Abstract
The amyloid cascade hypothesis has guided much of research into Alzheimer disease (AD) over the last 25 years. We argue that the hypothesis of beta amyloid (Aβ) as the primary cause of dementia may not be fully correct. Rather, we propose that decline in brain metabolic activity, which is tightly linked to synaptic activity, actually underlies both the cognitive decline and the deposition of Aβ. Aβ may further exacerbate metabolic decline and result in a downward spiral of cognitive function, leading to dementia. This novel interpretation can tie the disparate risk factors for dementia to a unifying hypothesis and present a roadmap for interventions to decrease the prevalence of dementia in the elderly population.
Keywords: Amyloid beta Protein, Apolipoprotein E, Metabolism, Dementia, Olfactory Pathways, Mitochondria, Etiology, Pathology, Therapeutics
INTRODUCTION
CURRENT HYPOTHESIS
The amyloid cascade hypothesis has guided much of research into Alzheimer disease (AD) over the last 20 years [1,2]. The amyloid cascade hypothesis posits some primary abnormality of processing of the amyloid precursor protein (AβPP) that causes an overproduction of the 4 kD Aβ fragment. This fragment spontaneously aggregates in a β-pleated sheet structure. The 4kD fragment is toxic to neurons and synapses either as an early soluble monomeric, dimeric or oligomeric form, or as an aggregated deposition, the senile or neuritic plaque. Toxic effects of Aβ are proposed to cause the dementia. The diagnostic requirement for both dementia and Aβ senile or neuritic plaques [3] for a diagnosis of AD, emphasizes the assumed causation of dementia by Aβ. Hence, major scientific effort has focused on inhibiting formation or facilitating clearance of Aβ from the brain.
We argue that, although these data are not incorrect per se, the hypothesis of Aβ directly causing dementia may not be fully correct. For example, multiple myeloma is often detected when a patient presents with kidney dysfunction [4], and although a kidney biopsy finds IgG forming β-pleated amyloid, and these deposits may directly cause renal dysfunction, the ultimate etiology of the renal dysfunction is a neoplastic process. We propose a causative event further upstream precedes Aβ deposition. This causative event may be a decline in central nervous system metabolic activity. Aβ is deposited as a secondary consequence of metabolic decline.
PROBLEMS WITH THE AMYLOID CASCADE HYPOTHESIS FOR DEMENTIA
Three major observations raise difficulties with the amyloid cascade hypothesis. The first observation is functional: Aβ is normally present in the brain and must serve some function other than causing dementia. Of note is that soluble Aβ concentration is higher in younger individuals than older in the absence of dementia [5]. One possible role for Aβ is modifying neuroplasticity [6,7,8,9]. The second observation is that individuals with fully penetrant mutations of either AβPP or one of the processing enzymes (gamma secretases), do not show dementia until 20–40 years of age [10]. The third observation is that 20–30% of cognitively intact individuals carry significant numbers of pre- or postmortem detected amyloid plaques [11,12]. Importantly, for this third observation, immunotherapy can remove Aβ deposition but does not significantly affect the progress of dementia [13,14] suggesting that Aβ alone may not be sufficient to cause dementia in AD.
These problems, among others, have led to questioning the accuracy of Aβ as the primary cause of AD [15,16]. The major problem with this skepticism was that, until recently, no alternative hypothesis could explain the cognitive decline, Aβ deposition, and numerous other changes characteristic of AD, such as abnormal tau accumulation or evidence of excessive reactive oxygen species.
AN ALTERNATIVE HYPOTHESIS
We propose an alternative hypothesis based on findings in the last few years which are discussed below. We hypothesize that a decline in brain metabolic activity regardless of etiology, is the underlying cause of cognitive decline and dementia. Decreased metabolic activity increases beta secretase (BACE) which, in turn, increases Aβ deposition as a secondary response.
