Abstract
IL-10 is an important regulatory cytokine which can modulate excessive immune mediated injury. Several distinct cell types have been demonstrated to produce IL-10 including most recently CD8+ cytotoxic T lymphocytes (CTL) responding to respiratory virus infection. Here we report that CD4+ T cell help in the form of IL-2 is required for IL-10 production by CTL, but not for the induction of CTL effector cytokines. We show that IL-2 derived from CD4+ TH cells cooperates with innate-derived IL-27 to amplify IL-10 production by CTL through a Blimp-1 dependent mechanism. These findings reveal a previously unrecognized pathway that coordinates signals derived from both innate and TH cells to control the production of a regulatory cytokine by CTL during acute viral infection.
IL-10 is an important anti-inflammatory cytokine that can suppress both innate and adaptive immune responses to infectious agents or auto-antigens1,2. Certain pathogens exploit IL-10 to suppress the host response and establish chronic infection2. However, during acute infections that induce strong inflammatory responses, IL-10 often acts beneficially to moderate excessive inflammation2.
While various innate and adaptive immune cell types have been identified as IL-10 producers3, there is increasing evidence that effector T cells are an important source of IL-10 especially during certain parasitic and viral infections4-6. Thus, the cells that often produce or enhance inflammation during infection can regulate their pro-inflammatory activity through IL-10 production7. However, the cellular and molecular mechanisms controlling in vivo IL-10 expression in T cells and particularly in effector T cells are imperfectly understood. In certain regulatory T-cells, stiThatmuli such as, dexamethasone plus vitamin D3, IL-27, IL-21, TGF-β and aryl hydrocarbon receptor (AhR) ligands as well as IL-10 itself promote IL-10 production3,8-12 while IL-27, IL-21 and IL-12 are reported to augment IL-10 production by CD4+ effector T cells13-17. However, despite the multiple signals identified to promote IL-10 production by T cells, the in vivo significance and relative contribution of these signals is poorly defined. Similarly the intracellular signaling intermediates activated by these IL-10 promoting signals in T cells remain under-explored, although STAT3, c-maf and recently AhR have been implicated in controlling IL-10 gene expression in CD4+ T cells3,11,12.
Anti-viral CD8+ CTL are a major producer of IL-10 in the respiratory tract during influenza infection 6. CTL-derived IL-10 plays a critical role in preventing excess inflammation during immune-mediated virus clearance6. In this report, we investigate the signals necessary to drive the production of IL-10 by CTL in the infected respiratory tract. Our data reveals a novel interplay between products of TH cells and infiltrating innate immune cells in shaping the function of anti-viral CTL at the site of infection.
Results
IL-10+ CTL development requires IL-27 and TH cells
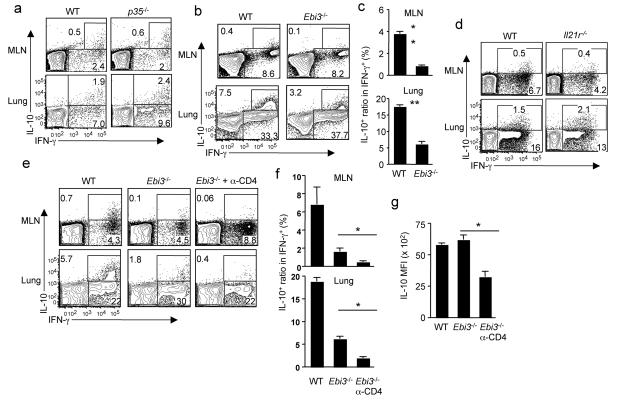
