., Kadowaki Y., Fukushige S., Shimizu S., Semba K., Yamanashi Y., Matsubara K., Toyoshima K., Yamamoto T. Identification of two novel members of erbA superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res. 1988 Dec 9;16(23):11057–11074. doi: 10.1093/nar/16.23.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen D. A., Chang T. C., Shapiro D. J. A highly sensitive, mixed-phase assay for chloramphenicol acetyltransferase activity in transfected cells. Anal Biochem. 1989 May 15;179(1):19–23. doi: 10.1016/0003-2697(89)90193-0. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M. alpha-Fetoprotein in cancer and fetal development. Adv Cancer Res. 1979;29:275–346. doi: 10.1016/s0065-230x(08)60849-0. [DOI] [PubMed] [Google Scholar]
- Sagami I., Tsai S. Y., Wang H., Tsai M. J., O'Malley B. W. Identification of two factors required for transcription of the ovalbumin gene. Mol Cell Biol. 1986 Dec;6(12):4259–4267. doi: 10.1128/mcb.6.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T. F., Compton R. S., Scott R. W., Tilghman S. M. Differential requirements for cellular enhancers in stem and differentiated cells. Nucleic Acids Res. 1988 Jan 25;16(2):487–500. doi: 10.1093/nar/16.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. J., Wu T. C. The effects of retinoic acid on the expression of alpha-fetoprotein and albumin genes in rat hepatoma cell lines. Differentiation. 1992 Jun;50(2):107–111. doi: 10.1111/j.1432-0436.1992.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Widom R. L., Ladias J. A., Kouidou S., Karathanasis S. K. Synergistic interactions between transcription factors control expression of the apolipoprotein AI gene in liver cells. Mol Cell Biol. 1991 Feb;11(2):677–687. doi: 10.1128/mcb.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. K., Dong J. M., Chiu J. F. Regulation of alpha-fetoprotein gene expression by antagonism between AP-1 and the glucocorticoid receptor at their overlapping binding site. J Biol Chem. 1991 May 5;266(13):8248–8254. [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]