BRAIN METABOLIC ACTIVITY AND COGNITIVE FUNCTION
Decreased brain metabolic activity measured either by PET or fMRI, has been shown to be associated with dementia [17,18]. The assumption of most brain metabolism studies in dementia is that cell death, plaques and neurofibrillary tangles cause the metabolic decline. However, brain metabolic decline is also correlated with cognitive decline in aged rodents [19,20,21] which do not produce Aβ that aggregates to form senile plaques. Thus, it is possible that declines in brain metabolic activity lead to or are in response to cognitive impairment.
Synaptic activity and brain metabolism are strongly correlated. Estimates suggest a close to one-to-one correlation between synaptic activity and glucose utilization. [22,23]. Glucose utilization is required for transmitter release and uptake, post-synaptic activity, axon depolarization and repolarization, and other processes associated with synaptic transmission. Importantly, severity of dementia correlated better with synaptic loss [24,25] and mitochondrial abnormalities [26] than did Aβ deposition. The observation of a tight coupling between brain metabolic and synaptic function and their correlation with dementia emphasizes the importance of evaluating the role of metabolism in the formation of AD lesions.
METABOLIC ACTIVITY REGULATES BACE ACTIVITY
Key elements in our hypothesis are the data indicating that synaptic activity and brain metabolic activity modulate BACE, the initiating enzyme in Aβ formation in rodent species. Expression of BACE and proteins derived from AβPP increase in response to decreased metabolic activity [27,28,29]. A recent study reported that chronically induced cerebral hypoperfusion in rats resulted in increased BACE and Aβ and poorer learning in a Morris water maze [30]. Synaptic control of BACE and AβPP products is especially well shown by unilateral closure of the external nares. Unilateral closure, blocking stimuli from reaching olfactory receptors in the olfactory epithelium, suppresses synaptic and metabolic activity both in the olfactory bulb (OB) and in downstream cortical sites [27,31]. The mitochondrial enzymes cytochrome oxidase and succinic dehydrogenase (indicators of brain metabolic activity) decline. Importantly, BACE is upregulated in the axon terminals of the olfactory receptor neurons as metabolism declines [27]. Upregulation of BACE results in ipsilateral increased production of c-terminal fragments of AβPP and the 4kD Aβ rodent homologue. Moreover, in mice lacking BACE, closure also suppresses cytochrome oxidase and succinic dehydrogenase showing that the metabolic deficits are a primary response to synaptic activity decline and not increased BACE and Aβ [27]. Hence, synaptic activity could dynamically control the expression of BACE and c-terminal fragments of AβPP by modulating metabolic activity.
THE ROLE OF BACE IN NORMAL COGNITIVE FUNCTION
What then is the role of BACE and Aβ in normal function of the brain? Data are limited, but we hypothesize that Aβ is integral to modulation of neuroplasticity. In preparations that maintain reasonably normal synaptic connectivity (e.g., brain slices), Aβ can either facilitate or inhibit long term potentiation and synaptic activity by a concentration-dependent mechanism [6,7,8,32]. Therefore, the olfactory bulb (OB) presents a model for understanding how the BACE/Aβ system might function to modify plasticity. The olfactory receptor neurons in the olfactory epithelium are replaced throughout life leading to a great deal of neuroplasticity [33]. New neurons grow an axon and form synapses in the OB [33,34]. Hence, evidence of synaptic plasticity is extremely high in the OB as is the expression of BACE [35]. Following unilateral naris closure, OB BACE significantly increases in the presynaptic terminals of the olfactory nerve ipsilateral to the closure. Moreover, changes can be reversed within days by simply opening the naris [27]. The products of BACE could modulate synaptogenesis in the OB when olfactory input is transiently lost following injury, disease, or during normal regeneration of olfactory receptor neurons. In addition, our unpublished studies of BACE localization in human and monkey tissues show that BACE is conspicuously absent in layer IV, where thalamic axons terminate and where plasticity may be disruptive to function. We suggest that BACE is part of a dynamic system to regulate axonal growth and synaptic plasticity.