To investigate the mechanisms regulating IL-10 production by anti-viral CTL during influenza infection, we first examined whether interferon-γ (IFN-γ) is required since the IL-10-producing CTL co-express IFN-γ (Supplementary Fig. 1). IFN-γ is dispensable for the induction of IL-10 producing CTL (Supplementary Fig. 1). Similarly, although IL-12 is important to induce IL-10-producing T cells in some settings17, it is not required for the induction of IL-10 producing CTL during influenza infection as CD8+ T cells from p35−/− mice (which lack both IL-12 and IL-3518) can produce IL-10 (Fig. 1a). IL-27 induces the development of IL-10 producing CD4+ T cells3, therefore we examined the role of IL-27 in controlling the induction of IL-10-producing CTL during influenza infection. Influenza infection induces IL-27 gene expression in the respiratory tract (Supplementary Fig. 2). We then infected Ebi3−/− mice (which lack both IL-27 and IL-3518) with influenza and measured IL-10 production by influenza specific CTL. EBI-3 deficiency does not affect the induction of IFN-γ producing CTL, but substantially impairs the induction of IL-10 producing CTL in both draining lymph nodes (i.e. mediastinal lymph nodes, MLN) and the infected lungs (Fig. 1b, c). Consistent with the impaired production of IL-10 by CTL, Ebi3−/− mice exhibited impaired IL-10 release in the airway and enhanced pulmonary inflammation during influenza infection (Supplementary Fig. 3). The induction of IL-10 producing CD4+ T cells by IL-27 is dependent on IL-21 signaling10, however the induction of IL-10 producing CTL during influenza infection is intact in mice lacking IL-21 receptor (Fig. 1d).
Figure 1. Induction of IL-10 producing CTL in vivo requires IL-27 and CD4+ T cells.
(a) WT or p35−/− mice were infected with influenza. At d7 p.i., the production of IL-10 and IFN-γ by CTL from MLN or lungs was measured by ICS. (b, c) WT or Ebi3−/− mice were infected with influenza. At d7 p.i., the production of IL-10 and IFN-γ by CTL from MLN or lungs was measured by ICS. (c) The normalized percentages of IL-10+ cells in influenza-specific CTL (IFN-γ+) from MLN or lungs of infected WT and Ebi3−/− mice are depicted. (d) WT or Il21r−/− mice were infected with influenza. At d7 p.i., the production of IL-10 and IFN-γ by CTL from MLN or lungs was measured by ICS. (e, f, g) WT, Ebi3−/− or CD4+ T cell-depleted (α-CD4) Ebi3−/− mice were infected with influenza. At d7 p.i., the production of IL-10 and IFN-γ by CTL from MLN or lungs was measured by ICS. (f) The normalized percentages of IL-10+ cells in influenza-specific CTL (IFN-γ+) from MLN or lungs of WT and Ebi3−/− mice are depicted. (g) The mean fluorescence intensity (MFI) of IL-10 in IL-10+ cells from infected lungs is depicted. Numbers are the percentages of cells in gated population. *, P <= 0.05; **, P <= 0.01. Data are from one experiment, but are typical of those obtained from at least two.
The absence of IL-27 did not completely abrogate IL-10 producing CTL, suggesting additional signals contribute to the induction of IL-10 producing CTL. Release of IL-10 in vivo is dependent on both CD8+ and CD4+ T cells6, suggesting a possible role of CD4+ T cells in the induction of IL-10 producing CTL. To this end, we have eliminated both IL-27 and CD4+ T cells in vivo and examined the induction of IL-10 producing CTL following influenza infection. Depletion of CD4+ T cells, together with EBI-3 deficiency, almost completely abrogated the induction of IL-10 producing CTL (Fig. 1e, f). Elimination of CD4+ T cells and IL-27 also compromised the production of IL-10 at the single cell level (Fig. 1g) in the few remaining IL-10+ CTL (Fig. 1g). Taken together, these data suggests that the induction of IL-10 producing CTL during influenza infection is dependent on the presence of both IL-27 and TH cells, but is independent of IL-12, IL-21, IL-35 and IFN-γ.
