Abstract
Alzheimer’s disease is associated with synapse loss, memory dysfunction and pathological accumulation of amyloid beta in plaques. However, an exclusively pathological role for amyloid beta is being challenged by new evidence for an essential function of amyloid beta at the synapse. Amyloid beta protein exists in different assembly states in the central nervous system and plays distinct roles ranging from synapse and memory formation to memory loss and neuronal cell death. Amyloid beta is present in the brain of symptom-free people where it likely performs important physiological roles. New evidence indicates that synaptic activity directly evokes the release of amyloid beta at the synapse. At physiological levels, amyloid beta is a normal, soluble product of neuronal metabolism that regulates synaptic function beginning early in life. Monomeric amyloid beta 40 and 42 are the predominant forms required for synaptic plasticity and neuronal survival. With age, some assemblies of amyloid beta are associated with synaptic failure and Alzheimer’s disease pathology, possibly targeting the N-methyl-D-aspartic acid (NMDA) receptor through the α7-nicotinic acetylcholine receptor (α7-nAChR), mitochondrial amyloid-β alcohol dehydrogenase (ABAD) and cyclophilin D. But emerging data suggests a distinction between age effects on the target response in contrast to the assembly state or the accumulation of the peptide. Both aging and beta amyloid independently decrease neuronal plasticity. Our laboratory has reported that amyloid beta, glutamate and lactic acid are each increasingly toxic with neuron age. The basis of the age-related toxicity partly resides in age-related mitochondrial dysfunction and an oxidative shift in mitochondrial and cytoplasmic redox potential. In turn, signaling through phosphorylated extracellular signal-regulated protein kinases (pERK) is affected along with an age-independent increase in phosphorylated cAMP response element-binding protein (pCREB) This review examines the long-awaited functional impact of amyloid beta on synaptic plasticity.
Keywords: Alzheimer’s disease, Synapse, Aging, mitochondria, survival signaling
1. Introduction
Alzheimer’s disease (AD) arises on a neuropathological background of amyloid plaques and neurofibrillary tangles (NFT) characterized by ongoing neurodegeneration in areas of brain involved in learning and memory. Synaptic pathology is an early marker of both AD and aging [1, 2, 3]. The best understood AD pathogenesis could be explained by a loss of plasticity [4, 5] that may adversely affect dendritic ramifications, synaptic remodeling, long-term potentiation (LTP), axonal sprouting, neurite extension, synaptogenesis, and neurogenesis. Plasticity, the process by which synapses modulate their strength and form new connections with other neurons plays a particularly important role in response to injury and disease [6]. Age is the most important and universal risk factor for AD perhaps because the biological capacity for plasticity decreases with age [7, 8]. With aging, a combination of subclinical AD pathology, inflammation, Lewy bodies, microinfarcts and vascular lesions in the cortical and hippocampal regions contribute to late-life brain atrophy and dementia in each individual [9]. Thus, as a consequence of age, the ability to sustain learning and memory are diminished or slowed [10]. Age could interact with other variables that influence neuroplasticity in at least two ways. Conventionally, aging may shift the complex balance of amyloid beta (Aβ) metabolism away from the potentially neurotrophic products of α secretase processing and toward the production of neurotoxic moieties containing the intact Aβ fragment [11]. Alternatively, aging could render the brain vulnerable to Aβ-protein neurotoxicity [12] an age-related susceptibility [13]. Aβ toxicity in vivo is also highly species-specific; toxicity is greater in aged rhesus monkeys than in aged marmoset monkeys, and is not significant in aged rats [12]. These results suggest that Aβ neurotoxicity in vivo is a pathological response of the aging brain, which is more pronounced in higher order primates. Thus, longevity may contribute to the unique susceptibility of humans to AD by rendering the brain vulnerable to Aβ neurotoxicity [12]. The pathological hallmark of Aβ deposits appears to precede the hallmark of phosphorylated tau in the brain [14]. Aβ1-42 at high doses impairs cognitive and memory functions in mouse models of AD. But the relationship of Aβ deposition to synapse loss is less clear in these models. Additionally, whether Aβ deposits might be the early symptom contributing to neurodegeneration or whether synaptic pathology might be an early event preceding amyloid deposition in AD is not clear. Synaptic loss might occur early in AD and molecular biomarkers such as tau hyperphosphorylation and Aβ deposits might be indicators of prolonged disease. Moreover, a central question is whether Aβ plays a direct role in the neurodegenerative process in AD. There are two schools of thought on involvement of Aβ in Alzheimer’s disease. In one, Aβ initiates the disease once produced in excess, which has motivated most AD clinical trials. Alternatively, Aβ does not initiate but rather is secondary to other pathogenic events as a protective response to neuronal insult [15].
Two models have emerged to explain the role of Aβ in normal and AD pathological state. According to the most popular model, oligomeric [16] and fibrillary Aβ deposits [17, 18] are responsible for the eventual neuronal degeneration involving disruption of glutamatergic synaptic function leading to the characteristic cognitive deficits [19, 20, 21, 22]. The second model dictates that Aβ may normally serve as a negative feedback signal that maintains neuronal activity within a normal dynamic range. In vivo studies on wild-type animals [23] and in vitro studies on wild-type [24] and knock-out [25] animals demonstrate that Aβ production significantly increases with increase in communication between brain cells [26] and this increased level depresses excitatory synapses and reduces neuronal activity. Aβ was proposed as a regulator of ion channel function [27] and as essential for neuronal health [28]. Aβ is secreted from neurons in response to synaptic activity and that Aβ, in turn, down regulates synaptic transmission [29]. This negative feedback loop could operate as a physiological homeostatic mechanism to limit levels of neuronal activity [30].
A youthful role for Aβ may enhance neuronal plasticity to help the remaining neural circuits compensate for lost or broken circuits and improve overall network performance and neurological function (Figure 1). Improving network activity may also help to prevent the inexorable loss of neuronal processes and cell bodies that occurs in AD and other neurodegenerative disorders. In the present paper we discuss the recent mechanistic link between Aβ function and synaptic plasticity. This review focuses on the interface between a physiological role of Aβ and toxicity in terms of molecular mechanisms of synaptic plasticity.
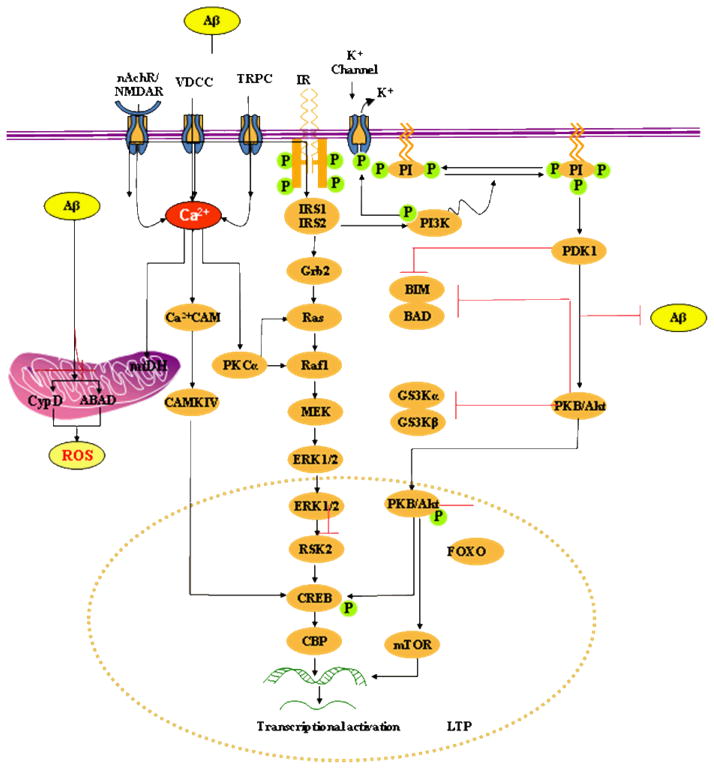
Fig. 1. Model of picomolar Aβ-induced insulin-PI3K-Akt-ERK signalling plus mitochondrial targets of intracellular Aβ.
Extracellular Aβ at picomolar concentration binds to the insulin receptor (IR) and activates PKB/Akt via PDK-1. PKB/Akt translocates into the nucleus and phosphorylates CREB. Activation of the lipid kinase PI3K is critical for the activation of PKB by PDK. PDK1 phosphorylates the activation loop of a number of protein serine/threonine kinases of the AGC kinase superfamily, including protein kinase B (PKB α; also called Akt1). Akt may also maintain the integrity of the mitochondria by a unknown mechanism or by a specific mechanism of Bad phosphorylation. Akt can also inhibit apoptosis by phosphorylation and inactivation of caspase-9. ERK1/2 are activated by upstream MAPKK, such as MEK1/2, and MAPKKK, such as c-Raf. MEK1/2 induce ERK1/2 activation via dual phosphorylation on threonine 202 and tyrosine 204 residues. Phosphorylation of ERK leads to the activation of a number of transcription factors, important in controlling differentiation, neuronal survival, learning and memory plasticity. For example, ERK activates pro-survival transcription factor CREB, by activating both p90RSK and MSK1/2.
Picomolar extracellular Aβ also binds nAChR, glutamate receptors (NMDAR) and Ca2+ ion channels (e.g. VDCCs, TRPC) and causes Ca2+ influx at controlled rates into the cytoplasm and mitochondria. Increased cytosolic calcium concentrations initiate the activation of several kinase-dependent signalling cascades including activation of PKC leading to CREB activation and phosphorylation at Ser133, a process critical for protein synthesis-dependent synaptic plasticity and LTP. PKC-α also activates ERK by interacting with Ras or Raf-1.
Mitochondria are critical targets of intracellular Aβ. Aβ interacts with CypD, a protein component of the membrane permeability transition pore (MPTP). The interaction of CypD with Aβ causes functional modification of this protein leading to MPTP opening. Aβ also binds with another mitochondrial protein, ABAD to distort the enzyme’s structure, rendering it inactive. This causes an increase in reactive oxygen species and oxidative stress leading to initiation of apoptosis.
2. Aβ formation and assembly
The Aβ peptide is derived by proteolytic processing of the amyloid precursor protein [APP; 31, 32], a type I integral membrane protein [33]. Aβ is a prominent constituent of the amyloid plaques present in AD brain [34]. In the beginning, APP is delivered to the plasma membrane where it is subjected to proteolytic processing by α-secretase. However, absent the α-secretase cleavage, APP molecules are internalized into endocytic compartments where they are subjected to cleavage by β-secretase (BACE) and γ-secretase to generate Aβ. Therefore, inhibition of the combined action of β- and γ-secretase that leads to Aβ peptide generation is regarded as a promising approach to treat AD. The Golgi apparatus and to a lesser extent the endoplasmic reticulum are also sources of a distinct population of Aβ peptides secreted into the extracellular space [35]. The majority of secreted Aβ peptides are 40 amino acids in length (Aβ40), whereas a smaller fraction of Aβ is cleaved to produce a 42 amino acid species (Aβ42). Aβ42 is the main amyloid peptide that drives production of amyloid fibrils [36] in AD patients. Aβ in turn can be degraded by proteases such as the insulin-degrading enzyme [37, 38] and neprilysin [39]. Aβ generated from axon-transported APP is released from presynaptic sites and subsequently accumulates close to the nerve terminal [40].
