Abstract
Changes in the elasticity of the vaginal walls, connective support tissues, and muscles are thought to be significant factors in the development of pelvic organ prolapse, a highly prevalent condition affecting at least 50% of women in the United States during their lifetimes. It creates two predominant concerns specific to the biomechanical properties of pelvic support tissues: how does tissue elasticity affect the development of pelvic organ prolapse and how can functional elasticity be maintained through reconstructive surgery. We designed a prototype of vaginal tactile imager (VTI) for visualization and assessment of elastic properties of pelvic floor tissues. In this paper, we analyze applicability of tactile imaging for evaluation of reconstructive surgery results and characterization of normal and pelvic organ prolapse conditions. A pilot clinical study with 13 patients demonstrated that VTI allows imaging of vaginal walls with increased rigidity due to implanted mesh grafts following reconstructive pelvic surgery and VTI has the potential for prolapse characterization and detection.
Index Terms: Elastography, tactile imaging, tissue elasticity, vaginal prolapse
I. Introduction
Pelvic organ prolapse (POP) is a highly prevalent condition affecting at least 50% of women in the US during their lifetimes [1]. A recent study including 27 342 women revealed that 40% of women aged 50–79 years have some form of pelvic organ prolapse [2]. Some loss of utero-vaginal support occurs in most adult women [3], however, the true etiology of prolapse and differences seen among individuals is not entirely understood. Changes in the elasticity of the vaginal walls, connective support tissues, and muscles are thought to be significant factors in the development of pelvic organ prolapse [4]–[6]. Consideration of these data creates two predominant concerns specific to the biomechanical properties of pelvic support tissues: how does tissue elasticity affect the development of pelvic organ prolapse and how can a functional elasticity be maintained through reconstructive surgery while providing enough strength for a durable repair.
Pop is the descent of the apex of the vagina or cervix (or vaginal vault after hysterectomy), anterior vaginal wall, and/or posterior vaginal wall [7], [8]. As prolapse progresses, pelvic organs can become displaced and even protrude outside the vaginal canal. It has been shown that the frequency of pelvic floor disorders (urinary and fecal incontinence, and pelvic organ prolapse) increases with age, affecting more than 40% of women from 60 to 79 years of age, and about 50% of women 80 and older in the United States [9]. The lifetime risk of undergoing surgery for prolapse or urinary incontinence is approximately 11% by age 80 years [10]. POP is the leading indication for hysterectomy in postmenopausal women and accounts for 15%–18% of procedures in all age groups [11]. Beyond the physical impact of POP, women with progressing pelvic organ prolapse score poorer on both generic and condition specific quality-of-life scales [12]. In addition, about one-third of sexually active women with pelvic organ prolapse report that their condition interferes with sexual function [13].
The high incidence of POP dictates the need for effective means of prolapse detection, characterization, and assessment results of reconstructive surgery.
A. Current Pelvic Organ Prolapse Evaluation Methods
Diagnosis of vaginal prolapse involves taking a medical history and performing a physical examination. In more severe cases, imaging tests such as ultrasound, MRI, and X-ray imaging is used to further assess the condition. Diagnostic tests may include bladder and rectum function tests.
1) Physical Examination
During a physical examination, the physician inspects the urogenital areas and rectum for masses and indication of reduced muscle tone. Many physicians simply describe POP as a small, medium, or large prolapse. Other physicians use a ruler to measure the vaginal protrusion. Some physicians rate POP using POP Quantification (POP-Q) system [14]. Even though, the POP-Q system is precise at describing POP, it has limited ability to quantify the prolapse itself, since it still classifies prolapse into four stages [15]. Though the physical examination helps the clinician to describe the extent of pelvic organ prolapse, it does not help in the evaluation of the risk of prolapse development from the normal condition. Digital palpation does not provide quantitative tissue characterization to compare with normal elasticity of vaginal walls. It has poor sensitivity and is highly subjective, making manual palpation an impractical tool for assessing underlying tissue properties.
2) Ultrasound
Various ultrasonic techniques are available for the assessment of pelvic floor organs in females. Perineal and introital ultrasounds have both become routine diagnostic techniques for assessing stress urinary incontinence and genitourinary prolapse [16]. Preoperative ultrasound yields information on the pathomorphology of the continence control system [17]. Many female patients seen in outpatient clinics possess not only stress urinary incontinence and genitourinary prolapse, but also voiding problems and recurrent urinary tract infections. Abdominal ultrasound is frequently used in these conditions, while transvaginal ultrasound is rarely applied. Intraurethral, Doppler, and 3-D ultrasound [18] have so far been used only within the framework of scientific investigations, due to the complex equipment required to perform these examinations and due to as yet unanswered questions regarding the interpretation of the findings [19]. For similar reasons, transrectal ultrasound has not gained ground as an imaging modality for assessing the female urogenital organs.
3) MRI
Great progress in magnetic resonance techniques has magnified the role and potential of MRI in female pelvic organ disorder characterization. The standard technique consists of T1-weighted (T1W) and T2-weighted (T2W) imaging with spin-echo sequences. Recent fast MRI has enabled the acquisition of serial images at an interval of a few seconds. Cine MRI is used for evaluating uterine contractility [20], including sustained contraction and peristalsis, in a variety of conditions and gynecologic disorders, and for evaluating pelvic-floor weakness [17]. However, the functional MRI of the female pelvic region is primarily used in research rather than in everyday clinical practice [21]. MRI is still not widely accepted by gynecologists also because of the lack of proper training, complexity of examination, and associated cost.