DELAYED DEMENTIA ONSET IN AUTOSOMAL DOMINANT DEMENTIA
The second problem, adult onset of autosomal dominant dementia in individuals with AβPP or gamma secretase mutations, can also be explained by a relationship between metabolic and BACE activity. At about 30 years of age, brain metabolic activity [36,37,38,39] and mitochondrial function [40] appear to decline in normal individuals. We hypothesize that this normal age-associated metabolic decline results in upregulation of BACE and over- or aberrant production of Aβ in those with the abnormal genotype. This leads to decline of synaptic activity and further metabolic decline. Onset during childhood does not occur because metabolic activity may be high enough to suppress BACE, BACE may not be functional during the maturational period of the brain, or other fundamental developmental processes (e.g. synaptogenesis) mask or neutralize Aβ production. After maturation, when brain metabolic activity declines [36,37,39], BACE increases enough to support Aβ deposition in those with a predisposing genotype. Hence, the development of dementia in the autosomal dominant forms of AD may occur as a consequence of maturation of synaptic connectivity in the brain and decreasing metabolic activity. Age-related decrease of metabolic activity would start the defective processing of Aβ and the downward course of cognitive decline.
THE ROLE OF Aβ AND COGNITIVE DECLINE
The apparent lack of efficacy of Aβ removal on stopping cognitive decline is the final problem to address. Although published studies showed that deposits of Aβ were substantially reduced, no slowing of the dementing process occurred [13,14]. Furthermore, 20–30% of elderly individuals with normal cognitive function have substantial amounts of Aβ plaques [11] or show brain amyloid binding in life [12]. We propose that these discordances represented the indirect relationship between Aβ and cognitive decline. Aβ deposition may not be the primary cause of dementia but rather a response to some other process that occurs. We propose that brain metabolic decline might be the principal cause of dementia. Deposition of Aβ may be a response to that decline.
A METABOLIC SCENARIO FOR ALZHEIMER TYPE DEMENTIA
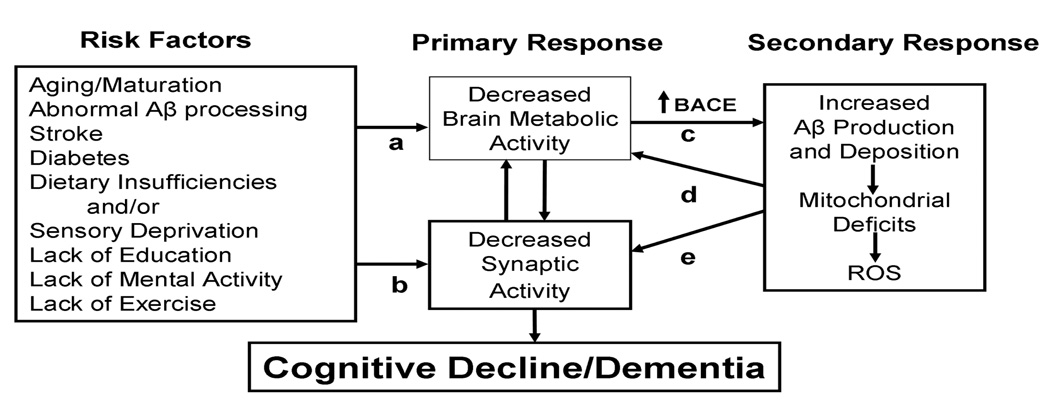
We propose the following scenario for cognitive dysfunction and Aβ deposition (Figure 1). Due to multiple, etiologically diverse and independent factors, metabolic activity in the adult brain declines. This may lead to mild cognitive decline and simultaneous BACE activation. Regardless of why metabolic/synaptic activity decreases, BACE expression and, hence, Aβ (and the rodent homologue that doesn’t aggregate) increase. At a slightly later stage, Aβ and other products from AβPP further adversely affect mitochondria and energy metabolism [41,42,43]. Compromised mitochondria may further suppress synaptic and metabolic activity and increase reactive oxygen species and injure mitochondria [15,44]. In sum, we would have a self-reinforcing system; any initial event suppressing metabolic/synaptic activity could amplify itself, leading to more BACE and Aβ [35]. A small perturbation could result in effects that would potentiate over time leading to the co-occurrence of dementia and Aβ.