The hydrophobicity of Aβ42 leads to a number of aggregation states [41]. This ability to self-associate [49, 50] into different assembly states ranges from dimers to soluble oligomers to insoluble aggregates of fibrils [51]. Initially, it was assumed that toxicity was mediated by fibrillar Aβ similar to that present in amyloid plaques. This together with the findings that monomer is innocuous and that amyloid plaques alone cannot account for disease has lead many to conclude, if it isn’t fibrillar Aβ and it isn’t Aβ monomer then it must be some other form of Aβ [52]. Soluble non-fibrillar Aβ assemblies [19, 53] are more toxic than the fibrillar form, but as yet the specific form of Aβ which causes injury to neurons in vivo has not been identified. These oligomers have been described in cultured cells [44] as well as in APP transgenic mouse brain and human brain [45, 46, 47, 48]. Synthetic oligomers specifically bind to synapses of hippocampal neurons [42, 43]. Soluble Aβ (sAβ) is found, at low concentrations, as a normal constituent of biological fluids [54, 55, 56]. As demonstrated by Piccini et al. [57], the composition as well as the aggregation and toxicity properties of soluble Aβ aggregates that accumulate in AD is different from those of normal aging. One such form of Aβ known as N-terminal truncated pyroglutamyl amyloid peptide (Aβpy3-42) is the predominant form of early aggregates that alter the membrane permeability, suggesting that they form pores in the membrane like other amyloidogenic peptides [57, 58]. Naturally occurring Aβ peptides can begin to assemble in vivo into metastable dimers, trimers and higher oligomers while still at low nanomolar levels [44, 59, 60]. Immunocytochemical approaches identified different forms of Aβ produced with age in an APP transgenic (Tg2576) mouse model of AD [61]. Initially Aβ40 and Aβ42, the most predominant form of Aβ, occurs in its soluble form. At 6–10 months, the soluble forms of Aβ decrease as SDS-insoluble forms of Aβ40 and Aβ42 increase exponentially. SDS-resistant Aβoligomers develop only in older Tg2576. After 11–12 months, Aβ is converted into diffuse plaques. At later ages, the Aβ accumulates in diffuse plaques, neuritic plaques with amyloid cores [61]. It seems reasonable that the synaptic and neuronal compromise seen at sites distant from plaques is mediated by an Aβ species that can readily diffuse and access the space in and surrounding the synaptic cleft [52]. The physiological level of Aβ is controlled by its production, degradation and clearance [23, 62] while a defect in clearance leads to the accumulation of Aβ [52].
3. Activity-dependent Aβ production
Since synaptic loss correlates better with memory loss than plaque burden and people with mutations in the APP gene who make more amyloid invariably develop AD, the critical question emerges, what is the role of Aβ in synaptic activity and progression of the disease process? The mechanism regulating Aβ production and its subsequent release by neurons is closely linked with synaptic activity [29]. Thus, understanding the factors that regulate Aβ levels has implications for disease pathogenesis as well as for developing therapeutics. In animal models as well as in humans, the activation of muscarinic M1 acetylcholine receptors increases α-secretase cleavage of APP and consequently reduces Aβ levels [63, 64] whereas activation of NMDARs decreases α-secretase cleavage, consequently increasing Aβ levels [65]. Stimulation with muscarinic agonists or activators of protein kinase C (PKC), such as phorbol esters causes the up-regulation of the α-secretase cleavage of APP [66]. Thus, regulation of the α-secretase contributes to regulation of Aβ peptide and toxicity in vivo [67]. Modulating synaptic transmission has been shown to alter extracellular soluble Aβ levels in organotypic brain slice [29]. Synaptic activity modulates interstitial fluid Aβ levels in vivo in APP transgenic and wild-type mice [23]. Neural activity regulates the trafficking of proteins at synaptic sites [68, 69]. Thus it is possible that the induction of neural activity promotes the endocytosis of surface APP, enhancing the accessibility of APP to BACE in endosomal/recycling compartments [29]. In acute brain slices, synaptic vesicle cycling alone, in the absence of neuronal depolarization, was sufficient to drive release of Aβ from neurons [23]. Similar increases in Aβ levels were demonstrated in hippocampal seizures induced by electrical stimulation. However, decreasing synaptic transmission using tetrodotoxin (TTX) or tetanus toxin rapidly reduced interstitial fluid Aβ levels by 30% and 80%, respectively [26]. Cirrito et al. [26] also show that synaptic activity-induced increase in endocytosis drives more APP into the endocytic compartment, ultimately resulting in increased Aβ production and release. Aβ produced in the endocytic pathway is then brought to the cell surface where it is released into the extracellular fluid [70]. Inhibition of endocytosis reduces APP internalization and reduces Aβ production and release in cell lines [71]. Support for a casual link between synaptic activity and Aβ levels in humans comes from recent brain imaging studies of regions that contain the most metabolic activity throughout life (and presumably have the highest levels of neuronal activity) are the same regions that degenerate and accumulate Aβ [72]. The increased synaptic activity enhances both oligomer formation and localization at synaptic sites in rat and mouse hippocampal slices and primary human cortical neurons in culture [73]. These oligomers appear to bind to NMDARs at the synapse [74] and induce internalization of NMDAR [75] and deregulation of NMDA signaling pathways [76]. In this regard, neuronal activity not only enhances oligomer targeting but also facilitates oligomer formation at synaptic sites [73]. These findings indicate that sustained synaptic activity causes an increase in oligomeric Aβ which accumulates with age and leads to synaptic dysfunction and neuronal death. There seems to be a homeostasis of a normal negative feedback function under normal physiological conditions where an increased neuronal activity produces more Aβ; the enhanced Aβ production depresses synaptic function; the depressed synaptic function will decrease neuronal activity [29]. Disturbances in this homeostatic mechanism of Aβ could produce the problems of AD patients. Persistently elevated neuronal activity if it is unchecked could lead to excitotoxicity [77], as well as higher levels of secreted Aβ peptides, which may convert to neurotoxic fibrils with consequent synaptic depression and neuronal toxicity [78, 79]. Thus Aβ toxicity might represent a disturbance of normal function with the net balance of production versus clearance determining a beneficial synaptic or toxic fate.
4. A normal physiologic role for Aβ
Aβ has been extensively studied because of its association with plaques in AD brains, interference with synaptic functionand possible pathogenesis of AD [60]. However, Aβ exists in normal individuals without any known pathology. Therefore, there has been a conscientious search for its normal physiological role in the brain, particularly its possible involvement in synaptic plasticity and neuronal survival. Aβ at physiological levels is essential for synaptic plasticity in normal individuals [80]. The physiological functions of Aβ, as well as the primary mechanisms that initiate early Aβ-mediated synaptic plasticity are now being revealed. We hypothesize that the protective or destructive effects of Aβ are determined by its relative concentration in addition to the age-related cellular environment. Physiologically low regulated concentrations of Aβ would play a critical function in mediating synaptic plasticity and improve cognitive functions whereas, accumulation of higher concentrations of Aβ together with age effects cause disruptions of this regulation with consequent synaptic dysfunction and loss, as seen in AD [76]. Other factors such as structural changes of Aβ due to pathological processing and/or post-translational dysregulations as well as age related changes in Aβ clearance cannot be excluded. The large body of evidence for activity-dependent production of Aβ strongly suggests a normal function for this peptide. Proposed functions of Aβ include control of synaptic activity and memory consolidation, trophic and neuronal survival, cholesterol transport and antioxidant functions.
4.1. Lower levels of Aβ modulate synapticplasticity
It is generally believed that plasticity, such as LTP and long-term depression (LTD), is important for learning and memory. Multiple signaling pathways, including several protein kinases and phosphatases, are required for the generation of LTP and LTD [81]. These same pathways have been shown to influence in vivo phenomena, such as learning and memory [82]. Aβ is released in lower amounts in normal brains throughout life during synaptic activity and seems to be beneficial for normal brain synaptic functions [83]. Aβ is normally produced in the brain, where the in vivo concentration in the rodent brain has been estimated to be in the picomolar range [84]. Picomolar Aβ is present in both cerebrospinal fluid and plasma of healthy individuals throughout life [55]. Recent results indicate that Aβ serves an essential role at the synapse and in synaptic structure-functional plasticity critical to learning and memory. A necessary role of Aβ in synaptic plasticity and memory in normal brain is supported by the observation that APP knock-out (KO) mice show impaired LTP and memory [85]. The impaired synaptic plasticity and memory found in BACE1 KO mice also suggest a necessary role of Aβ [86]. Similarly, the necessary role of Aβ in synaptic plasticity and memory is seen from loss of presenillin function (γ-secretase) [87]. Puzzo et al. [83] show thatpicomolar concentrations (200 pM) of both Aβ42 monomers and oligomers cause a marked increase in long-term potentiation, whereas high nanomolar concentrations (200 nanomolar) lead to the well established reduction of potentiation in the hippocampus. Picomolar levels of Aβ42 also produce a pronounced enhancement of both reference and contextual fear memory. Thus, lower concentrations play a novel positive modulatory role on neurotransmission and memory, whereas high concentrations are associated with neuronal cell death. The lower concentrations of aged Aβ42used by Puzzo et al.[83] are close to those found in the normal brain [88, 89, 90, 91] and shown to enhance LTP and memory. Increase in synaptic activity will increase Aβ production [29] while lowering synaptic activity minimizes Aβ production. Similarly specific stimulation of NMDA receptors promotes Aβ production [65] and Aβ in turn depresses synaptic activity. Thus indirectly Aβ also plays a role in suppressing excessive glutamate release. Interestingly, Aβ may play an important role during synapse elimination [92] and stimulatation of neurogenesis in the hippocampus during brain development [93]. These studies provide convincing evidence to show that physiological levels of Aβ have a role in controlling synaptic activity.
4.2. Neuronal survival
Aβ42 is normally produced and secreted by cells in much lower quantities than Aβ40, which represents ~90% of the total secreted Aβ [94]. However both species of Aβ are necessary for neuronal survival. Both Aβ40 and Aβ42 at physiological concentrations are important in neuronal survival and memory (Table 1). Physiological levels of Aβ also have trophic and neuroprotective actions in trophic deprived conditions [95]. Many Aβ has a physiological role in normal synapse function. In organotypic hippocampal slices, BACE activity is increased by synaptic activity and the resulting Aβ peptides depress excitatory transmission through α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) and N-methyl-D-aspartic acid (NMDA) receptors, suggesting a role for Aβ in homeostatic plasticity [29]. Aβ may have an important physiological role in synapse elimination during brain development [reviewed in reference 92]. Inhibition of endogenous Aβ production by exposure to inhibitors either of β- or γ-secretases in primary neuronal cultures caused neuronal cell death [28]. Thus targeting Aβ formation pharmacologically, or immunologically, could be deleterious [96]. Both Aβ40 and Aβ42 have been shown to be protective. Synthetic Aβ42 monomers at 30–100 nM support the survival of developing neurons under conditions of trophic deprivation and protect mature neurons against excitotoxic death [97]. In cultured neural stem cells, Aβ42 increased the number of newborn neurons [93]. Aβ42 exhibited highly protective effects not only when combined with NMDA (100 nM), but also when applied before or after the NMDA pulse [97]. The later evidence excludes a direct interaction between Aβ42 monomers and NMDARs. Monomers of Aβ40 were also fully protective against NMDA toxicity. Neurotrophic function of Aβ40 was obtained in a cell culture treated with picomolar levels of Aβ40 [98, 28]. Cells treated with such picomolar levels of Aβ40 reverse the toxicity of secretase inhibition. These findings provide compelling evidence for a role for Aβ in neuronal survival. The pro-survival role of Aβ is summarized in Fig. 1.