4) X-Ray Imaging
Generally, radiographic assessment to establish extent or characteristics of a patient’s prolapse is unnecessary. Some clinicians have advocated use of imaging procedures such as contrast radiography to describe the location of pelvic-support defects before attempting surgical repair [22]. However, a scarcity of standardized radiological criteria currently exists for diagnosis of pelvic organ prolapse, and the clinical benefit of such radiographic imaging has yet to be defined. Radiological imaging might be needed for further diagnostic assessment only in certain individual cases, which can be used together with urodynamic testing and endoscopic examinations in the framework of the special urogynecological outpatient service after carefully weighing their risks and benefits [23].
B. Elasticity Imaging and Assessment of Soft Human Tissues
In the last decade, a new modality for tissue characterization termed Elasticity Imaging (EI) or Elastography has emerged. EI allows visualization and assessment of mechanical properties of soft tissue. Mechanical properties of tissues, i.e., elastic modulus and viscosity, are highly sensitive to tissue structural changes accompanying various physiological and pathological processes. A change in Young’s modulus of tissue during the development of a tumor could reach thousands of percent [24]–[26]. EI is based on generating a stress in the tissue using various static or dynamic means and the measuring resulting strain by ultrasound or MRI [27]–[33]. The current increasing flow of publications from many countries all over the world on Elastography covers practically all-key human organs [34]–[40]. Tactile imaging (TI), a branch of EI, yields a tissue elasticity map, similarly to other elastographic techniques. At the same time, TI, which is also called “stress imaging,” “computerized palpation,” or “mechanical imaging” [41]–[46], most closely mimics manual palpation, because the TI probe, with a pressure sensor array mounted on its face, acts similarly to human fingers during a clinical examination by slightly compressing soft tissue with the probe and detecting resulting changes in the pressure pattern.
The main purpose of the present paper is the development of vaginal tactile imager (VTI) and evaluation of its applicability for imaging and assessment of mechanical properties of vaginal walls.
II. Vaginal Tactile Imager
A. System Overview
We designed and built a proof-of-concept prototype of the VTI, which includes a transvaginal probe, an electronic unit, and a laptop computer with a data acquisition card. The vaginal probe comprises of a tactile sensor array and a tilt sensor. The tactile sensor array is installed on the probe head surface contacting with the vaginal wall during the examination procedure. The probe head measures 48 mm in length, 20 mm in width, and 14 mm in height having ellipsoidal cross section. The tactile sensor array (Pressure Profile Systems, Inc., CA) comprises of 120 capacitive pressure sensors, which provide 2-D pressure pattern being contacted with vaginal wall. In average, each pressure sensor has sensitivity of 50 Pa and reproducibility is about 200 Pa. We used a two-axis acceleration sensor ADXL202 (Analog Devices, MA) as the tilt sensor, which was incorporated inside the probe. During examination, the VTI probe is covered by a disposable elastic sheath (Sheathing Technology, CA) designed for ultrasonic transrectal probes. Fig. 1 shows the basic structural components of the VTI probe. As shown in Fig. 1, during the pressings on the anterior and posterior vaginal walls, the probe was rotating relatively unmovable horizontal axis passing through the tilt sensor, which is distanced about 15 cm from the probe head.
Fig. 1.

Tactile sensor array mounted on the tip of the VTI probe allows recording the stress patterns on the vaginal walls under manually applied pressure as a function of the angular position of the probe detected by the orientation sensor.
We assumed that the slope of a peak pressure value related to specified zone inside a tactile image versus total applied force to the probe head (scanhead) characterizes relative elasticity of a hard inclusion placed inside soft tissue against which the scanhead has been pressed.
We accepted that the slope (Es) of the total applied force to the scanhead versus elevation angle of the probe characterizes an elasticity of the vaginal wall against which the probe head has been pressed. Further, we will call this slope Es (kilogram per degree) as an elasticity index. We used a least-square linear fit to estimate the slopes.
The electronic unit provides data acquisition from the tactile sensor array, the tilt sensor, and communicating with a laptop computer through a universal serial bus (USB) port. The data acquisition rate is about 25 tactile pressure patterns per second.
The implemented VTI software supports three operational modes: data acquisition, data management, and device management. The implemented software allows real-time visualization of the pressure pattern on the probe head and storing the data in a digital format. All data post-processing is done with the use of MATLAB and Borland C++ compiler. The tactile image processing included: 1) removing negative sensor signals; 2) lowpass noise-cutting filtration based on calculation of the exponential moving average for each tactile sensor with the time constant of 0.3 s; 3) edge smoothing by linear filtration (weighed averaging of 3 × 2 pixels/sensors along the tactile sensor array boundary); 4) 2-D noise-removal filtration (weighted averaging of 3 × 3 pixels/sensors inside the frame); and 5) 2-D linear interpolation. All these steps are described in details in [46].
B. Examination Procedure
The VTI examination was performed on a patient in a standard position for physical examination of a vagina used in gynecologic office. A lubricating jelly manufactured by Chester Labs (Cincinnati, OH) was applied to the outer surface to ensure comfortable insertion. The examination procedure for elasticity imaging of female pelvic floor comprised the following steps.
Inserting the probe head into the first one third of the vagina and imaging the first one third of the vagina by pressing the probe head against an anterior and posterior vaginal wall.
Sliding the probe deeper to a medium part of the vagina and the pressings were repeated as in Step 1.
Sliding the probe head on maximum depth until a cervix contact and pressing again.
C. Population Description
In a period from August 1, 2007 to February 1, 2008, 13 women were enrolled in the study and underwent transvaginal TI evaluation. Among them six women were with normal pelvic support, three of whom had had prior reconstructive pelvic surgery, and seven women with current pelvic organ prolapse. The clinical protocol was approved by the Institutional Review Board. All women gave written informed consent. The study was done in compliance with the Health Insurance Portability and Accountability Act. The imaging protocol was performed at the time of scheduled routine gynecologic visits at The Institute of Female Pelvic Medicine (Allentown, PA) and at the outpatient gynecologic clinics at St. Luke’s Hospital (Bethlehem, PA).