TH cells selectively induce IL-10+ CTL development
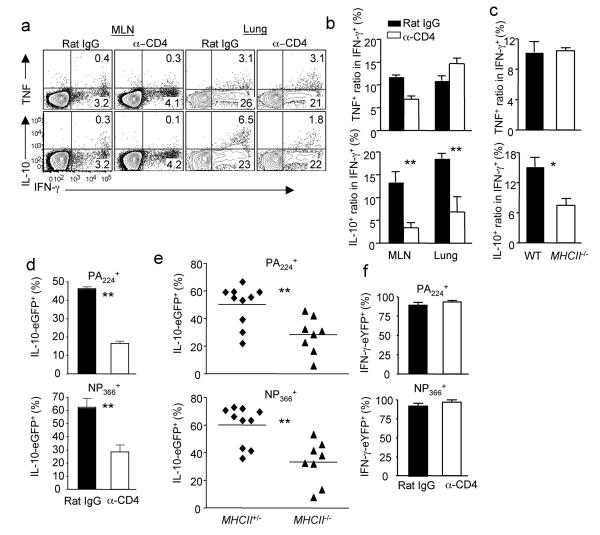
The finding that the induction of IL-10 producing CTL in vivo requires the presence of CD4+ T cells raised the possibility that CD4+ T cell “help” is required for the physiologic CTL responses to influenza infection. We therefore examined the role of CD4+ T cells in the accumulation and differentiation of CTL in the respiratory tract during influenza infection. The depletion of CD4+ T cells severely impairs the production of IL-10, but not IFN-γ nor tumor necrosis factor (TNF) by CTL (Fig. 2a, b). These data agree with reported findings19,20 and suggest that CD4+ T cell “help” is minimally required for the differentiation and accumulation of CTL. CD4+ T cell depletion at various time points impaired the development of IL-10 producing CTL even at d 6 p.i., i.e. when T cells have entered the infected lungs, suggesting that CD4+ T cells are able to help CD8+ T cells in the lung as well as in the MLN (Supplementary Fig. 4).
Figure 2. CD4+ T cell “help” is selectively required for the induction of IL-10 producing CTL in vivo.
(a, b) WT mice were injected with Rat IgG or anti-CD4 (α-CD4) depleting Ab and infected with influenza. At d8 p.i., the production of IL-10, IFN-γ and TNF by CTL from MLN or lungs was measured by ICS. (b) The normalized percentages of IL-10+ or TNF+ cells in influenza-specific CTL (IFN-γ+) from MLN or lungs are depicted. (c) WT or MhcII−/− mice were infected with influenza. At d7 p.i., the normalized percentages of IL-10+ or TNF+ cells in influenza-specific CTL (IFN-γ+) from lungs of WT and MhcII−/− are depicted. (d) Vert-X mice were injected with Rat IgG or anti-CD4 Ab and infected with influenza. At d7 p.i., the percentages of IL-10-eGFP+ cells in influenza-specific PA224 or NP366 tetramer+ cells are depicted. (e) MhcII+/−-Vert-X or MhcII−/−-Vert-X mice were infected with influenza. At d7 p.i., the percentages of IL-10-eGFP+ cells in influenza-specific lung PA224 or NP366 tetramer+ cells are depicted. (f) Yeti mice were injected with Rat IgG or α-CD4 Ab and infected with influenza. At d7 p.i., the percentages of IFN-γ-eYFP+ cells in influenza-specific lung PA224 or NP366 tetramer+ cells are depicted. Numbers are the percentages of cells in gated population. *, P <= 0.05; **, P <= 0.01. (a-d, f) Data are from one experiment, but are typical of three. (e) Data are pooled from total of four experiments.