Table 1.
Neuron survival and memory functions of Aβ peptides.
| Peptide | Form | Source | Concentration (nM) | Function | Reference |
|---|---|---|---|---|---|
| Aβ1-40 | monomer | Synthetic | 10 μM | Inhibits formation of Aβ1-42 β-sheets | 94 |
| Aβ1-40 | monomer | Synthetic | 0.1–10 | Neurotrophic for E18 rat neurons | 98 |
| Aβ1-40 & Aβ1-42 | Monomer or mixed | Synthetic | 0.01–1 | Increases rat cortical neuronal cell viability | 28 |
| Aβ1-40 & Aβ1-42 | Mono + oligomers; anti-Aβ antibody | Synthetic | 0.1 | Rat memory formation & consolidation | 248 |
| Aβ1-40 & Aβ1-42 | monomer | Synthetic | 1–10; 1–1000 | Increased survival (PI- 3K, AKT) and protection from NMDA toxicity | 97 |
| Aβ1-42 | monomer | Synthetic | 0.2 | Enhanced LTP in mouse hippocampal slices + improves mouse memory by ICV injection | 83 |
| Aβ1-42 | Monomers +oligomers | Synthetic | 0.01–1 | Inhibited nicotine- evoked increases of Ca2+ in rat synaptosomes | 153 |
4.2.1. Insulin like growth factor-I (IGF-I) and insulin signaling
The underlying mechanism of neuronal survival with Aβ is emerging. Neuronal synapses and astrocytes of memory-processing brain regions possess insulin receptors (IRs) [99] which when activated by insulin facilitate synaptic plasticity in normal brain [100]. IR and Insulin-like growth factor I (IGF-I) receptors consist of α-subunits and transmembrane β-subunits. Binding of insulin or IGF-I to the α-subunit increases the intrinsic tyrosine kinase activity of the β-subunit, and causes autophosphorylation of the β-subunit, thus triggering tyrosine phosphorylation of insulin receptor substrate (IRS)-1 and IRS-2, as well as Shc [101] as an important pathway of cell survival. To protect against Aβ toxicity, the tyrosine-phosphorylated sites create binding sites for various signal-transducing molecules containing Src homology-2 domain, such as phosphoinositide 3-kinase (PI3K) and growth factor receptor-bound protein 2 (Grb2), thus activating PI3K/phosphoinostide-dependent kinase 1 (PDK1)/Akt (protein kinase B)/glycogen synthase kinase (GSK)-3α/-3β and Ras/Raf-1/mitogen-activated protein kinase/extracellular-signal regulated kinase (MEK/ERK) signaling pathways [101]. In normal brain, IGF-I and insulin promote glucose utilization, energy metabolism, and neuronal survival [102], largely through PI3K/Akt/GSK-3β signaling [103, 104]. Consistent with positive effects of insulin on synaptic plasticity [105], acute insulin treatment improved memory in rats [106] and also in normaladults and AD patients [107] by strongly activating ERK and Akt and blocking c-Jun N-terminal kinase (JNK) activation in a PI3K-dependent manner [108]. The mechanism involves many steps beginning with Aβ activation of IGF-1/insulin receptors by locally produced IGF-1 or, possibly, Aβ monomers may bind to IGF-1/insulin receptors, as already shown for Aβ oligomers [109, 110].
4.2.2. The PI3K/Akt signaling
PI3K, a membrane-associated second messenger protein, and its downstream kinase, Akt, are associated with neuronal survival [111] and plasticity [112] via activation of transcription pathways and protein synthesis. PI3K pathway, which required the activation of IGF-1/insulin receptors, is the most convincing prosurvival effect of Aβ42 monomers. The PI3K signaling pathway is important in the transmission of survival signals in many cell types including neurons [113, 114]. The PI3K-Akt signaling cascade, initiated by IRS, is phosphorylated by stimulated insulin- andIGF-receptor tyrosine kinases [115]. One of the kinases known to lie downstream of PI3K is Akt, which can be directly activated by products of PI3K [116] by promoting its phosphorylation at Ser473 and Thr308 [117]. Activated Akt, in turn, phosphorylates a wide range of substrates activating anti-apoptotic (survival) factors and inactivating pro-apoptotic factors [114, 117]. Certain proapoptotic mediators, such as the transcription factor forkhead (FOXO), the tau kinase GSK-3β, and the Bcl2 antagonist BAD proteins, are inactivated by Akt [118, 119]. Akt substrates such as mammalian target of rapamycin (mTOR; Ser2448) and decreased levels of cell-cycle inhibitors (p27kip1) are found in AD temporal cortex when compared to controls [120]. Akt downregulates the activities of GSK-3α and GSK-3β by phosphorylating the former at Ser21 and the latter at Ser9 [118]. GSK-3α has been implicated in the production of Aβ peptide [117, 121] while increased GSK-3β activity has been implicated in tau hyperphosphorylation [122, 123, 124] and neuronal cell death [124, 125]. Phosphorylation/inactivation of GSK-3β, suppresses GSK-3β-dependent phosphorylation of tau at residues overphosphorylated in AD and prevents apoptosis of confluent cells. Treatment of cortical neurons with Aβ42 monomers increased Ser9 phosphorylation (inhibition) of the Akt substrate, GSK- 3β [97]. Inhibition of GSK-3β promotes cell survival through a variety of mechanisms including a reduced degradation of β-catenin, which then translocates into the nucleus and activates the transcription of protective genes [126].
4.2.3. Extracellular-signal regulated kinase 1/2 signaling (ERK1 and ERK2)
The mitogen-activated protein kinase (MAPK) family of protein kinases is traditionally viewed as important kinases in transmitting extracellular membrane signals intothe nucleus. The 44 kDa ERK1 and 42 kDa ERK2 are members of the MAPK superfamily that specifically respond to Aβ in brain cells [127]. ERK1 and ERK2 are known to be activated through dual phosphorylation by the MAPK/ERK on threonine and tyrosine in the Thr-Glu-Tyr sequence of the activation loop [128, 129]. ERK signaling is critical for memory and tightly regulated by many proteins. ERKs are critical for human learning as revealed by human mental retardation syndromes [130]. They are also known to contribute to molecular information processing in dendrites, to stabilize structural changes in dendritic spines and to interact with scaffolding and structural proteins at the synapse [131]. ERK is an important neuronal marker for activity through activation by cytosolic calcium and depolarization of the membrane [132, 133]. On phosphorylation and activation, ERKs phosphorylate other cytoplasmic effectors and are translocated into the nucleus where they phosphorylate transcription factors such as Myc, Fos, Jun, and Elk1 [104, 134]. Direct substrates of the ERKs includetwo members of the RSK family of protein serine-threonine kinases, RSK1 and RSK2. The transcription factor CREB is phosphorylated on serine 133 in vivo by RSK2 in NGF-stimulated PC12 cells [135]. The dependence of CREB phosphorylation on activation of the ERK pathway is suggested by inhibition of Aβ-induced phosphorylation of CREB by piceatannol and the MEK inhibitor PD98059. Other kinases, such as protein kinase A (PKA) or Ca2+/calmodulin-dependent protein kinases [CAM kinases; 136] may also contribute to phosphorylation of cyclic AMP response element (CRE)-binding protein (CREB) in response to Aβ but the complete inhibition of CREB phosphorylation by PD98059 suggests that the ERK pathway is the main signaling pathway elicited by Aβ leading to transcriptional activation through CREB [137]. These data provide a mechanism by which Aβ alters gene expression through the transcription factor CREB [137], possibly resulting in a ceiling of activation that limits further formation of new memories.
4.2.3. Protein kinase C
PKC is part of a multigene family of serine-threonine kinases central to many signal transduction pathways [138] with a prominent role in memory [139]. It is likely that Aβ-induced increases in cytosolic Ca2+ signals are transmitted to PKC for PKC-mediated transcriptional activation. In addition, PKC activates ERK by interacting with Ras or Raf-1 [140] to initiate CREB phosphorylation. While PKC levels decline in AD [141], their activation restores K+ channel function in cells from AD patients [142]. In addition, activation of PKC directly or indirectly enhances the α-processing cleavage of APP [143]. The activation of PKC has also been shown to prevent Aβ toxicity in rat primary hippocampal neurons [144].
4.2.4. Calcium signaling
Synaptic activity is required for neurons to survive[145] by entry of appropriate amounts of Ca2+ through synaptic NMDA receptors and other Ca2+ channels [146]. The process implicates key protein effectors, such as CaMKs, MAPK/ERKs, and CREB. Properly controlled homeostasis of calcium signaling not only supports normal brain physiology but also maintains neuronal integrity and long-term cell survival. Ca2+ signaling pathways can suppress apoptosis and promote survival through two mechanistically distinct processes. One process involves the PI3K/AKT signaling pathway which promotes survival [147]. The other pathway requires the generation of calcium transients in the cell nucleus which offers long-lasting neuroprotection [146, 148]. Malfunctioning of calcium signaling to the cell nucleus may lead to neurodegeneration and neuronal cell death [149].
Dysregulation of intracellular calcium signaling has been implicated in the pathogenesis of Alzheimer’s disease [150]. Aβ is known to act through multiple targets [151] including Ca2+ channels and various receptors in membranes. Synthetic Aβ binds to the calcium permeable nAChRs with high affinity [152]. Aβ42 administered in the low picomolar range activates nAChRs at presynaptic nerve endings of synaptosomes [83, 153]. Under normal conditions, activation of nAChRs is necessary for the Aβ-induced increase in synaptic plasticity and memory [23]. However, it remains to be determined whether these effects are mediated by a direct physical interaction of the peptide with the nAChR. In addition, Aβ enhances transmitter release by transient increase of glutamate release from the presynaptic terminal that results from brief periods of high frequency stimulation with Ca2+ buildup within the terminal that triggers mechanisms of short-term synaptic plasticity [154].
Aβ directly interacts with Ca2+ channels such as voltage-dependent calcium channels (VDCC) and TRP cation channels (TRPC) to produce a transient increase in Ca2+ necessary for synaptic plasticity and neuronal survival. Aβ interacts directly with the recombinant L-type Ca2+ channel (α1C) subunit to increase Ca2+ channel protein at the cell membrane and hence increased Ca2+ conductance [80]. Within the TRPC subfamily, TRPC3 and 6 have been shown to protect cerebellar granule neurons against serum deprivation–induced cell death in cultures and promote neuronal survival in rat brain [155]. A neuronal survival mechanism of Aβ may also involve altered expression of K+ channels [80]. In cerebellar granule neurons, 24-h pre-incubation with 1 μM unaggregated Aβ protein resulted in a 60% increase in the ‘A’-type component of K+ current possibly reflecting Ca2+-mediated gene expression [156]. A full understanding of these signal transduction pathways of Ca2+ may lead to refined pharmacological strategies that minimize deadly effects of Ca2+ entry and optimize its growth- andsurvival-promoting properties.