Total workflow comprised of the following steps.
Recruiting women who routinely undergo vaginal examination as a part of their diagnostic treatment of concerned areas.
Performing a VTI examination of the concerned area.
Acquisition of clinical diagnostic information related to the studied cases by standard clinical means.
Analyzing tactile images and evaluating vaginal wall elasticity from the recorded VTI examination data.
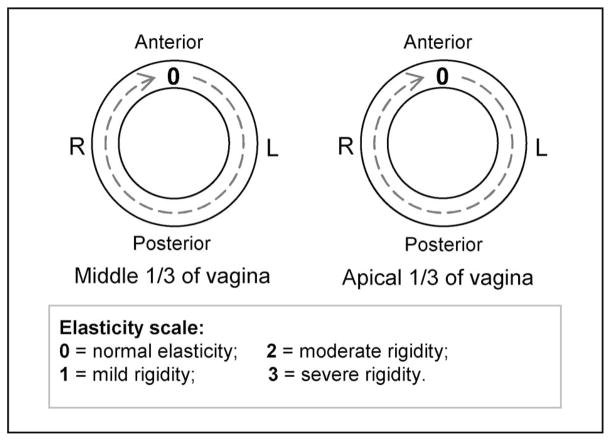
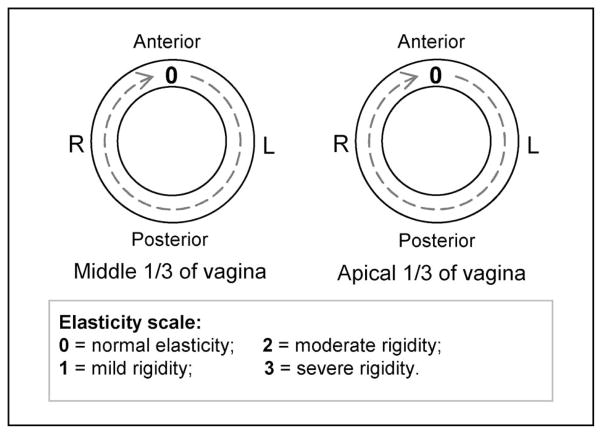
Prior to taking the VTI examination, a standard gynecologic vaginal examination was performed by a practicing urogynecologist. Attention was given to vaginal softness, elasticity, and any abnormalities and the results were recorded to map the vaginal surface at the level of the middle one third of the vagina and the upper one third of the vagina (the areas most affected in prolapse and in sexual function). A three-point grading system for vaginal elasticity was used [47] to assign mild, moderate, or severe rigidity scores to any area of the vagina with a palpably decreased elasticity. For prolapse description, we used POP-Q system [14]. According to the POP-Q system, prolapse conditions are classified in one of the four stages (grades) for anterior and posterior vaginal walls.
D. Statistical Analysis
A statistical assessment of the diagnostic significance of the elasticity index was completed with the aid of the statistical toolbox in MATLAB 6.1 (MathWorks, Natick, MA). For visual evaluation of the analyzed clinical data distributions within the normal and prolapse patient samples, we used boxplots for data representation. In descriptive statistics, the boxplot is a convenient and widely accepted way of graphically depicting groups of numerical data or data samples [61]. Boxplots are able to visually show distinctions of data samples without making any assumptions about the underlying statistical distribution. We have used a notched boxplot [62] showing a confidence interval for the median value. The spacings between the different parts of the box help to compare variance. The boxplot also identifies skewness (asymmetry) and outliers. The intersection or divergence of confidence intervals for two patient samples is a visual analog of the paired t-test. We used also a formal t-test (t-test2 function in MATLAB) to determine whether two samples (normal versus prolapse) could have the same mean when the standard deviations are unknown but assumed equal. The test result is 1 if the test rejects the null hypothesis (both samples have the same means) at the 0.05 significance level (alpha) and 0 otherwise.
III. Results of Clinical Studies
The objectives of the clinical study were to assess the VTI capabilities in vaginal wall imaging and elasticity characterization. Below, we consider three representative cases out of 13 cases to illustrate imaging capability and elasticity evaluation approach, and further, we summarize the elasticity data for all 13 cases.
In all three cases with reconstructive pelvic surgery, the VTI allowed observing increased rigidity at sites with implanted mesh grafts. Case 1 presents an example of tactile images of both anterior and posterior vaginal walls after reconstructive surgery.
A. Case 1
A 59-year-old woman with history of pelvic reconstructive surgery using a mesh graft behind the anterior vaginal wall (Anterior Prolift) and posterior vaginal colporrhaphy (linear, longitudinal incision, and repair of the posterior vaginal wall using traditional midline suture repair). The surgery was performed in August of 2007. The TI scan was performed in January of 2008.
Physical examination revealed a palpable foreign body sensation of the underlying mesh at the anterior wall, particularly, at 70 degrees off of midline bilaterally as shown in Fig. 2. The posterior wall is normal on palpation at the apex, with a mild rigidity appreciated at the site of the prior posterior colporrhaphy from the middle to lower vagina.
Fig. 2.
Physician examination sheet for case 1.
VTI allowed visualization of increased rigidity at both implanted mesh grafts as it might be seen on the tactile images (red-colored zones) presented in Fig. 3. The color distribution on the tactile image represents a contact pressure distribution or tactile feedback under applied load to the tissue. The spatial X, Y -coordinates for the color images show the transverse (X) and longitudinal (Y) direction for a vaginal wall over which the elasticity is being characterized. Tactile imaging also shows a small rigidity increase (yellow-colored zone) at the posterior vagina wall after the posterior vaginal colporrhaphy. To provide quantitative evaluation of the most rigid tissue, we presented 2-D plot where the horizontal axis is proportional to the total applied force to the scanhead, while the vertical axis is a peak pressure related to maximum measured value inside the observed zone (see Fig. 3). The slope of this kind of a “load” curve characterizes the elasticity deviation of a lesion or a hard inclusion placed inside soft tissue [46], [48].