This figure summarizes our hypothesis about the causes of cognitive decline leading to Alzheimer Disease. The first column (Risk Factors) includes conditions implicated in cognitive decline. The first five are “physical” factors. The latter four could be considered life-style factors although we propose substantial overlap of the two categories. The second column (Primary Response) represents the response of the CNS to the Risk Factors that decrease either metabolic (a) OR synaptic activity (b). Although presented as separate factors, metabolic and synaptic activity are closely tied. Critically, decreased synaptic activity leads to cognitive decline. BACE (c) is substantially regulated by metabolic activity; decreased metabolic activity increases BACE leading to increased production of Aβ. The final column (Secondary Factors) indicates that Aβ, either as soluble or fibrillary amyloid, may disrupt metabolic activity by suppressing mitochondrial activity or producing ROS (d) which further disrupts metabolic activity. Alternatively, Aβ can also disrupt synaptic function (e) that could feed back on metabolic activity. The key to the progressive nature of AD is that we propose that the Primary and Secondary factors are a self-reinforcing cycle that can exacerbate effects of the numerous Risk Factors on metabolism and synaptic activity.
RISK FACTORS FOR DEMENTIA
Factors that cause the initial metabolic decline could include maturational changes, cerebral vascular aging, chronic diseases (e.g., diabetes), injury or sedentary lifestyle selection. Since none of these conditions acts in isolation, we would expect synergistic effects on metabolism/synaptic activity and cognitive function decline, progressing to dementia.
Maturational changes would be characterized by declines of metabolic activity that occur with normal maturation [36,38,39,40], which could underlie the onset of autosomal dominant forms of AD. Chronic diseases, such as Type I and type II diabetes would also be a logical etiology for cognitive compromise if they chronically decrease CNS metabolic activity [45,46,47]. Of note are studies that propose a decrease of brain glucose utilization in long-term diabetics [48], decreased brain metabolic activity during mild hyperglycemia [49] and cognitive compromise in Type I diabetics [50]. Moreover, the increased risk for cognitive decline in type II diabetes is not associated with a greater density of Aβ deposition than non-diabetics but with evidence for more small strokes [51].
Acute injuries, such as stroke, can result in both local and remote changes. Local effects occur in response to vascular insufficiency and from resulting neuronal death. Brain-wide effects (diaschisis), represent decreased brain activity remote from the infarct [52]. Decreased metabolic activity could start a progressive decline leading to further decline. Even the role of apolipoprotein E, apart from its role in transport of Aβ, fits this schema, since the ε4 isoform of apoE is less effective than either the ε2 or ε3 isoforms in facilitating neuronal process regeneration [33,53]. If neurite regeneration and synaptogenesis are slowed, then recovery from insults (e.g., strokes) would be impeded and synaptic activity/metabolic activity may not recover. Delayed regeneration/restoration of function would leave neurons deafferented and dysfunctional for longer periods and would further exacerbate decreased metabolic/synaptic activity.
Finally, lifestyle factors would fit into this scenario. Educational achievement and cognitive/social engagement seem to decrease the risk for dementia [54,55]. Physical exercise, which has been shown to increase brain activity, and diet can be protective against onset/progression of dementia [56,57,58]. Even estradiol replacement in the perimenopausal period appears to decrease the risk of developing dementia later in life [59], and estradiol replacement increases synaptic activity [60]. Hence, lifestyle choices influence the production of BACE and Aβ.
CONCLUSION
We propose that the underlying cause of dementia is decreased brain synaptic/metabolic activity independent of the etiology. Decreased activity leads to cognitive decline and also, indirectly to Aβ deposition. Aβ may cause further declines in synaptic/metabolic activity, but critically, at least in the earliest stages of the disease, it is the synaptic loss and metabolic decline that cause the dementia. Aβ production is a secondary phenomenon that may, as in multiple myeloma, result in further clinical decline. The obvious interventional approach, if this hypothesis is correct, is to identify and address factors that cause decreased metabolic/synaptic activity, neuronal death and dysfunction. A proximate goal would be to find and emphasize ways to increase brain metabolic activity. These interventions would, ideally, occur prior to the onset of dementia. Maintenance rather than intervention may be the optimal direction of future behavioral and pharmacological interventions but study of these would require a significant investment in long term studies.