4.2.5. Transcriptional activation
CREB is one of the best characterized stimulus-induced transcription factors that activate transcription of target genes in response to a diverse array of stimuli, including neuronal activity, a variety of protein kinases such as protein kinase A (PKA), MAPK/ERKs, pp90 ribosomal S6 kinase (RSK), and Ca2+/calmodulin-dependent protein kinases (CaMKs). These kinases all phosphorylate CREB at a particular residue, serine 133 (Ser133) which is required for CREB-mediated transcription [157]. In contrast to CaMKs, ERKs cannot directly phosphorylate CREB. Two related RSKs and mitogen- and stress-activated protein kinases (MSKs) transmit the signal from activated ERKs to CREB [158]. CREB has been shown to be involved in certain types of hippocampal LTP as well as long-term memory, neurogenesis and synaptogenesis [159, 160]. Transcriptional activation of CREB recruits a multiprotein assembly called a transcriptional co-activator complex. These often include proteins with intrinsic acetyltransferase activity [161]. Among the best characterized transcriptional co-activator proteins is CREB binding protein (CBP) [162]. A role for CBP in memory storage was first demonstrated in a mouse model of Rubinstein-Taybi Syndrome [163]. There is no direct evidence indicating how lower levels of Aβ might initiate CREB phosphorylation principally by Ca2+ signaling and/or through PKA/Atk/ERK pathways. However, exceeding physiological levels of Aβ could deregulate Ca2+ signaling mechanism by excessive accumulation of Ca2+ in the cytoplasm and cytoplasmic organelles such as mitochondria. Since hippocampal neuronal calcium is one of the most potent signals in neuronal gene expression [149], Aβ-induced Ca2+ deregulation may lead to compromised synaptic function. Consistence with this hypothesis, AD has been associated with impaired cAMP signaling which may contribute to the pathophysiology of the disease. Levels of the activated (i.e. phosphorylated) form of CREB are reduced in AD compared to that of an age-matched healthy control group [164]. Calcium signaling to the cell nucleus is the key inducer of CREB phosphorylation on its activator site serine 133 [165]. Experiments in aged neurons show altered calcium signaling at the level of either calcium signal generation and/or calcium signal propagation [166]. These studies indicate a critical role of calcium in Aβ-induced synaptic activity and memory formation by regulating specific signal transduction pathways.
4.3. Cholesterol transport
High cholesterol levels have been linked to overproduction of Aβ and are a risk factor for AD. One of the physiological functions of Aβ has been suggested to control cholesterol transport [167]. Prevalence of AD is reduced among people treated with inhibitors of cholesterol biosynthesis, statins [168, 169] and animal studies support these results [170]. In vitro and in vivo studies have shown that cholesterol modulates APP processing and affects APP mRNA expression [171]. Another mechanism is the increased binding of Aβ to ApoE4 over non-E4 alleles. ApoE is a lipid and cholesterol transport protein responsible for the efflux of cholesterol from neurons to form stable complexes both in vitro and in vivo [172]. Allele ApoE4 is a major risk factor in AD [173]. This relationship might promote synaptogenesis, since in vitro studies have demonstrated that cholesterol released by astroglia increases synaptogenesis [174, 175] with resulting modulation of spike rates [176]. Together, this evidence indicates that one of the physiological functions of APP might be to control cholesterol movement across neuronal membranes [167].
4.4. Antioxidant
The three histidine residues in Aβ control the redox activity of iron, indicating that Aβ is likely to be an important antioxidant. Aβ40 at 5 μM was found to protect primary neuronal cultures from the neurotoxicity of iron [94]. Nakamura et al. [177] found that Aβ40 or Aβ42 inhibits Fe3+ or Cu+2-catalyzed ascorbate oxidation and hydroxyl radical generation. Nanomolar concentrations of Aβ can block neuronal apoptosis following oxidative damage, which suggests that Aβ has a protective role against oxidative stress [178] and is essential for neuronal survival [28, 94]. Monomeric Aβ40 was found to protect neurons cultured in a medium containing 1.5 μM Fe2+ without antioxidant molecules. However, the antioxidant protection of monomeric Aβ40 depends on the type of oxidant used. Aβ40 inhibits cultured neurondeath caused by Cu2+, Fe2+, and Fe3+ but does not protect neurons against H2O2-induced damage [94]. In cerebral cortical neuronal cultures, monomeric Aβ40 inhibits the reduction of Fe3+ induced by vitamin C and the generation of superoxides and prevents lipid peroxidation induced by Fe2+ [94]. Moreover, monomeric forms of Aβ42 also exhibited antioxidant and neuroprotective effects. However, oligomeric or aggregated Aβ40 and Aβ42 were devoid of such antioxidant activity and their neuroprotective activity was demolished. Thus, depriving neurons of the protective activity of Aβ42 monomers may also be an important factor in neurodegeneration [97]. These findings provide novel insights on a normal antioxidant role of Aβ and indicate that monomeric Aβ protects neurons by quenching metal-inducible oxygen radical generation and thereby inhibits neurotoxicity.
5. Effects of Aβ on dendritic spine plasticity and synaptic function
Synapse loss is thestrongest anatomical correlate of the degree of clinical impairment in AD [179]. Loss of dendritic spines at the sites of excitatory synaptic transmission may be the major pathological mechanism in Alzheimer’s disease. However, issues regarding the level of Aβ concentration, type of Aβ species as well as the mechanisms of its production and actions that lead to synaptic loss remained poorly understood. Continuous overproduction of Aβ at dendrites or axons acts locally to reduce the number and plasticity of synapses [76, 180]. The majority of excitatory synapses in the brain are made on the heads of dendritic spines [181]. Initially, synapse degeneration begins at the level of dendritic spines, the loci of memory-initiating mechanisms [182, 183, 184]. As seen in AD and transgenic mouse AD models, significant decreases occur in spine density [185, 186, 187, 188], molecules involved in spine signaling [189, 190] and control of filamentous actin (F-actin) [191]. In a mouse model for AD, the vicinity of amyloid plaques is characterized by highly dysmorphic neurites and spine turnover [192, 193] causing a net loss of spines. These abnormalities in dendritic spines develop even before appearance of clinical symptoms in AD, likely because of cognitive reserve [187]. This phenotype could be caused by Aβ oligomers, which have been shown to block LTP and directly induce LTD, spine loss and memory loss [50]. Soluble oligomers of Aβ have a direct synaptotoxic effect at nanomolar concentrations [51]. In hippocampal culture, the soluble Aβ produced abnormalities in spine composition, shape, and abundance that strongly support the hypothesis that soluble Aβ initiates toxic mechanisms in AD brains that account for synaptic damage [74]. Continued exposure to Aβ caused abnormal spine morphology, with induction of long thin spines, loss of spine cytoskeletal protein drebin and a significant decrease in spine density [74]. In a direct investigation of the acute effects of extracellular and intracellular Aβ42 peptides on synaptic transmission, Moreno et al. [194] noticed inhibition of synaptic transmission by nanomolar concentrations of intra-axonal oligomeric Aβ42, but not oligomeric Aβ40 or extracellular oligomeric Aβ42. Similar nanomolar levels of Aβ disrupt hippocampal LTP [60, 195]. Importantly, physiological concentrations of Aβ in extracellular fluids are picomolar [196]. Thus, local dendritic and axonal abnormalities associated with amyloid deposits lead to loss of synapses and the breakage of dendrites and axons in AD [187, 193]. As dendritic spines are the major connecting elements of one neuron with another in the brain, changes in spine plasticity would have a detrimental impact on disease pathogenesis and progression. Overall, the accumulation of soluble or fibrillar amyloid deposits in AD causes disruption of synaptic connections on a permanent basis and this likely contributes to the cognitive decline and memory [197]. This decreased synaptic activity leads to the elimination of synapses and loss of network activity [198, 199].
The molecular mechanism of spine loss by Aβ is not clear. Electron microscopic studies demonstrate that oligomeric Aβ is localized within the synaptic compartment [200] or that it is bound to the extracellular surface of the spine suggesting that oligomeric Aβ may interact directly at the synapse to cause dysfunction and spine collapse [201]. Soluble Aβ causes abnormal expression of Arc, a synaptic memory related protein that causes abnormal spine shape and glutamate receptor trafficking [42, 202]. Aβ treatment of cultured hippocampal neurons leads to the inactivation of PKA and persistence of its regulatory subunit PKAIIα [203]. Since glutamate treatment reduces phosphorylated CREB phosphorylation and the decrease is reversed by rolipram (a phosphodiesterase inhibitor that raises cAMP and leads to the dissociation of the PKA catalytic and regulatory subunits), a similar mechanism may inhibit LTP by Aβ. Later studies confirmed the activation of the PKA/CREB pathway in both cultured neurons and murine hippocampal slices after inhibition of LTP by Aβ [204, 205]. Interestingly, the toxicity of micromolar fibrillar Aβ on cultured neurons correlates with an age-related increase in phosphorylated extracellular signal-regulated kinase (pERK) as well as an age-independent over-activation of pCREB [7]. Aβ-induced activation of ERK1/2 may reduce mitochondrial respiration and ATP production by decreasing complex I activity and substrate oxidation through complex I [206]. Oligomers can also compromise synaptic function by altering the permeability of neuronal membranes and disrupting ion homeostasis [207, 208]. None of these studies of action of Aβ on protein kinases have identified the proximal target of Aβ. However, these observations suggest that Aβ acts directly on the pathways involved in the formation of late LTP. Agents that enhance the cAMP/PKA/CREB-signaling pathway have potential for the treatment of AD [203]. These studies clearly support the emerging view that impaired synaptic function may be more important for the development of AD than neuronal cell death which occurs at later stages of the disease [199]. However, the major question of how abnormal spine dynamics and alterations in spine plasticity contribute to the disease progression in AD is still not very clear. The major challenge to prevent such loss in spine plasticity could prove invaluable for the treatment of neurodegenerative diseases.
6. Molecular targets of Aβ induced synaptic dysfunction
The search for a mechanism by which Aβ impairs synaptic plasticity has led to the identification of the cell surface receptors and signaling pathways mediating Aβ-induced synaptoxicity. Cell surface interaction sites reported for Aβ include receptor for Advanced Glycation End products (RAGE) and NMDAR [152, 209]. Aβ has been variously reported to directly affect the activity of NMDAR, possibly by binding to nAChRs, or intracellular mitochondrial cyclophilin D (CypD), mitochondrial Aβ alcohol dehydrogenase (ABAD) or certain protein kinases. Examination of the evidence for these multiple activities of Aβ and their affinity constants may distinguish direct binding partners from downstream effectors.
6.1. NMDA receptors
It is well known that excitatory synapses contain AMPA and NMDA ionotropic glutamate receptors as well as metabotropic type glutamate receptors (mGluRs) positioned on dendritic spines [210, 211]. Aβ-induced synaptic dysfunction has been attributed to the synaptic removal of AMPA receptors (AMPARs); however, it is unclear how Aβ induces this loss [212]. Glutamatergic processes are strongly implicated in causing and mediating the symptoms of AD [213]. The clinical use of memantine in treatment of AD provides direct support for the involvement of NMDARs in the cognitive deficits [214]. Memantine acts as a low-to-moderate affinity open channel uncompetitive inhibitor of NMDARs at therapeutic concentrations [215, 216]. Aβ promotes glutamatergic excitotoxicity and potently disrupts glutamatergic synapses and plasticity providing an explanation for the cognitive deficits in AD [217]. Aβ alters the glutamatergic transmission system by inducing marked reductions in levels of AMPA and NMDA receptors at the neuronal plasma membrane [74, 75, 218, 219]. At physiological levels, Aβ selectively enhances NMDAR-mediated currents and synaptic transmission [220, 221]. While increased Aβ application promotes endocytosis of NMDARs in cortical neurons and produces a rapid and persistent depression of NMDA-evoked currents in cortical neurons [75]. This reduction of NMDAR-dependent currents is thought to result from Aβ-mediated activation of nAChRs [75]. Aβ can also promote increased Ca2+ influx and elevate the levels of potentially toxic reactive oxygen species in an NMDAR-dependent manner [221, 222]. In general, brief periods of high synaptic activity open NMDARs, leading to a long-lasting increase in postsynaptic AMPAR number, spine growth and LTP of synaptic transmission [223, 224, 225]. Snyder et al. [75] reported a pathway by which Aβ reduces glutamatergic transmission and NMDAR – dependent LTP. The application of Aβ42 to cultured cortical neurons promoted endocytosis of NMDARs, effectively reducing the density of NMDARs at synapses. At higher concentrations, Aβ is known to enhance activation of NMDARs [226] and cause NMDAR agonist-induced delayed cognitive dysfunction [227]. It is apparent that excessive or inappropriate activation of NMDAR can block LTP [228, 229].