Fig. 3.
Tactile imaging of anterior and posterior vaginal walls allows visualization and quantitative elasticity evaluation of increased rigidity at both mesh grafts.
Further, we selected two typical cases for normal and vaginal prolapse conditions to illustrate the approach we used for evaluation of the vaginal wall elasticity.
B. Case 2
A 39-year-old woman with no prolapse findings or abnormalities on exam. No prior surgeries.
Physical examination revealed normal elasticity of all vaginal walls (see Fig. 4), no prolapse findings or abnormalities.
Fig. 4.
Physician examination sheet for case 2.
VTI scan provided quantitative evaluation of elasticity of the vaginal walls. The elasticity index is Es = 0.18 kg/deg. for anterior wall, and Es = 0.11 kg/deg. for the posterior vaginal wall (see Fig. 5). We defined the elasticity index in Section II-A. Relatively linear dependence of the total applied force to the probe head versus elevation angle allowed assessment of a slope of the imposed line (see Figs. 5 and 7, respectively). This kind of a “load” curve characterizes an average elasticity of the compressed soft tissue [44], [46].
Fig. 5.

Loading curves obtained in case 2 examination (see text). The slope (Es) of the scanhead applied force versus elevation angle of the probe characterizes an elasticity of the vaginal wall against which the scanhead has been pressed.
Fig. 7.
Loading curves obtained in case 3 examination (see text). The slope (Es) of applied force versus elevation angle is the elasticity index characterizing elastic properties of the vaginal wall.
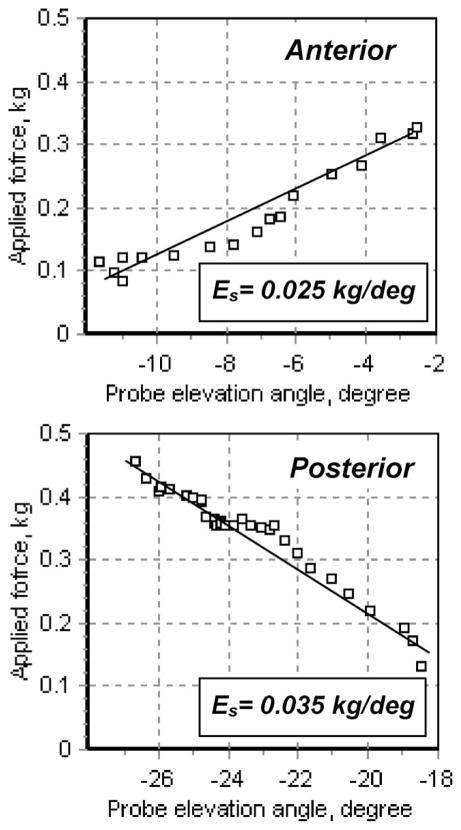
C. Case 3
A 69-year-old woman with moderate-severe (Grade 3) prolapse of the anterior vaginal wall, moderate (Grade 2) prolapse of the posterior vaginal wall.
Physical examination revealed normal elasticity of all vaginal walls (see Fig. 6). Both anterior and posterior vaginal walls were easily distendable with palpation, consistent with prolapse. Grade 3 prolapse of the anterior vaginal wall and Grade 2 prolapse of the posterior vaginal wall was appreciated.
Fig. 6.
Physician examination sheet for case 3.
VTI scan provided quantitative evaluation of elasticity of the anterior vaginal wall Es = 0.025 kg/deg., and the posterior vaginal wall Es = 0.035 kg/deg. (see Fig. 7).
Figs. 8 and 9 represent vaginal wall elasticity data for all 13 patients enrolled in the clinical study. The elasticity data obtained by VTI are shown versus the stage of the prolapse development assessed by physical examination for anterior (see Fig. 8) and posterior (see Fig. 9) vaginal walls. Vertical bars in figures denote calculated standard deviations for each experimental value as an average for three to five compressions of the scanhead against vaginal wall. In our pilot clinical study, no patient had a prolapse stage higher than three.
Fig. 8.

Anterior vaginal wall elasticity index (Es) measured by VTI versus patient vaginal anterior condition from normal to prolapse (Grades 1–3) evaluated by physical examination.
Fig. 9.
Posterior vaginal wall elasticity index (Es) measured by VTI versus patient vaginal posterior condition from normal to prolapse (Grades 1–3) evaluated by physical examination.
Fig. 10 shows discrimination of normal from prolapse conditions. The results of t-test statistics for anterior vaginal wall (left-hand side graph) allowed rejecting the null hypothesis. The significance is 0.015, which means that we would have observed by chance these values of t in only 15 out of 1000 experiments. The results of t-test statistics for posterior vaginal wall (right-hand side graph) allowed also rejecting the null hypothesis (the significance is 0.022). That means the VTI data demonstrate statistically significant quantitative decrease in the vaginal walls elasticity for prolapsed conditions, which were defined by physical examination.
Fig. 10.
Box plots demonstrating discrimination of normal from prolapse vaginal wall conditions assessed by VTI.
IV. Discussion
We may see that VTI can clearly visualize the increased rigidity at both the mesh graft site and the posterior vaginal site (see Fig. 3). These images may be considered as documentation of the current elasticity state of the vaginal walls. Any significant changes in the elasticity pattern of vaginal walls in time (months or years) might be observed by repetitive TI scanning after establishing confidence intervals for quantitative values. The collected TI data for sites with increased hardness, such as mesh graft “arms” after surgery, allow elasticity assessment of these sites by calculating the slope of the peak value inside the pressure pattern versus total applied force to the scanhead.