Acknowledgements
We wish to sincerely thank Drs. Andrzej Bartke and Gregory Rose who contributed valuable advice in the writing of the review.
Footnotes
Disclosures:
RG Struble: none
T Ala: Dr. Ala is a principal investigator in Springfield for a Pfizer-funded clinical test of bapineuzumab,
P Patrylo: none
GJ Brewer: none
X-X Yan: none
Literature Cited
- 1.Selkoe DJ. The genetics and molecular pathology of Alzheimer's disease: roles of amyloid and the presenilins. Neurol Clin. 2000;18:903–922. doi: 10.1016/s0733-8619(05)70232-2. [DOI] [PubMed] [Google Scholar]
- 02.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 03.NIA-Reagan Consensus Diagnosis. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 04.Fauci Anthony S, Braunwald Eugene, Kasper Dennis L, Hauser Stephen L, Longo Dan L, Jameson J Larry, Loscalzo Joseph, editors. Harrison's Principles of Internal Medicine. 17th edition. NY: McGraw Will; 2008. Ch. 324, Amyloidosis. [Google Scholar]
- 05.van Helmond Z, Miners JS, Kehoe PG, Love S. Higher Soluble Amyloid beta Concentration in Frontal Cortex of Young Adults than in Normal Elderly or Alzheimer's Disease. Brain Pathol. 2010 doi: 10.1111/j.1750-3639.2010.00374.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 06.Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 07.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 08.Morley JE, Farr SA, Banks WA, Johnson SN, Yamada KA, Xu L. A Physiological Role for Amyloid-beta Protein: Enhancement of Learning and Memory. J Alzheimers Dis. 2010;19:441–449. doi: 10.3233/JAD-2009-1230. [DOI] [PubMed] [Google Scholar]
- 09.Tampellini D, Rahman N, Gallo EF, Huang Z, Dumont M, Capetillo-Zarate E, Ma T, Zheng R, Lu B, Nanus DM, Lin MT, Gouras GK. Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations. J Neurosci. 2009;29:9704–9713. doi: 10.1523/JNEUROSCI.2292-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bird TD. Genetic aspects of Alzheimer disease. Genet Med. 2008;10:231–239. doi: 10.1097/GIM.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 12.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 14.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M. Bapineuzumab (201 Clinical Trial Investigators. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol. 2008;67:523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 17.Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer's disease. J AlzheimersDis. 2002;4:225–232. doi: 10.3233/jad-2002-4312. [DOI] [PubMed] [Google Scholar]
- 18.Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gage FH, Kelly PA, Björklund A. Regional changes in brain glucose metabolism reflect cognitive impairments in aged rats. J Neurosci. 1984;4:2856–2865. doi: 10.1523/JNEUROSCI.04-11-02856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tack W, Wree A, Schleicher A. Local cerebral glucose utilization in the hippocampus of old rats. Histochemistry. 1989;92:413–419. doi: 10.1007/BF00492499. [DOI] [PubMed] [Google Scholar]
- 21.Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. (2001) [DOI] [PubMed] [Google Scholar]
- 24.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 25.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 26.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 27.Yan XX, Xiong K, Luo XG, Struble RG, Clough RW. beta-Secretase expression in normal and functionally deprived rat olfactory bulbs: inverse correlation with oxidative metabolic activity. J Comp Neurol. 2007;501:52–69. doi: 10.1002/cne.21239. [DOI] [PubMed] [Google Scholar]
- 28.Xiong K, Cai H, Luo XG, Struble RG, Clough RW, Yan XX. Mitochondrial respiratory inhibition and oxidative stress elevate beta-secretase (BACE1) proteins and activity in vivo in the rat retina. Exp Brain Res. 2007;181:435–446. doi: 10.1007/s00221-007-0943-y. [DOI] [PubMed] [Google Scholar]
- 29.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhiyou C, Yong Y, Shanquan S, Jun Z, Liangguo H, Ling Y, Jieying L. Upregulation of BACE1 and beta-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer's disease. Neurochem Res. 2009;34:1226–1235. doi: 10.1007/s11064-008-9899-y. [DOI] [PubMed] [Google Scholar]