To further confirm the impact of CD4+ T cell depletion, we infected MhcII−/− mice (which lack CD4+ T cells) with influenza and found that CD8+ T cells from MhcII−/− mice were likewise impaired in IL-10 but not IFN-γ or TNF production (Fig. 2c). The absence of CD4+ T cells only minimally affected the expression of the cytolytic molecule Granzyme B by CTL (Supplementary Fig. 5). We next directly examined the role of CD4+ T cell “help” in the in vivo induction of IL-10 producing CTL by utilizing IL-10 reporter mice (Vert-X)21. IL-10 gene expression in CTL is primarily restricted to the site of infection i.e. the lungs (Supplementary Fig. 6). Importantly, the depletion of CD4+ T cells significantly impaired the development of IL-10-expressing cells among both total lung-infiltrating CD8+ T cells as well as gated influenza antigen specific cells (Fig. 2 d and Supplementary Fig. 6). We also bred Vert-X mice to MhcII−/− mice and infected Vert-X-MhcII−/− mice with influenza. Compared to the influenza-specific CD8+ T cells from littermate Vert-X-MhcII+/− mice, influenza-specific T cells from Vert-X-MhcII−/− mice had diminished IL-10-expressing cells (Fig. 2e) and IL-10 expression intensity at the single cell level (Supplementary Fig. 7). We also verified in vivo that CD4+ T cell help is not required for the induction of IFN-γ-expressing cells using IFN-γ eYFP reporter mice (Yeti) which were constructed similarly to the Vert-X mice (Fig. 2 f and Supplementary Fig. 8)22. Collectively, these data suggest that CD4+ T cell “help” is critical for inducing an optimal anti-inflammatory cytokine profile (i.e. IL-10 production) but not for the activation and differentiation of the effector features of influenza specific CTL in vivo.
IL-2 provides the “help” for IL-10+ CTL development
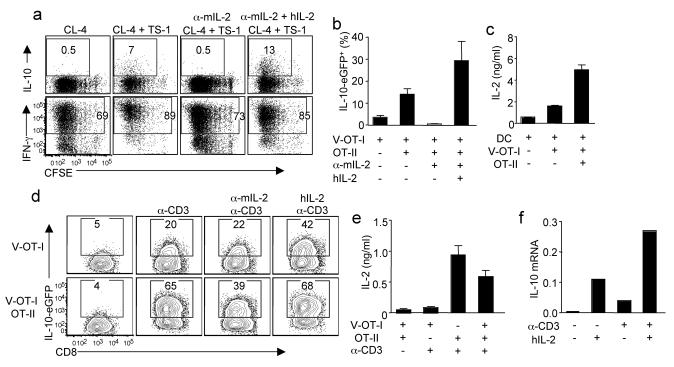
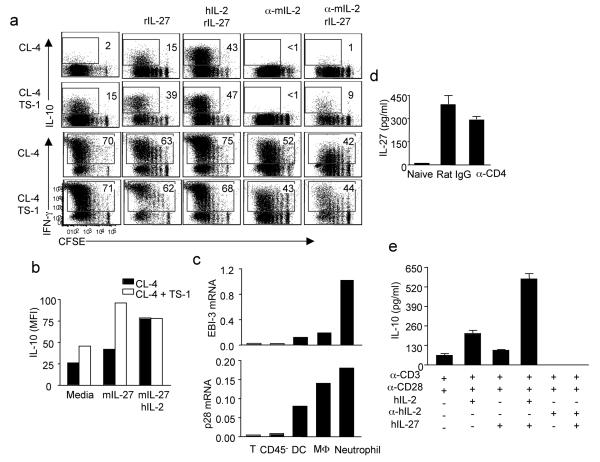
To identify the molecule(s) that mediate the “help” from CD4+ T cells, we developed an in vitro T cell-DC co-culture system. We labeled influenza HA-specific CD8+ T cell receptor (TCR) transgenic T cells (CL-4) with CFSE and stimulated them with influenza-infected DC in the absence or presence HA-specific CD4+ TCR transgenic T cells (TS-1). CL-4 T cells stimulated with influenza-infected DC expanded and expressed IFN-γ but failed to produce IL-10 (Fig. 3a). However, when CD4+ TS-1 cells were included in the culture, CL-4 cells acquired the ability to produce IL-10. Importantly, all IL-10-producing cells were also IFN-γ+ (Supplementary Fig. 9). These data recapitulate our in vivo findings that CD4+ T cells are able to provide the “help” to CD8+ T cells to induce the development of IL-10 producing CTL.