Figs. 5 and 7 support the claim that TI may be used to assess the underlying connective tissue properties of vaginal tissue and this approach may be used to assess the risk of developing pelvic organ prolapse. We see that the slope (Es) of the scanhead-applied force versus elevation angle, which is proportional to the probe head displacement, can characterize an elasticity of the vaginal wall. Data summaries for cases 2 and 3 are shown in Table I. From this table, we see that the tissue elasticity ratio of normal-to-diseased vaginal walls corresponding to Grade 3 and Grade 2 prolapse are 7.2 and 3.1, respectively. Such significant difference in wall elasticity can be easily detected.
TABLE I.
Comparison of Elasticity Data Obtained for a Patient With No Prolapse and the Patient With Prolapse
| Case 2 | Case 3 | Ratio of normal-to-diseased tissue elasticity | |
|---|---|---|---|
| Physical Exam findings | Women 39 year old with no prolapse findings | Women 69 year old with Grade 3 anterior and Grade 2 posterior vaginal prolapse. | |
| VTI anterior elasticity index | 0.180±0.028 kg/deg | 0.025±0.009 kg/deg | 7.2 |
| VTI posterior elasticity index | 0.110±0.042 kg/deg | 0.035±0.007 kg/deg | 3.1 |
The VTI data also demonstrate the difference in the elasticity of the anterior and posterior vaginal walls in healthy woman (see Table I, Figs. 8–10). The vaginal axis is actually tilted back (towards the sacrum) and so the anterior vaginal wall would receive the pressures of lifting, straining, coughing, jumping, etc., with greater forces than the posterior vaginal wall. Also, the posterior vaginal wall is resting on the rectum and pelvic floor muscles, which may “cushion” forces transmitted to the pelvis, as opposed to the anterior vaginal wall. That means we could expect higher Young’s modulus for anterior than posterior vaginal walls in healthy woman which is in agreement with the data presented in Figs. 8 and 9.
Despite the observed data variability presented in Figs. 8 and 9, which might be caused by probe linear displacements during vaginal tissue compression, by patient movements, and spatial displacement of the rotating axis, we may clearly see from Fig. 10 that the elasticity index is decreased from 0.11 to 0.03 kg/deg. for anterior and from 0.10 to 0.03 kg/deg for posterior vaginal walls. This difference is statistically significant as demonstrated by both the visual comparison of the confidence interval for the presented sample means in Fig. 10 (normal versus prolapse) and the results of the t-test for anterior and posterior. For the combined data (anterior and posterior), we received significant value for t-test less than 0.001 with the 95% confidence interval [0.028, 0.093] on the difference in means of elasticity indexes for normal versus prolapse condition. We believe that such a significant change in the elasticity index can be reliably detected and quantitatively assessed with the use of VTI, and the measurement reproducibility might be further improved. Quantitative elasticity data may help to differentiate tissue transformations and correlate them with other data such as pelvic floor muscle condition, congenital defects, the impact of smoking, childbirth, age, lifestyle, and obesity.
A critical review of published data on the urogynecologic aspects of female sexual dysfunction demonstrates a lack of standardized instruments for assessing biomechanical conditions of the pelvic floor. Clinicians highlight the need for studies to assess the anatomical, physiological, and sensory mechanisms related to female sexual dysfunction [49]. Published studies measuring vaginal tissue elasticity properties involve testing of vaginal biopsies obtained at the time of pelvic surgery [50], use of animal models, or use of cadaveric tissue [51]–[53]. There are several obvious disadvantages of these laboratory measurements including testing of only local areas of the vagina with an inability to correlate with overall vaginal properties and removal of the specimen from the living body and the underlying tissues. Other measurements that have been reported include use of suction pressure to deform the vaginal tissue inward and measuring the amount of stretch obtained with a specified pressure application, balloon distention pressure measurements, and subjective physician examiner assessment of elasticity based on a scaled scoring system [54].
Several research groups have tested ultrasound elastography for elasticity assessment of uterine, cervical, or pelvic floor tissue. A group from the University of Wisconsin [55], [56] proposed to use this approach for differentiation between fibroids and adenomyosis in the uterine wall. A group from Germany [57] investigated the basic tissue elasticity properties of the cervix in pre- and postmenopausal healthy women and compared these findings with the results from a group of patients with focal pathology of the cervix. They found that computer-assisted and subjective evaluation of cervical ultrasound elastography allows differentiation of malignancy from normal findings while cervical tissue is of medium hardness and does not change with age.
The overall surgical goal for prolapse surgery is to give the most functional repair, while preventing recurrence of the condition and minimizing complications incurred by the repairs. Recurrence is one of the barriers in surgical correction most frustrating to both the surgeon and the patient. Failure rates have been cited as high as 20%–40% after surgical repair, with over 50% occurring within the first three years [58]. In attempts to minimize recurrence, with the understanding that many patients with POP have inherent deficient or defective connective tissue, many reconstructive surgeons have turned to the use of adjuvant materials for vaginal support, including the use of synthetic, allogenic, xenogenic, or autologous materials [59]. Currently, at least ten synthetic materials are available for vaginal use [60]. Unfortunately, none of the currently available graft materials is ideal for restoration of both optimal support and functionality of the vaginal walls.
How to evaluate the quality of the surgical repair of pelvic organ support is not known. If there were a way to reproducibly measure elasticity of pelvic floor tissues and to record 3-D elasticity image of the vagina after reconstructive surgery, it may be possible to quantitative characterize the effectiveness of the surgical approach and behavior of materials used for vaginal support in vivo. We believe that VTI may give answers on these questions.
V. Conclusion
A pilot clinical study with 13 patients demonstrated that tactile imaging allows quantitative evaluation of elastic properties of vaginal walls by means of the elasticity index and has a potential for differentiation of normal tissue and diseased tissue under prolapse condition. VTI allows imaging of a vaginal wall with increased rigidity due to implanted mesh grafts following reconstructive pelvic surgery.