Alternatively, low levels of synaptic stimulation can activate NMDARs to produce NMDA-dependent or mGluR-dependent LTD. These two forms of LTD can induce removal of postsynaptic AMPARs and loss of spines [230, 231, 232, 233]. Interestingly, sublethal NMDAR activation increased the production and secretion of Aβ [65, 234]. However, some of the conceptually and biomedically most important questions that have arisen from these novel insights concern the molecular mechanisms by which Aβ and the glutamatergic transmission system cooperate at the synapse to synergistically regulate and control synaptic transmission. Is excitotoxicity that results from excessive glutamate receptor activation the main trigger that increases the secretion of Aβ during synaptic transmission or is Aβ solely a pathologic product directing cell demise? It is possible that the increased Aβ production causes an increase in NMDA activation and increased NMDA activation in turn increases the Aβ production within limits. This process would have a negative impact on synaptic function if there were a homeostatic balance between NMDA activation and Aβ production. However, excess NMDA activation or excess Aβ generation both are harmful for synaptic plasticity that could lead to the cognitive impairment in AD.
Treatment with Aβ oligomers also causes reduction of post-synaptic density-95 (PSD-95), an adaptor protein that plays a critical role in synaptic plasticity and in the stabilization of AMPAR and NMDAR at synapses [218]. Dysregulation of NMDAR function causes excessive neuronal Ca2+ influx, oxidative stress [222], and inhibition of the Wnt/β-catenin signaling pathway [235, 236].
6.2. Nicotinic acetylcholine receptors (nAChRs)
Activation of the neuronal pentameric nAChRs is involved in diverse brain functions including synaptic plasticity and memory [237, 238] and enhances transmitter release in several brain regions including the hippocampus [239], the spinal cord dorsal horn [240], the olfactory bulb, and the amygdala [241]. The increase of synaptic plasticity by Aβ requires activation of nAChRs [242]. Because activation of nAChRs is necessary for the Aβ-induced increase of synaptic plasticity and memory under normal conditions, Aβ may modify glutamate release with a mechanism dependent upon activation of nAChRs [83]. However, several reports of the effect of Aβ42 on nAChRs are conflicting. Some studies have reported that Aβ42 activates nAChRs [243, 244], while others indicate that Aβ42 inhibits nAChRs [245, 246]. For example, physiological levels of Aβ can activate while toxic levels inhibit presynaptic nAChR and evoke changes in presynaptic Ca2+ levels in rat hippocampus and neocortex [244]. Interestingly, picomolar concentrations of Aβ42 were effective in activating nAChRs while higher levels of Aβ produced inhibitory action. The disparity may depend on the nanomolar Aβ1–42 inhibition of nicotine-induced Ca2+ responses while picomolar Aβ42 directly evokes sustained increases in presynaptic Ca2+ via nAChRs [244]. Aβ42 binds to the nAChR with picomolar affinity [247]. This binding can modulate presynaptic, glutamate-mediated synaptic transmission or glutamate release, suggesting that Aβ42-dependent cholinergic modulation activates signal transduction mechanisms that ultimately result in synaptic transmission and memory consolidation [248]. However, it remains to be determined whether these effects are mediated by a direct physical interaction of the Aβ peptide with the nAChRs. Immunohistochemical studies on human sporadic AD brains show that Aβ42 and nAChR, are both present in neuritic plaques and co-localize in individual cortical neurons suggesting that Aβ could be tightly associated with nAChR [247]. Alternatively, Aβ might be responsible for regulation of nAChR function through strong binding with membrane lipids [249]. Picomolar or higher Aβ42 acting through nAChRs, can elicit ERK MAPK activation in hippocampal cultures [245, 250] possibly triggered by Ca2+ influx [243, 251]. ERK is known to regulate transcription factors such as CREB and Elk-1 by phosphorylation [140], which help initiate transcription of memory-associated genes that contain their respective regulatory elements [252]. Therefore, over-activation of nAChRs and excessive Ca2+ influx or dysregulation of Ca2+ homeostasis provide a molecular mechanism for the cholinergic dysfunction that is a hallmark of AD [253, 254].
6.3. Mitochondrial cyclophilin D
Mitochondria serve as direct targets of neuronal toxicity in which Aβ associates with the outer mitochondrial membrane, inter-membrane space, inner mitochondrial membrane, and the matrix [255]. Progressive accumulation of Aβ in cortical mitochondria in AD patients and also in brains from transgenic AD type mouse models suggests a role for mitochondrial Aβ in the pathogenesis or development of the disease. Once inside the mitochondria, Aβ is able to interact with a number of targets, including the mitochondrial proteins ABAD and cyclophilin-D (CypD) [256]. Opening the mitochondrial permeability transition pore (MPTP) to depolarize mitochondria and release cytochrome C may be central to mitochondrial and neuronal malfunction in AD patients [257]. CypD, an integral part of the MPTP, whose opening leads to cell death, interacts with Aβ peptide within the mitochondria of AD patients and a Tg mouse model of AD [258]. MPTP causes mitochondrial swelling, outer membrane rupture, release of cell death mediators and enhances production of reactive oxygen species (ROS). Computer simulation studies show that Aβ interacts with both ANT and CypD [259]. CypD/Aβ interaction causes an oxidative stress and increased MPTP opening that triggers neurodegeneration [258]. CypD-deficient cortical mitochondria are resistant to Aβ- and Ca2+-induced mitochondrial swelling and MPTP opening [257]. Adenine nucleotide translocase (ANT) is a transport protein for ADP and ATP and component of MPTP that binds directly to CypD [260]. This interaction may facilitate its anchoring in the inner membrane and disturbance of the mitochondrial membrane potential, mitochondrial swelling and cell death [259]. Interestingly, the MPTP also requires the participation of members of the Bcl-2 family proteins but a clear understanding of the interaction of Aβ with CypD together with both proapoptotic or antiapoptotic Bcl-2 family proteins in AD has not been made. The ability of CypD to protect neurons from Aβ- and oxidative stress-induced cell death and its role in improvement of synaptic and cognitive functions has been suggested to provide a new therapeutic approach for the treatment of conditions associated with AD. Together these studies provide new mechanisms for Aβ targets that link the MPTP to synaptic stress and the neurodegeneration seen in AD.
6.4. Mitochondrial Aβ-binding alcohol dehydrogenase (ABAD)
ABAD is a member of the short chain dehydrogenase reductase family in mitochondria that binds Aβ [261]. Binding of Aβ to ABAD distorts the enzyme’s structure, rendering it inactive. Binding also promotes mitochondrial generation of free radicals. In neurons, ABAD is predominately localized to mitochondria. Upon binding ABAD, Aβ triggers events leading to neuronal apoptosis through a mitochondrial pathway [262, 263]. Mitochondrial Aβ levels in the 3xTg-AD mouse increase significantly at 9 months and temporally correlate with increased levels of Aβ binding to ABAD [264]. Interestingly, mitochondrial ABAD is upregulated in neurons from AD patients [263]. The ABAD-Aβ complex has been hypothesized to induce oxidant stress and mitochondrial dysfunction [265]. Increased expression of ABAD exacerbates Aβ-mediated mitochondrial and neuronal stress [263, 266]. Aβ binding to ABAD causes free radical production and neuronal apoptosis [267]. Neurons cultured from transgenic mice with targeted overexpression of a mutant form of amyloid precursor protein and ABAD (Tg mAPP/ABAD) displayed spontaneous generation of hydrogen peroxide and superoxide anion, and decreased ATP, as well as subsequent release of cytochrome c from mitochondria and induction of caspase-3-like activity followed by DNA fragmentation and loss of cell viability [266]. In addition, cytochrome c oxidase (COX) activity was selectively decreased in neurons cultured from mAPP/ABAD mice. In vivo, mAPP/ABAD mice displayed reduced levels of brain ATP and COX activity, diminished glucose utilization, as well as electrophysiological abnormalities in hippocampal slices compared with mAPP mice [266]. ABAD-Aβ binding and enhanced generation ofoxidants in brain mitochondria of transgenic mice results in exaggerating neuronal stress and impaired learning and memory [268]. Analysis of hippocampal slices from mAPP/ABAD mice were shown to display diminished LTP compared with other genotypes [266]. However, positive effects of low levels of Aβ have not been studied. Similar to CypD, the ABAD-Aβ interaction may also represent a novel treatment target against AD. Other intra-mitochondrial targets of Aβ remain to be discovered. These data suggest that mitochondrial ABAD, ordinarily a contributor to metabolic homeostasis, has the capacity to become a pathogenic factor in an Aβ rich environment.
7. Redox and phosphorylative energetic exhaustion: Aβ-induced mitochondrial and synaptic dysfunction
Increasing evidence indicates that the mitochondrial dysfunction is an important early factor in the development of AD-like pathology [264]. Mitochondria are known to accumulate in synapses [269, 270] and mitochondrial trafficking to synapses is dynamic and regulated by synaptic activity [271]. However, increasing evidence indicates that accumulation of Aβ in mitochondria occurs before extracellular amyloid deposition and increases with age. Aβ has been found in the mitochondria of both AD brain and transgenic mouse models of AD overexpressing Aβ [255, 256, 272,]. Aβ has been detected in mitochondria from postmortem brain specimens of AD patients [255] and also in isolated mitochondria from the cerebral cortex of APP transgenic mice [272]. APP and its derivatives, monomeric and oligomeric forms of Aβ, interact with mitochondrial membranes [263, 272, 273, 274] or mitochondrial matrix protein ABAD [264] leading to mitochondrial dysfunction. Accordingly, Aβ is linked to the mitochondrial malfunction observed in the Alzheimer’s disease brain and mouse models of AD [255, 272]. Substantial evidence indicates that mitochondria serve as direct targets for Aβ protein mediated neuronal toxicity.