Figure 3. IL-2 provides the “help” from CD4+ T cell to CTL for IL-10 production in vitro.
(a) CFSE labeled CD8+ CL-4 cells were stimulated with influenza-infected DC in the presence or absence of CD4+ TS-1 cells for 4d. The cultured cells were treated with indicated conditions. Then the production of IL-10 and IFN-γ by CL-4 cells were measured through ICS. (b, c) CD8+ Vert-X-OT-I (V-OT-I) cells were stimulated with influenza-OVA-infected DC in the absence or presence of OT-II cells. (b) The percentages of IL-10-eGFP+ cells in V-OT-I cells are depicted. (c) The release of IL-2 into medium after 2d in culture was measured by ELISA. (d, e) V-OT-I or OT-II cells were activated separately by influenza-OVA-infected DC for 4d. The activated V-OT-I cells were unmanipulated or co-cultured with the activated OT-II cells. Cells were then either left unstimulated or stimulated with plate-bound anti-CD3 (α-CD3). The cultured cells were treated as indicated. (d) The expression of IL-10-eGFP by V-OT-I cells after 2d in culture was measured by flow cytometry. (e) The release of IL-2 into medium after overnight in culture was measured by ELISA. (f) OT-I cells were stimulated with influenza-OVA-infected DC. After 4 d in culture, OT-I cells were treated with hIL-2 or hIL-2 plus plate-bound α-CD3 for 4h. The expression of IL-10 was measured by quantitative RT-PCR. Numbers are the percentages of cells in the gated population. Data are from one experiment, but are representatives of at least 3 replicates.
IL-2 is one of the major cytokine produced by CD4+ T cells and has been shown to support expansion and differentiation of CTL23,24. We therefore evaluated the impact of IL-2 on IL-10 expression by CL-4 T-cells in the co-culture system. Neutralization of murine IL-2 completely blocked the induction of IL-10 producing CL-4 CTL in vitro but only modestly affected cell proliferation and IFN-γ production by CTL (Fig. 3a). Importantly, addition of human IL-2 to cultures reversed the effects of mIL-2 neutralization (Fig. 3a). In companion experiments, we purified CD8+ T cells from ovalbumin (OVA)-specific TCR transgenic OT-I-Vert-X double transgenic mice (V-OT-I) and cultured the CD8+ T cells with DC simultaneously infected with recombinant influenza strains expressing MHC I or MHC II OVA epitopes (influenza-ova) in the presence or absence of OVA-specific CD4+ transgenic OT-II T cells. The inclusion of OT-II cells enhanced the development of IL-10-producing V-OT-I CTL which was dependent on IL-2 but independent of IL-6, IL-12 or IL-21 (Fig. 3b and Supplementary Fig. 10a). We further established that CD4+ T cells are the major IL-2 producing cells in the culture (Fig. 3c). IL-2 is therefore likely the primary molecule derived from CD4+ T cells which provides “help” to CD8+ T cells to drive the development of IL-10 producing CTL.