Acknowledgments
The authors would like to thank L. Lipetskaia, M.D. of St. Luke’s Hospital of Pennsylvania for data collection in the clinical study; S. Kanilo, Ph.D., Senior Programmer of Artann Laboratories for the development and implementation of a core data acquisition engine for the VTI software; S. Ayrapetyan, M.D., Vice President for clinical research and general management of Artann Laboratories; and N. Sarvazyan, Ph.D., CEO of Artann Laboratories for organizational support in clinical study.
Biographies
 Vladimir Egorov (M’08) received the M.S. degree in biophysics from the Moscow Institute of Physics and Technology, Moscow, Russia, and the Ph.D. degree in biology from K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Sciences, Moscow.
Vladimir Egorov (M’08) received the M.S. degree in biophysics from the Moscow Institute of Physics and Technology, Moscow, Russia, and the Ph.D. degree in biology from K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Sciences, Moscow.
He is currently the Vice President for Technology Development at Artann Laboratories, Trenton, NJ. His research interests include tissue elasticity, imaging, algorithms, and instrumentation development.
 Heather van Raalte received the B.S. degree in biology and the M.D. degree in medicine from Vanderbilt University, Nashville, TN, in 1996, and 2001, respectively. She completed her residency in obstetrics and gynecology at State University of New York, Stony Brook, and the fellowship training in urogynecology at the Institute for Female Pelvic Medicine, Allentown, PA.
Heather van Raalte received the B.S. degree in biology and the M.D. degree in medicine from Vanderbilt University, Nashville, TN, in 1996, and 2001, respectively. She completed her residency in obstetrics and gynecology at State University of New York, Stony Brook, and the fellowship training in urogynecology at the Institute for Female Pelvic Medicine, Allentown, PA.
She is currently at the Institute for Female Pelvic Medicine, Allentown, PA and also the Director of a Private Urogynecology Practice Center in Princeton, NJ. She holds an academic appointment with the Department of Urology, Robert Wood Johnson Medical Center, New Brunswick, NJ, and is a Volunteer Staff Surgeon for fistula repairs with Mercy Ships International, Lindale, TX. Her research interests include clinical outcomes using mesh graft material for reconstructive surgery and the biomechanical properties of pelvic support structures.
 Armen P. Sarvazyan (M’04) received the Ph.D. degree in biophysics and the D.Sc. degree in bioacoustics from the Institute of Biophysics, USSR Academy of Science, Moscow, Russia, in 1969 and 1983, respectively.
Armen P. Sarvazyan (M’04) received the Ph.D. degree in biophysics and the D.Sc. degree in bioacoustics from the Institute of Biophysics, USSR Academy of Science, Moscow, Russia, in 1969 and 1983, respectively.
He is currently the Founder and Chief Scientist at Artann Laboratories, Trenton, NJ. He is also an Adjunct Professor at Surgery Department, Robert Wood Johnson Medical School, NJ, and International Professor Emeritus of the Physics Department, Moscow State University, Moscow, Russia. His research interests include imaging, medical ultrasound, and time reversal acoustics. He has developed new modalities of medical imaging: mechanical imaging and shear wave elasticity imaging. He is the holder of more than 100 international patents on ultrasonic and biomechanical methods of medical diagnostics, and is the author or coauthor of more than 200 research papers.
Footnotes
Color versions of one or more of the figures in this paper are available online at http://ieeexplore.ieee.org.
Contributor Information
Vladimir Egorov, Email: vegorov@artannlabs.com, Artann Laboratories, Trenton, NJ 08618 USA.
Heather van Raalte, Email: heathermcg@msn.com, Institute for Female Pelvic Medicine, Allentown, PA 18104 USA.
Armen P. Sarvazyan, Email: armen@artannlabs.com, Artann Laboratories, Trenton, NJ 08618 USA.
References
- 1.Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Amer J Obstet Gynecol. 2000;183:277–285. doi: 10.1067/mob.2000.107583. [DOI] [PubMed] [Google Scholar]
- 2.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Amer J Obstet Gynecol. 2002;186(6):1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 3.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369(9566):1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 4.Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Amer J Obstet Gynecol. 2008;198(5):590.e1–596.e1. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietz HP, Shek KL. The quantification of levator muscle resting tone by digital assessment. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(11):1489–1493. doi: 10.1007/s00192-008-0682-z. [DOI] [PubMed] [Google Scholar]
- 6.Epstein LB, Graham CA, Heit MH. Systemic and vaginal biomechanical properties of women with normal vaginal support and pelvic organ prolapse. Amer J Obstet Gynecol. 2007;197(2):165.e1–165.e6. doi: 10.1016/j.ajog.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: Report from the Standardization Subcommittee of the International Continence Society. Neurol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 8.Borello-France DF, Handa VL, Brown MB, Goode P, Kreder K, Scheufele LL, Weber AM. Pelvic-floor muscle function in women with pelvic organ prolapse. Phys Therapy. 2007;87(4):399–407. doi: 10.2522/ptj.20060160. [DOI] [PubMed] [Google Scholar]