Studies of postmortem brains from AD patients and transgenic mouse models of AD suggest that mitochondria are involved in oxidative damage induced by Aβ early in AD progression [reviewed in reference 275]. Lower levels of ROS are required for synaptic signaling with ROS acting as messenger molecules in the process of LTP [276]. However, high levels of ROS have been implicated as damaging toxic molecules in the age-related impairments of LTP [276, 277]. Our previous work shows that ROS levels increase with age of neurons in parallel with an age-related decline in transmembrane potential [278]. As mitochondrial transmembrane potential is a driving force for cellular production of ATP, its decline in neurons will have a long term effect in many important energy driven reactions. Increased oxidative stress, coupled with dysregulation of calcium homeostasis and resulting apoptosis of vulnerable neuronal populations, are proposed to underlie the loss of synaptic activity and associated cognitive decline [275]. From these deficiencies emerges the concept of synaptic energy exhaustion in AD, both phosphorylative (ATP) and redox (NAD[P]H) energies. Our previous work shows that hippocampal NAD(P)H and glutathione (GSH) decline with age in association with increased susceptibility to glutamate toxicity in neurons of old-age [279]. Thus, an age-related decline in neuronal reducing currency (NAD[P]H) and reducing buffer (GSH) will surely promote oxidative stress and excess ROS. It is noteworthy that in the early stages of AD, there is already a reduction in the number of mitochondria [280] and the activities of tricarboxylic acid cycle enzymes [281] and cytochrome C oxidase [282]. However, how ROS are produced at the synapse in response to Aβ oligomers is not fully known. Excessive ROS are locally generated in response to synaptic Aβ oligomer binding [222]. This ROS formation can be totally blocked by the mitochondrial uncoupler, 2,4-dinitrophenol which suggests a central role of mitochondria in Aβ-induced oxidative stress [222]. Many studies suggest the possible involvement of oxidative stress and calcium dysfunction in Aβ toxicity [283, 284]. Experiments with Caenorhabditis elegans containing inducible Aβ42 indicate that oxidative stress can precede fibrillogenesis [285]. These reports were strengthened by findings that Aβ toxicity at the synapse is dependent on the presence of a functional mitochondrial electron transport chain which is a principal site of ROS formation as well as a major target for their deleterious effects [286].
The question as to why brain synaptic ROS levels increase with age is uncertain, but may involve lack of use [287] followed by acute overstimulation of excitatory NMDARs that leads to excessive ROS, related to excess Ca2+ entry into mitochondria [288]. Dysregulation of NMDAR function induced by Aβ binding to neuronal synapses may lead to synaptic mitochondrial dysfunction and excessive ROS formation [222, 289]. Memory mechanisms might be directly compromised by elevated ROS, which could explain the connection between AD and oxidative stress [222]. The increase in oxidative damage exhibited by synaptic mitochondria will damage synapses, affect neurotransmission and might be ultimately responsible for cognitive decline in AD patients. Taken together these studies provide convincing evidence for the concept that mitochondria have a pivotal role in Aβ-induced synaptic dysfunction and neuronal stress. Improved function of mitochondria is an effective way of reducing effects of aging and may inhibit neuronal cell death in AD [287].
8. Conclusions
The present review highlights some important physiological roles for Aβ in the CNS during normal function and AD pathogenesis. Given the important role that Aβ plays in various activities at the synapse, Aβ should not be regarded merely as a toxic factor that requires eradication to avoid dementia. There is enough evidence to suggest an essential activity-dependent role of Aβ in modulation of synaptic activity and neuronal survival. However, dissociation of the synaptic effects of aging from Aβ remains to be investigated. Several extracellular and intracellular synaptic receptors are important targets of Aβ oligomers. Alterations in receptor characteristics and synaptic dysfunction are crucial to the early memory deficit and cognitive decline in AD. Alterations in synaptic plasticity, mitochondrial function and neurotransmission by Aβ can affect activity-dependent signaling and gene expression, resulting in the disintegration of neural networks and, ultimately, the failure of neural functions [290]. These ongoing discussions provide new insights into strategies for development of AD therapy that not only reduce the amount of Aβ but also inhibit Aβ aggregation and restore mitochondrial and redox energy for synaptic function.
Acknowledgments
This work was supported in part by NIH grants R01 AG032431, R56 AG013435 and the Kenneth Stark Endowed Chair for Alzheimer Research to GJB.
Abbreviations
- ABAD
mitochondrial Aβ-binding alcohol dehydrogenase
- Ca2+CAM
Calcium calmodulin
- CAMKIV
Calcium/calmodulin-dependent protein kinase type IV
- CREB
Cyclic AMP response element (CRE)-binding protein
- CBP
CREB binding protein
- CypD
cyclophilin D
- ERK
extracellular signal-regulated kinase
- Fas
FS7-associated cell surface antigen
- FOXO
forkhead transcription factor
- GSK
glycogen synthase kinase
- Grb2
growth factor receptor-bound protein 2
- IR
insulin receptor
- IRS-1
insulin receptor substrate-1
- IRS-2
insulin receptor substrate-2
- LTP
Long term potentiation
- mitDH
mitochondrial dehydrogenases
- MEK
MAPK kinase, MAPK, mitogen-activated protein kinase
- MPTP
membrane permeability transition pore opening
- mTOR
mammalian target of rapamycin
- α7-nAChR
α7-nicotinic acetylcholine receptor
- NMDAR
N-methyl-D-aspartic acid receptor
- PDK1
3-Phosphoinositide-dependent kinase 1
- PI3K
phosphoinositide 3′ kinase
- PKB/Akt
protein kinases B
- ROS
reactive oxygen species
- VDCC
voltage-dependent calcium channels
- TRPC
TRP cation channel
References
- 1.Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978;202:1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- 2.Agnati LF, Benfenati F, Solfrini V, Biagini G, Fuxe K, Guidolin D, Carani C, Zini I. Brain aging and neuronal plasticity. Ann N Y Acad Sci. 1992;673:180–186. doi: 10.1111/j.1749-6632.1992.tb27451.x. [DOI] [PubMed] [Google Scholar]
- 3.Martin LJ, Pardo CA, Cork LC, Price DL. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am J Pathol. 1994;145:1358–1381. [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer GJ. Neuronal plasticity and stressor toxicity during aging. Exp Gerontol. 2000;35:1165–1183. doi: 10.1016/s0531-5565(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 5.Buell SJ, Coleman PD. Dendritic growth in the aged human brain and failure of growth in senile dementia. Science. 1979;206:854–856. doi: 10.1126/science.493989. [DOI] [PubMed] [Google Scholar]
- 6.Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 7.Brewer GJ, Torricelli JR, Lindsey AL, Kunz EZ, Neuman A, Fisher DR, Joseph JA. Age-related toxicity of amyloid-beta associated with increased pERK and pCREB in primary hippocampal neurons: reversal by blueberry extract. J Nutr Biochem. 2010 doi: 10.1016/j.nutbio.2009.08.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 9.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 10.Lanahan A, Lyford G, Stevenson GS, Worley PF, Barnes CA. Selective alteration of long-term potentiation-induced transcriptional response in hippocampus of aged, memory-impaired rats. J Neurosci. 1997;17:2876–2885. doi: 10.1523/JNEUROSCI.17-08-02876.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasternack JM, Palmert MR, Usiak M, Wang R, Zurcher-Neely H, Gonzalez-De Whitt PA, Fairbanks MB, Cheung T, Blades D, Heinrikson RL. Alzheimer’s disease and control brain contain soluble derivatives of the amyloid protein precursor that end within the beta amyloid protein region. Biochemistry. 1992;31:10936–10940. doi: 10.1021/bi00159a038. [DOI] [PubMed] [Google Scholar]
- 12.Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat Med. 1998;4:827–831. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- 13.Brewer GJ. Age-related toxicity to lactate, glutamate, and beta-amyloid in cultured adult neurons. Neurobiol Aging. 1998;19:561–568. doi: 10.1016/s0197-4580(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 14.LaFerla FM, Oddo S. Alzheimer’s disease: Abeta, tau and synaptic dysfunction. Trends Mol Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Lee HG, Zhu X, Castellani RJ, Nunomura A, Perry G, Smith MA. Amyloid-beta in Alzheimer disease: the null versus the alternate hypotheses. J Pharmacol Exp Ther. 2007;321:823–829. doi: 10.1124/jpet.106.114009. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selkoe DJ. Amyloid protein and Alzheimer’s disease. Sci Am. 1991;265:68–71. doi: 10.1038/scientificamerican1191-68. [DOI] [PubMed] [Google Scholar]
- 18.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 19.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 22.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Ting JT, Kelley BG, Lambert TJ, Cook DG, Sullivan JM. Amyloid precursor protein overexpression depresses excitatory transmission through both presynaptic and postsynaptic mechanisms. Proc Natl Acad Sci U S A. 2007;104:353–358. doi: 10.1073/pnas.0608807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsden M, Henderson Z, Pearson HA. Modulation of Ca2+ channel currents in primary cultures of rat cortical neurones by amyloid beta protein (1-40) is dependent on solubility status. Brain Res. 2002;956:254–261. doi: 10.1016/s0006-8993(02)03547-3. [DOI] [PubMed] [Google Scholar]
- 28.Plant LD, Boyle JP, Smith IF, Peers C, Pearson HA. The production of amyloid beta peptide is a critical requirement for the viability of central neurons. J Neurosci. 2003;23:5531–5535. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 30.Reinhard C, Hebert SS, De Strooper B. The amyloid-beta precursor protein: integrating structure with biological function. EMBO J. 2005;24:3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selkoe DJ, Yamazaki T, Citron M, Podlisny MB, Koo EH, Teplow DB, Haass C. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- 32.Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurobiol. 2007;82:11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 34.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 35.Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, Checler F, Sisodia SS, Greengard P, Xu H. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc Natl Acad Sci U S A. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 37.Leissring MA. The AbetaCs of Abeta-cleaving proteases. J Biol Chem. 2008;283:29645–29649. doi: 10.1074/jbc.R800022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 40.Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 42.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- 44.Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995;270:9564–9570. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- 45.Enya M, Morishima-Kawashima M, Yoshimura M, Shinkai Y, Kusui K, Khan K, Games D, Schenk D, Sugihara S, Yamaguchi H, Ihara Y. Appearance of sodium dodecyl sulfate-stable amyloid beta-protein (Abeta) dimer in the cortex during aging. Am J Pathol. 1999;154:271–279. doi: 10.1016/s0002-9440(10)65273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawarabayashi T, Shoji M, Younkin LH, Wen-Lang L, Dickson DW, Murakami T, Matsubara E, Abe K, Ashe KH, Younkin SG. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2004;24:3801–3809. doi: 10.1523/JNEUROSCI.5543-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 48.Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR. Morphology and toxicity of Abeta-(1-42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem. 1996;271:20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- 49.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 50.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 52.Shankar GM, Walsh DM. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48–62. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 55.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 56.Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 57.Piccini A, Russo C, Gliozzi A, Relini A, Vitali A, Borghi R, Giliberto L, Armirotti A, D’Arrigo C, Bachi A, Cattaneo A, Canale C, Torrassa S, Saido TC, Markesbery W, Gambetti P, Tabaton M. Beta-amyloid is different in normal aging and in Alzheimer disease. J Biol Chem. 2005;280:34186–34192. doi: 10.1074/jbc.M501694200. [DOI] [PubMed] [Google Scholar]
- 58.Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med. 2003;81:678–699. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 59.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 60.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 61.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32:177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- 63.Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 64.Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 2000;48:913–918. [PubMed] [Google Scholar]
- 65.Lesne S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci. 2005;25:9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooper NM, Turner AJ. The search for alpha-secretase and its potential as a therapeutic approach to Alzheimer s disease. Curr Med Chem. 2002;9:1107–1119. doi: 10.2174/0929867023370121. [DOI] [PubMed] [Google Scholar]