- 31.Kim HH, Puche AC, Margolis FL. Odorant deprivation reversibly modulates transsynaptic changes in the NR2B-mediated CREB pathway in mouse piriform cortex. J Neurosci. 2006;26:9548–9559. doi: 10.1523/JNEUROSCI.1727-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Song L, Laird F, Wong PC, Lee HK. BACE1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. J Neurosci. 2008;28:8677–8681. doi: 10.1523/JNEUROSCI.2440-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 34.Nathan BP, Nisar R, Short J, Randall S, Grissom E, Griffin G, Switzer PV, Struble RG. Delayed olfactory nerve regeneration in ApoE-deficient mice. Brain Res. 2005;1041:87–94. doi: 10.1016/j.brainres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Cai Y, Xiong K, Cai H, Luo X, Feng J, Clough RW, Struble RG, Patrylo PR, Yan X. b-Secretase-1 elevation in transgenic mouse models of Alzheimer's disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2009.07017.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeSanti S, de Leon MJ, Convit A, Tarshish C, Rusinek H, Tsui WH, Sinaiko E, Wang GJ, Bartlet E, Volkow N. Age-related changes in brain: II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subjects. Psychiatr Q. 1995;66:357–370. doi: 10.1007/BF02238755. [DOI] [PubMed] [Google Scholar]
- 37.Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996;16:385–398. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Petit-Taboué MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7:176–184. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- 39.van Bogaert P, Wikler D, Damhaut P, Szliwowski HB, Goldman S. Regional changes in glucose metabolism during brain development from the age of 6 years. Neuroimage. 1998;8:62–68. doi: 10.1006/nimg.1998.0346. [DOI] [PubMed] [Google Scholar]
- 40.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 41.Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 42.Uemura E, Greenlee HW. Amyloid beta-peptide inhibits neuronal glucose uptake by preventing exocytosis. Exp Neurol. 2001;170:270–276. doi: 10.1006/exnr.2001.7719. [DOI] [PubMed] [Google Scholar]
- 43.Atamna H, Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion. 2007;7:297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Brewer GJ. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol. 2010;45:173–179. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoyer S. The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: an update. J Neural Transm. 2002;109:341–360. doi: 10.1007/s007020200028. [DOI] [PubMed] [Google Scholar]
- 46.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer's disease. J Alzheimers Dis. 2009;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler D, Langen KJ, Herzog H, Kuwert T, Mühlen H, Feinendegen LE, Gries FA. Cerebral glucose metabolism in type 1 diabetic patients. Diabet Med. 1994;11:205–209. doi: 10.1111/j.1464-5491.1994.tb02021.x. [DOI] [PubMed] [Google Scholar]
- 49.Kawasaki K, Ishii K, Saito Y, Oda K, Kimura Y, Ishiwata K. Influence of mild hyperglycemia on cerebral FDG distribution patterns calculated by statistical parametric mapping. Ann Nucl Med. 2008;22:191–200. doi: 10.1007/s12149-007-0099-7. [DOI] [PubMed] [Google Scholar]
- 50.Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, Werther GA, Cameron FJ. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32:445–450. doi: 10.2337/dc08-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 52.Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1149–1165. doi: 10.1097/00004647-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Teter B, Xu PT, Gilbert JR, Roses AD, Galasko D, Cole GM. Human apolipoprotein E isoform-specific differences in neuronal sprouting in organotypic hippocampal culture. J Neurochem. 1999;73:2613–2616. doi: 10.1046/j.1471-4159.1999.0732613.x. [DOI] [PubMed] [Google Scholar]
- 54.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 55.Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72:460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog Neurobiol. 2000;61:397–441. doi: 10.1016/s0301-0082(99)00057-x. [DOI] [PubMed] [Google Scholar]
- 57.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA MIRAGE Study Group. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joels M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]