- 9.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J, Brody DJ Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. J Amer Med Assoc. 2008;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 11.Kesharvarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance – United States 1994–1999. MMWR Surveill Summ. 2002;51(SS05):1–8. [PubMed] [Google Scholar]
- 12.Jelovsek JE, Barber MD. Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. Amer J Obstet Gynecol. 2006;194:1455–1461. doi: 10.1016/j.ajog.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 13.Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99:281–289. doi: 10.1016/s0029-7844(01)01727-6. [DOI] [PubMed] [Google Scholar]
- 14.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 15.Sda, Lemos NL, Auge AP, Lunardelli JL, Carramão S, Faria AL, Aoki T. Validation of the pelvic organ prolapse quantification index (POP-Q-I): A novel interpretation of the POP-Q system for optimization of POP research. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(7):995–997. doi: 10.1007/s00192-007-0556-9. [DOI] [PubMed] [Google Scholar]
- 16.Dietz HP, Haylen BT, Broome J. Ultrasound in the quantification of female pelvic organ prolapse. Ultrasound Obstet Gynecol. 2001;18:511–514. doi: 10.1046/j.0960-7692.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara T, Togashi K, Yamaoka T, Nakai A, Kido A, Nishio S, Yamamoto T, Kitagaki H, Fujii S. Kinematics of the uterus: Cine mode MR imaging. Radiographics. 2004;24:e19. doi: 10.1148/rg.e19. [DOI] [PubMed] [Google Scholar]
- 18.Umek W, Obermair A, Stutterecker D, Hausler G, Leodolter S, Hanzal E. Three-dimensional ultrasound of the female urethra: Comparing transvaginal and transrectal scanning. Ultrasound Obstet Gynecol. 2001;17:425–430. doi: 10.1046/j.1469-0705.2001.00416.x. [DOI] [PubMed] [Google Scholar]
- 19.Tunn R, Petri E. Introital and transvaginal ultrasound as the main tool in the assessment of urogenital and pelvic floor dysfunction: an imaging panel and practical approach. Ultrasound Obstet Gynecol. 2003;22(2):205–213. doi: 10.1002/uog.189. [DOI] [PubMed] [Google Scholar]
- 20.Nakai A, Togashi K, Yamaoka T, Fujiwara T, Ueda H, Koyama T, Kobayashi H, Kagimura T, Fujii S, Konishi J. Uterine peristalsis shown on cine MR imaging using ultrafast sequence. J Magn Reson Imag. 2003;18:726–733. doi: 10.1002/jmri.10415. [DOI] [PubMed] [Google Scholar]
- 21.Koyama T, Togashi KJ. Functional MR imaging of the female pelvis. Magn Reson Imag. 2007;25(6):1101–1112. doi: 10.1002/jmri.20913. [DOI] [PubMed] [Google Scholar]
- 22.Brubaker L, Bump RC, Fynes M. Surgery for pelvic organ pro-lapse. In: Abrams P, Cordozo L, Koury S, Wein A, editors. Third International Consultation on Incontinence. Paris: Health Publication; 2005. [Google Scholar]
- 23.Tunn R, Perucchini D. Morphologische Diagnostik in der Urogyn akologie. Zentralbl Gynakol. 2001;123:1–8. doi: 10.1055/s-2001-20017. [DOI] [PubMed] [Google Scholar]
- 24.Skovoroda AR, Klishko AN, Gusakyan DA, Mayevskii YE, Yermilova VD, Oranskaya GA, Sarvazyan AP. Quantitative analysis of the mechanical characteristics of pathologically changed soft biological tissues. Biophsics. 1995;40(6):1359–1364. [PubMed] [Google Scholar]
- 25.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason Imag. 1998;20(4):260–274. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 26.Sarvazyan AP. Elastic properties of soft tissue. In: Levy M, Bass HE, Stern RR, editors. Handbook of Elastic Properties of Solids, Liquids and Gases. III. San Diego, CA: Academic; 2001. pp. 107–127. ch. 5. [Google Scholar]
- 27.Parker KJ, Huang SR, Musulin RA, Lerner RM. Tissue response to mechanical vibrations for “sonoelasticity” imaging. Ultrasound Med Biol. 1990;16(3):241–246. doi: 10.1016/0301-5629(90)90003-u. [DOI] [PubMed] [Google Scholar]
- 28.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrasonic Imag. 1991;13:111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 29.Sarvazyan AP, Skovoroda AR, Emelianov SY, Fowlkes JB, Pipe JG, Adler RS, Buxton RB, Carson PL. Biophysical bases of elasticity imaging. In: Jones JP, editor. Acoustical Imaging. Vol. 21. New York/London: Plenum Press; 1995. pp. 223–240. [Google Scholar]
- 30.Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, Felmlee JP, Greenleaf JF, Ehman RL. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5:237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 31.Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: In vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28(2):227–235. doi: 10.1016/s0301-5629(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 32.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 33.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging—A new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 34.Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38(4):344–348. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 35.Kiss MZ, Hobson MA, Varghese T, Harter J, Kliewer MA, Hartenbach EM, Zagzebski JA. Frequency-dependent complex modulus of the uterus: preliminary results. Phys Med Biol. 2006;51(15):3683–3695. doi: 10.1088/0031-9155/51/15/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bensamoun SF, Ringleb SI, Littrell L, Chen Q, Brennan M, Ehman RL, An KN. Determination of thigh muscle stiffness using magnetic resonance elastography. J Magn Reson Imag. 2006;23(2):242–247. doi: 10.1002/jmri.20487. [DOI] [PubMed] [Google Scholar]