- 67.Lichtenthaler SF, Haass C. Amyloid at the cutting edge: activation of alpha-secretase prevents amyloidogenesis in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1384–1387. doi: 10.1172/JCI21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 69.Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 70.Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J Cell Sci. 1996;109:991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- 71.Carey RM, Balcz BA, Lopez-Coviella I, Slack BE. Inhibition of dynamin-dependent endocytosis increases shedding of the amyloid precursor protein ectodomain and reduces generation of amyloid beta protein. BMC Cell Biol. 6:30–40. doi: 10.1186/1471-2121-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deshpande A, Kawai H, Metherate R, Glabe CG, Busciglio J. A role for synaptic zinc in activity-dependent Abeta oligomer formation and accumulation at excitatory synapses. J Neurosci. 2009;29:4004–4015. doi: 10.1523/JNEUROSCI.5980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 76.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu Rev Neurosci. 2000;23:217–247. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]
- 78.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 80.Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol. 2006;575:5–10. doi: 10.1113/jphysiol.2006.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- 82.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O’Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, Van der Ploeg LH, Sirinathsinghji DJ. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- 86.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, III, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 88.Rozmahel R, Huang J, Chen F, Liang Y, Nguyen V, Ikeda M, Levesque G, Yu G, Nishimura M, Mathews P, Schmidt SD, Mercken M, Bergeron C, Westaway D, George-Hyslop P. Normal brain development in PS1 hypomorphic mice with markedly reduced gamma-secretase cleavage of betaAPP. Neurobiol Aging. 2002;23:187–194. doi: 10.1016/s0197-4580(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 89.Phinney AL, Drisaldi B, Schmidt SD, Lugowski S, Coronado V, Liang Y, Horne P, Yang J, Sekoulidis J, Coomaraswamy J, Chishti MA, Cox DW, Mathews PM, Nixon RA, Carlson GA, George-Hyslop P, Westaway D. In vivo reduction of amyloid-beta by a mutant copper transporter. Proc Natl Acad Sci U S A. 2003;100:14193–14198. doi: 10.1073/pnas.2332851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pawlik M, Sastre M, Calero M, Mathews PM, Schmidt SD, Nixon RA, Levy E. Overexpression of human cystatin C in transgenic mice does not affect levels of endogenous brain amyloid Beta Peptide. J Mol Neurosci. 2004;22:13–18. doi: 10.1385/JMN:22:1-2:13. [DOI] [PubMed] [Google Scholar]
- 91.Mastrangelo P, Mathews PM, Chishti MA, Schmidt SD, Gu Y, Yang J, Mazzella MJ, Coomaraswamy J, Horne P, Strome B, Pelly H, Levesque G, Ebeling C, Jiang Y, Nixon RA, Rozmahel R, Fraser PE, George-Hyslop P, Carlson GA, Westaway D. Dissociated phenotypes in presenilin transgenic mice define functionally distinct gamma-secretases. Proc Natl Acad Sci U S A. 2005;102:8972–8977. doi: 10.1073/pnas.0500940102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wasling P, Daborg J, Riebe I, Andersson M, Portelius E, Blennow K, Hanse E, Zetterberg H. Synaptic retrogenesis and amyloid-beta in Alzheimer’s disease. J Alzheimers Dis. 2009;16:1–14. doi: 10.3233/JAD-2009-0918. [DOI] [PubMed] [Google Scholar]
- 93.Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zou K, Gong JS, Yanagisawa K, Michikawa M. A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J Neurosci. 2002;22:4833–4841. doi: 10.1523/JNEUROSCI.22-12-04833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whitson JS, Selkoe DJ, Cotman CW. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989;243:1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- 96.Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, Dickson DW, Golde T, McGowan E. Abeta40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 99.Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- 100.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 101.Sugano T, Yanagita T, Yokoo H, Satoh S, Kobayashi H, Wada A. Enhancement of insulin-induced PI3K/Akt/GSK-3beta and ERK signaling by neuronal nicotinic receptor/PKC-alpha/ERK pathway: up-regulation of IRS-1/-2 mRNA and protein in adrenal chromaffin cells. J Neurochem. 2006;98:20–33. doi: 10.1111/j.1471-4159.2006.03846.x. [DOI] [PubMed] [Google Scholar]
- 102.Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- 103.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 104.Lee HK, Kumar P, Fu Q, Rosen KM, Querfurth HW. The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell. 2009;20:1533–1544. doi: 10.1091/mbc.E08-07-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- 106.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 107.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 108.Wei W, Wang X, Kusiak JW. Signaling events in amyloid beta-peptide-induced neuronal death and insulin-like growth factor I protection. J Biol Chem. 2002;277:17649–17656. doi: 10.1074/jbc.M111704200. [DOI] [PubMed] [Google Scholar]
- 109.Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22:RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 111.Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- 112.Izzo E, Martin-Fardon R, Koob GF, Weiss F, Sanna PP. Neural plasticity and addiction: PI3-kinase and cocaine behavioral sensitization. Nat Neurosci. 2002;5:1263–1264. doi: 10.1038/nn977. [DOI] [PubMed] [Google Scholar]
- 113.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 114.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 115.Asano T, Yao Y, Shin S, McCubrey J, Abbruzzese JL, Reddy SA. Insulin receptor substrate is a mediator of phosphoinositide 3-kinase activation in quiescent pancreatic cancer cells. Cancer Res. 2005;65:9164–9168. doi: 10.1158/0008-5472.CAN-05-0779. [DOI] [PubMed] [Google Scholar]
- 116.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 117.Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 119.Zheng WH, Kar S, Quirion R. Insulin-like growth factor-1-induced phosphorylation of the forkhead family transcription factor FKHRL1 is mediated by Akt kinase in PC12 cells. J Biol Chem. 2000;275:39152–39158. doi: 10.1074/jbc.M002417200. [DOI] [PubMed] [Google Scholar]
- 120.Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 121.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 122.Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 123.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 124.Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 127.Abe K, Hisatomi R, Misawa M. Amyloid beta peptide specifically promotes phosphorylation and nuclear translocation of the extracellular signal-regulated kinase in cultured rat cortical astrocytes. J Pharmacol Sci. 2003;93:272–278. doi: 10.1254/jphs.93.272. [DOI] [PubMed] [Google Scholar]
- 128.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 129.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 130.Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 131.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 132.Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 133.Lever IJ, Pezet S, McMahon SB, Malcangio M. The signaling components of sensory fiber transmission involved in the activation of ERK MAP kinase in the mouse dorsal horn. Mol Cell Neurosci. 2003;24:259–270. doi: 10.1016/s1044-7431(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 134.Blenis J. Signal transduction via the MAP kinases: Proceed at your own RSK. Proc Natl Acad Sci U S A. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 136.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 137.McDonald DR, Bamberger ME, Combs CK, Landreth GE. beta-Amyloid fibrils activate parallel mitogen-activated protein kinase pathways in microglia and THP1 monocytes. J Neurosci. 1998;18:4451–4460. doi: 10.1523/JNEUROSCI.18-12-04451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 139.Alkon DL, Rasmussen H. A spatial-temporal model of cell activation. Science. 1988;239:998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- 140.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 141.Cole G, Dobkins KR, Hansen LA, Terry RD, Saitoh T. Decreased levels of protein kinase C in Alzheimer brain. Brain Res. 1988;452:165–174. doi: 10.1016/0006-8993(88)90021-2. [DOI] [PubMed] [Google Scholar]
- 142.Bhagavan S, Ibarreta D, Ma D, Kozikowski AP, Etcheberrigaray R. Restoration of TEA-induced calcium responses in fibroblasts from Alzheimer’s disease patients by a PKC activator. Neurobiol Dis. 1998;5:177–187. doi: 10.1006/nbdi.1998.0195. [DOI] [PubMed] [Google Scholar]
- 143.Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van dA I, Wera S, Qiao L, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci U S A. 2004;101:11141–11146. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garrido JL, Godoy JA, Alvarez A, Bronfman M, Inestrosa NC. Protein kinase C inhibits amyloid beta peptide neurotoxicity by acting on members of the Wnt pathway. FASEB J. 2002;16:1982–1984. doi: 10.1096/fj.02-0327fje. [DOI] [PubMed] [Google Scholar]
- 145.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 146.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 147.Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- 149.Zhang SJ, Zou M, Lu L, Lau D, Ditzel DA, Delucinge-Vivier C, Aso Y, Descombes P, Bading H. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 2009;5:e1000604. doi: 10.1371/journal.pgen.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 151.Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and Abeta toxicity: from top to bottom. Nat Rev Neurosci. 2001;2:595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- 152.Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta(1–42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J Neurochem. 2000;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- 153.Dougherty JJ, Wu J, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 155.Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- 156.Ramsden M, Plant LD, Webster NJ, Vaughan PF, Henderson Z, Pearson HA. Differential effects of unaggregated and aggregated amyloid beta protein (1-40) on K(+) channel currents in primary cultures of rat cerebellar granule and cortical neurones. J Neurochem. 2001;79:699–712. doi: 10.1046/j.1471-4159.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 157.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 158.Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gass P, Riva MA. CREB, neurogenesis and depression. Bioessays. 2007;29:957–961. doi: 10.1002/bies.20658. [DOI] [PubMed] [Google Scholar]
- 160.Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bayly R, Chuen L, Currie RA, Hyndman BD, Casselman R, Blobel GA, LeBrun DP. E2A-PBX1 interacts directly with the KIX domain of CBP/p300 in the induction of proliferation in primary hematopoietic cells. J Biol Chem. 2004;279:55362–55371. doi: 10.1074/jbc.M408654200. [DOI] [PubMed] [Google Scholar]
- 162.Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 163.Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- 164.Yamamoto-Sasaki M, Ozawa H, Saito T, Rosler M, Riederer P. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res. 1999;824:300–303. doi: 10.1016/s0006-8993(99)01220-2. [DOI] [PubMed] [Google Scholar]
- 165.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 166.Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 167.Yao ZX, Papadopoulos V. Function of beta-amyloid in cholesterol transport: a lead to neurotoxicity. FASEB J. 2002;16:1677–1679. doi: 10.1096/fj.02-0285fje. [DOI] [PubMed] [Google Scholar]
- 168.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 169.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 170.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer’s disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 172.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 173.Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 174.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 175.Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 176.Brewer GJ, Boehler MD, Jones TT, Wheeler BC. NbActiv4 medium improvement to Neurobasal/B27 increases neuron synapse densities and network spike rates on multielectrode arrays. J Neurosci Methods. 2008;170:181–187. doi: 10.1016/j.jneumeth.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nakamura M, Shishido N, Nunomura A, Smith MA, Perry G, Hayashi Y, Nakayama K, Hayashi T. Three histidine residues of amyloid-beta peptide control the redox activity of copper and iron. Biochemistry. 2007;46:12737–12743. doi: 10.1021/bi701079z. [DOI] [PubMed] [Google Scholar]
- 178.Curtain CC, Ali F, Volitakis I, Cherny RA, Norton RS, Beyreuther K, Barrow CJ, Masters CL, Bush AI, Barnham KJ. Alzheimer’s disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J Biol Chem. 2001;276:20466–20473. doi: 10.1074/jbc.M100175200. [DOI] [PubMed] [Google Scholar]
- 179.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 180.Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- 182.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 183.Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 184.Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 185.Ferrer I, Gullotta F. Down’s syndrome and Alzheimer’s disease: dendritic spine counts in the hippocampus. Acta Neuropathol. 1990;79:680–685. doi: 10.1007/BF00294247. [DOI] [PubMed] [Google Scholar]