- 37.Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Mai JJ, Pellot-Barakat C, Insana MF, Brill AB, Saga T, Hiraoka M, Togashi K. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237(1):202–211. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 38.Goss BC, McGee KP, Ehman EC, Manduca A, Ehman RL. Magnetic resonance elastography of the lung: Technical feasibility. Magn Reson Med. 2006;56(5):1060–1066. doi: 10.1002/mrm.21053. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Dominguez E, Mendoza J, Rubio S, Moreno-Monteagudo JA, Garcia-Buey L, Moreno-Otero R. Transient elastography: A valid alternative to biopsy in patients with chronic liver disease. Aliment Pharmacol Ther. 2006;24(3):513–518. doi: 10.1111/j.1365-2036.2006.02999.x. [DOI] [PubMed] [Google Scholar]
- 40.Baldewsing R, Schaar J, Mastik F, van der Steen A. Local elasticity imaging of vulnerable atherosclerotic coronary plaques. Adv Cardiol. 2007;44:35–61. doi: 10.1159/000096719. [DOI] [PubMed] [Google Scholar]
- 41.Wellman PS. PhD thesis presented to Harvard Univ Div Eng Appl Sci. 1999. Tactile imaging. [Google Scholar]
- 42.Sarvazyan AP. Mechanical imaging: A new technology for medical diagnostics. Int J Med Inf. 1998;49:195–216. doi: 10.1016/s1386-5056(98)00040-9. [DOI] [PubMed] [Google Scholar]
- 43.Kaufman CS, Jacobson L, Bachman B, Kaufman L. Digital documentation of the physical examination: moving the clinical breast exam to the electronic medical record. Amer J Surg. 2006;192:444–449. doi: 10.1016/j.amjsurg.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Egorov V, Ayrapetyan S, Sarvazyan AP. Prostate Mechanical Imaging: 3-D image composition and feature calculations. IEEE Trans Med Imag. 2006 Oct;25(10):1329–1340. doi: 10.1109/tmi.2006.880667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss RE, Egorov V, Ayrapetyan S, Sarvazyan N, Sarvazyan AP. Prostate mechanical imaging: a new method for prostate assessment. Urology. 2008;71(3):425–429. doi: 10.1016/j.urology.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egorov V, Sarvazyan AP. Mechanical imaging of the breast. IEEE Trans Med Imag. 2008 Sep;27(9):1275–1287. doi: 10.1109/TMI.2008.922192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leiblum S, Bachmann G, Kammann E, Colburn D, Swartzman L. Vaginal atrophy in the postmenopausal woman. J Amer Med Assoc. 1983;249:2195–2198. [PubMed] [Google Scholar]
- 48.Egorov V, Kearney T, Pollak SB, Rohatgi C, Sarvazyan N, Airapetian S, Browning S, Sarvazyan A. Differentiation of benign and malignant breast lesions by mechanical imaging. Breast Cancer Res Treat. 2009;118(1):67–80. doi: 10.1007/s10549-009-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalpiaz O, Kerschbaumer A, Mitterberger M, Pinggera GM, Colleselli D, Bartsch G, Strasser H. Female sexual dysfunction: A new urogynaecological research field. BJU Int. 2008;101(6):717–721. doi: 10.1111/j.1464-410X.2007.07442.x. [DOI] [PubMed] [Google Scholar]
- 50.Lei L, Song Y, Chen R. Biomechanical properties of prolapsed vaginal tissue in pre- and postmenopausal women. J Int Urogynecol. 2007;18:603–607. doi: 10.1007/s00192-006-0214-7. [DOI] [PubMed] [Google Scholar]
- 51.Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Amer J Obstet Gynecol. 2008;198(5):590.e1–590.e6. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prantil RL, Jankowski RJ, Kaiho Y, de Groat WC, Chancellor MB, Yoshimura N, Vorp DA. Ex vivo biomechanical properties of the female urethra in a rat model of birth trauma. Amer J Physiol Renal Physiol. 2007;292(4):F1229–F1237. doi: 10.1152/ajprenal.00292.2006. [DOI] [PubMed] [Google Scholar]
- 53.Rubod C, Boukerrou M, Rousseau J, Viard R, Brieu M, Dubois P. A biomechanical model of the pelvic cavity: first steps. Conf Proc IEEE Eng Med Biol Soc. 2006;1:968–971. doi: 10.1109/IEMBS.2006.260236. [DOI] [PubMed] [Google Scholar]
- 54.Bo K, Finckenhagen HB. Vaginal palpation of pelvic floor muscle strength: inter-test reproducibility and comparison between palpation and vaginal squeeze pressure. Acta Obstet Gynecol Scand. 2001;80(10):883–887. doi: 10.1034/j.1600-0412.2001.801003.x. [DOI] [PubMed] [Google Scholar]
- 55.Varghese T, Kliewer MA, Zagzebski JA. Method and apparatus for imaging the cervix and uterine wall. 7297116. US Patent. 2007
- 56.Kiss MZ, Hobson MA, Varghese T, Harter J, Kliewer MA, Hartenbach EM, Zagzebski JA. Frequency-dependent complex modulus of the uterus: preliminary results. Phys Med Biol. 2006;51(15):3683–3695. doi: 10.1088/0031-9155/51/15/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas A, Kümmel S, Gemeinhardt O, Fischer T. Real-time sonoelastography of the cervix: tissue elasticity of the normal and abnormal cervix. Acad Radiol. 2007;14(2):193–200. doi: 10.1016/j.acra.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 58.Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Amer J Obstet Gynecol. 2003;189(6):1612–1618. doi: 10.1016/s0002-9378(03)00929-3. [DOI] [PubMed] [Google Scholar]
- 59.Bako A, Dhar R. Review of synthetic mesh-related complications in pelvic floor reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(1):103–111. doi: 10.1007/s00192-008-0717-5. [DOI] [PubMed] [Google Scholar]
- 60.Sung VW, Rogers RG, Schaffer JI, Balk EM, Uhlig K, Lau J, Abed H, Wheeler TL, Morrill MY, Clemons JL, Rahn DD, Lukban JC, Lowenstein L, Kenton K, Young SB. Graft use in transvaginal pelvic organ prolapse repair: A systematic review. Obstet Gynecol. 2008;112(5):1131–1142. doi: 10.1097/AOG.0b013e3181898ba9. [DOI] [PubMed] [Google Scholar]
- 61.Tukey JW. Exploratory Data Analysis. Princeton, NJ/Reading, MA: Princeton Univ. Press/Addison-Wesley; 1977. [Google Scholar]
- 62.McGill R, Tukey JW, Larsen WA. Variations of box plots. Amer Statist. 1978;32:12–16. [Google Scholar]