- 186.Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol. 2004;33:377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- 187.Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sze C, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. N-Methyl-D-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer’s disease. J Neurol Sci. 2001;182:151–159. doi: 10.1016/s0022-510x(00)00467-6. [DOI] [PubMed] [Google Scholar]
- 190.Mishizen-Eberz AJ, Rissman RA, Carter TL, Ikonomovic MD, Wolfe BB, Armstrong DM. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer’s disease pathology. Neurobiol Dis. 2004;15:80–92. doi: 10.1016/j.nbd.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 191.Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer’s disease. J Neurosci Res. 1996;43:87–92. doi: 10.1002/jnr.490430111. [DOI] [PubMed] [Google Scholar]
- 192.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 194.Moreno H, Yu E, Pigino G, Hernandez AI, Kim N, Moreira JE, Sugimori M, Llinas RR. Synaptic transmission block by presynaptic injection of oligomeric amyloid beta. Proc Natl Acad Sci U S A. 2009;106:5901–5906. doi: 10.1073/pnas.0900944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 198.Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 199.Varghese K, Molnar P, Das M, Bhargava N, Lambert S, Kindy MS, Hickman JJ. A new target for amyloid beta toxicity validated by standard and high-throughput electrophysiology. PLoS One. 2010;5:e8643. doi: 10.1371/journal.pone.0008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer’s beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mokin M, Lindahl JS, Keifer J. Immediate-early gene-encoded protein Arc is associated with synaptic delivery of GluR4-containing AMPA receptors during in vitro classical conditioning. J Neurophysiol. 2006;95:215–224. doi: 10.1152/jn.00737.2005. [DOI] [PubMed] [Google Scholar]
- 203.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 205.Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci U S A. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291:F840–F855. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 208.Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66:S74–S78. doi: 10.1212/01.wnl.0000192103.24796.42. [DOI] [PubMed] [Google Scholar]
- 209.Snow AD, Kinsella MG, Parks E, Sekiguchi RT, Miller JD, Kimata K, Wight TN. Differential binding of vascular cell-derived proteoglycans (perlecan, biglycan, decorin, and versican) to the beta-amyloid protein of Alzheimer’s disease. Arch Biochem Biophys. 1995;320:84–95. doi: 10.1006/abbi.1995.1345. [DOI] [PubMed] [Google Scholar]
- 210.Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 211.Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 212.Gu Z, Liu W, Yan Z. {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem. 2009;284:10639–10649. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hu NW, Klyubin I, Anwy R, Rowan MJ. GluN2B subunit-containing NMDA receptor antagonists prevent Abeta-mediated synaptic plasticity disruption in vivo. Proc Natl Acad Sci U S A. 2009;106:20504–20509. doi: 10.1073/pnas.0908083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 215.Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets. 2007;8:621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- 216.Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 217.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 218.Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P, Morabito M, Almeida OF. Soluble beta-amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J Neurosci. 2005;25:11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Domingues A, Almeida S, Da Cruz e Silva EF, Oliveira CR, Rego AC. Toxicity of beta-amyloid in HEK293 cells expressing NR1/NR2A or NR1/NR2B N-methyl-D-aspartate receptor subunits. Neurochem Int. 2007;50:872–880. doi: 10.1016/j.neuint.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 221.Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- 222.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 223.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 224.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 225.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kawamoto EM, Lepsch LB, Boaventura MF, Munhoz CD, Lima LS, Yshii LM, Avellar MC, Curi R, Mattson MP, Scavone C. Amyloid beta-peptide activates nuclear factor-kappaB through an N-methyl-D-aspartate signaling pathway in cultured cerebellar cells. J Neurosci Res. 2008;86:845–860. doi: 10.1002/jnr.21548. [DOI] [PubMed] [Google Scholar]
- 227.Nakamura S, Murayama N, Noshita T, Katsuragi R, Ohno T. Cognitive dysfunction induced by sequential injection of amyloid-beta and ibotenate into the bilateral hippocampus; protection by memantine and MK-801. Eur J Pharmacol. 2006;548:115–122. doi: 10.1016/j.ejphar.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 228.Coan EJ, Irving AJ, Collingridge GL. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci Lett. 1989;105:205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- 229.Zorumski CF, Izumi Y. Modulation of LTP induction by NMDA receptor activation and nitric oxide release. Prog Brain Res. 1998;118:173–182. doi: 10.1016/s0079-6123(08)63207-0. [DOI] [PubMed] [Google Scholar]
- 230.Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- 231.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 232.Xiao MY, Zhou Q, Nicoll RA. Metabotropic glutamate receptor activation causes a rapid redistribution of AMPA receptors. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 233.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 234.Kaminsky YG, Marlatt MW, Smith MA, Kosenko EA. Subcellular and metabolic examination of amyloid-beta peptides in Alzheimer disease pathogenesis: evidence for Abeta (25–35) Exp Neurol. 2010;221:26–37. doi: 10.1016/j.expneurol.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 235.Inestrosa NC, Varela-Nallar L, Grabowski CP, Colombres M. Synaptotoxicity in Alzheimer’s disease: the Wnt signaling pathway as a molecular target. IUBMB Life. 2007;59:316–321. doi: 10.1080/15216540701242490. [DOI] [PubMed] [Google Scholar]
- 236.Magdesian MH, Carvalho MM, Mendes FA, Saraiva LM, Juliano MA, Juliano L, Garcia-Abreu J, Ferreira ST. Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J Biol Chem. 2008;283:9359–9368. doi: 10.1074/jbc.M707108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 238.Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- 239.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 240.Genzen JR, McGehee DS. Short- and long-term enhancement of excitatory transmission in the spinal cord dorsal horn by nicotinic acetylcholine receptors. Proc Natl Acad Sci U S A. 2003;100:6807–6812. doi: 10.1073/pnas.1131709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Girod R, Barazangi N, McGehee D, Role LW. Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology. 2000;39:2715–2725. doi: 10.1016/s0028-3908(00)00145-3. [DOI] [PubMed] [Google Scholar]
- 242.Hu M, Waring JF, Gopalakrishnan M, Li J. Role of GSK-3beta activation and alpha7 nAChRs in Abeta(1-42)-induced tau phosphorylation in PC12 cells. J Neurochem. 2008;106:1371–1377. doi: 10.1111/j.1471-4159.2008.05483.x. [DOI] [PubMed] [Google Scholar]
- 243.Dineley KT, Bell KA, Bui D, Sweatt JD. beta -Amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- 244.Dougherty JJ, Wu J, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pettit DL, Shao Z, Yakel JL. beta-Amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci. 2001;21(1–5):RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wu J, Kuo YP, George AA, Xu L, Hu J, Lukas RJ. beta-Amyloid directly inhibits human alpha4beta2-nicotinic acetylcholine receptors heterologously expressed in human SH-EP1 cells. J Biol Chem. 2004;279:37842–37851. doi: 10.1074/jbc.M400335200. [DOI] [PubMed] [Google Scholar]
- 247.Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 248.Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learn Mem. 2009;16:267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Small DH, Maksel D, Kerr ML, Ng J, Hou X, Chu C, Mehrani H, Unabia S, Azari MF, Loiacono R, Aguilar MI, Chebib M. The beta-amyloid protein of Alzheimer’s disease binds to membrane lipids but does not bind to the alpha7 nicotinic acetylcholine receptor. J Neurochem. 2007;101:1527–1538. doi: 10.1111/j.1471-4159.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 250.Liu Q, Kawai H, Berg DK. beta -Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci U S A. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 254.Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- 255.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 256.Muirhead KE, Borger E, Aitken L, Conway SJ, Gunn-Moore FJ. The consequences of mitochondrial amyloid beta-peptide in Alzheimer’s disease. Biochem J. 2010;426:255–270. doi: 10.1042/BJ20091941. [DOI] [PubMed] [Google Scholar]
- 257.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Du H, Yan SS. Mitochondrial permeability transition pore in Alzheimer’s disease: cyclophilin D and amyloid beta. Biochim Biophys Acta. 2010;1802:198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Singh P, Suman S, Chandna S, Das TK. Possible role of amyloid-beta, adenine nucleotide translocase and cyclophilin-D interaction in mitochondrial dysfunction of Alzheimer’s disease. Bioinformation. 2009;3:440–445. doi: 10.6026/97320630003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schubert A, Grimm S. Cyclophilin D, a component of the permeability transition-pore, is an apoptosis repressor. Cancer Res. 2004;64:85–93. doi: 10.1158/0008-5472.can-03-0476. [DOI] [PubMed] [Google Scholar]
- 261.Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A. 2009;106:20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ren Y, Xu HW, Davey F, Taylor M, Aiton J, Coote P, Fang F, Yao J, Chen D, Chen JX, Yan SD, Gunn-Moore FJ. Endophilin I expression is increased in the brains of Alzheimer disease patients. J Biol Chem. 2008;283:5685–5691. doi: 10.1074/jbc.M707932200. [DOI] [PubMed] [Google Scholar]
- 263.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 264.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Alikhani N, Ankarcrona M, Glaser E. Mitochondria and Alzheimer’s disease: amyloid-beta peptide uptake and degradation by the presequence protease, hPreP. J Bioenerg Biomembr. 2009;41:447–451. doi: 10.1007/s10863-009-9244-4. [DOI] [PubMed] [Google Scholar]
- 266.Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 267.Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, Fang F, Chen JX, Yan SD, Gunn-Moore FJ. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer’s disease patients and a transgenic Alzheimer’s disease mouse model. Mol Cell Neurosci. 2007;35:377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 269.Rizzuto R. Calcium mobilization from mitochondria in synaptic transmitter release. J Cell Biol. 2003;163:441–443. doi: 10.1083/jcb.200309111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 271.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 273.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mungarro-Menchaca X, Ferrera P, Moran J, Arias C. beta-Amyloid peptide induces ultrastructural changes in synaptosomes and potentiates mitochondrial dysfunction in the presence of ryanodine. J Neurosci Res. 2002;68:89–96. doi: 10.1002/jnr.10193. [DOI] [PubMed] [Google Scholar]
- 275.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 277.Thiels E, Klann E. Hippocampal memory and plasticity in superoxide dismutase mutant mice. Physiol Behav. 2002;77:601–605. doi: 10.1016/s0031-9384(02)00900-9. [DOI] [PubMed] [Google Scholar]
- 278.Parihar MS, Brewer GJ. Simultaneous age-related depolarization of mitochondrial membrane potential and increased mitochondrial reactive oxygen species production correlate with age-related glutamate excitotoxicity in rat hippocampal neurons. J Neurosci Res. 2007;85:1018–1032. doi: 10.1002/jnr.21218. [DOI] [PubMed] [Google Scholar]
- 279.Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J Neurosci Res. 2008;86:2339–2352. doi: 10.1002/jnr.21679. [DOI] [PubMed] [Google Scholar]
- 280.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 282.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 283.Butterfield DA. Amyloid beta-peptide [1-42]-associated free radical-induced oxidative stress and neurodegeneration in Alzheimer’s disease brain: mechanisms and consequences. Curr Med Chem. 2003;10:2651–2659. doi: 10.2174/0929867033456422. [DOI] [PubMed] [Google Scholar]
- 284.Mattson MP. Metal-catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders. Ann N Y Acad Sci. 2004;1012:37–50. doi: 10.1196/annals.1306.004. [DOI] [PubMed] [Google Scholar]
- 285.Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 286.Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J. 2001;15:1439–1441. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- 287.Brewer GJ. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol. 2010;45:173–179. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Abramov AY, Duchen MR. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta. 2008;1777:953–964. doi: 10.1016/j.bbabio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 289.Jurgensen S, Ferreira ST. Nicotinic receptors, amyloid-beta, and synaptic failure in Alzheimer’s disease. J Mol Neurosci. 2010;40:221–229. doi: 10.1007/s12031-009-9237-0. [DOI] [PubMed] [Google Scholar]
- 290.Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]