Our finding (Supplementary Fig. 4) that depletion of CD4+ T-cells as late as day 6 p.i. inhibited IL-10 production by CTL in the infected lungs suggested that CD4+ T cell-derived IL-2 may not only act at the induction phase of the CD8+ T cell response in the draining MLN but also on previously activated CTL in the lungs. To further explore this, we purified V-OT-I and OT-II cells and activated the transgenic CD8+ and CD4+ T-cells independently in culture for 4 days and then assessed the impact of IL-2 supplementation and/or activated CD4+ T cell addition on IL-10 production by the V-OT-I CTL (Supplementary Fig. 10b). Antibody-mediated TCR activation of V-OT-I alone modestly upregulated IL-2-independent expression of IL-10 (Fig. 3d, top row). IL-10 expression was further enhanced by the addition of exogenous IL-2 (Fig. 3d, top row). By contrast, co-cultures of previously activated CD8+ and CD4+ T cells resulted in robust IL-10 expression by CTL following TCR cross-linking (Fig. 3d, bottom row) which is inhibited by IL-2 neutralization (Fig. 3d, bottom row). This finding supports the view that activated CD4+ T cells acting via IL- 2 can promote the production of IL-10 by differentiated effector CD8+ T cells (CTL). In keeping with this concept, only effector CD4+ T cells exhibited robust production of IL-2 (Fig. 3e). When these in vitro activated CD8+ CTL were treated with IL-2 alone in short-term culture (4h) IL-2 exposure like TCR engagement can stimulate IL-10 expression by CTL but the IL- 2 effect was more potent when signaling in co-operation with TCR engagement (Fig. 3f), suggesting that IL-2 can directly promote the expression of IL-10 independent of its role in promoting CTL proliferation and survival.
IL-2 signaling is required to induce IL-10+ CTL in vivo
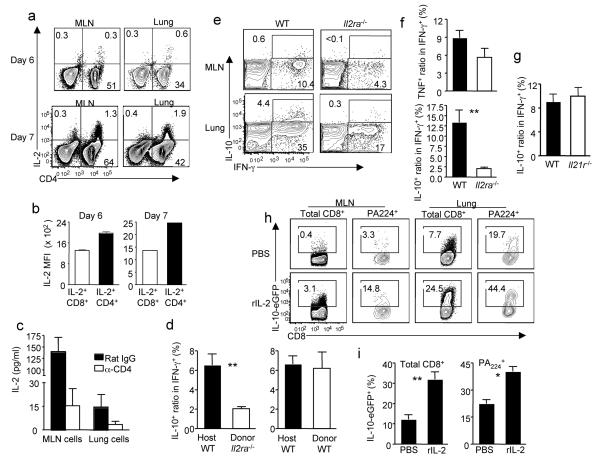
We next assessed the contribution of CD4+ T cell-derived IL- 2 and IL- 2 signaling to the induction of IL-10 production by anti-viral CTL in vivo during influenza infection. We first established in vivo that CD4+ T cells are the dominant source of IL-2 (Fig. 4 a, b and Supplementary Fig. 11a) and the depletion of CD4+ T cells abrogates most IL-2 production in both MLN and the lung (Fig. 4c). Furthermore, the absence of CD4+ T cells impaired IL-10 but not IL-2 production by CD8+ T cells (Supplementary Fig. 11b), suggesting that the amount of IL-2 produced by CD8+ T cells is not sufficient to drive the maximal production of IL-10 by CTL in vivo. In addition, co-transfer of wild-type (WT) CD4+ T cells but not Il2−/− CD4+ T cells with WT CD8+ T cells into Rag2−/− mice resulted in augmented IL-10 production by CTL (albeit at lower levels than observed with CTL from infected WT mice, likely due to non-specific homeostatic proliferation of transferred CD8+ T cells in Rag2−/− recipient), suggesting CD4+ T cells-derived IL-2 is required for the optimal generation of IL-10 producing CTL in vivo (Supplementary Fig. 12). In addition, we determined that IL-10-expressing CTL in the infected lungs are IL-2Rα (CD25)+, indicating that they are competent for IL-2 signaling (Supplementary Fig. 13). To directly establish the requirement for IL-2 receptor signaling, we transferred either WT or Il2ra−/− T cells into Thy1 mis-matched congenic mice and then infected the recipient mice with influenza. While transferred WT CD8+ T cells or endogenous WT CD8+ T cells produced IL-10 in the infected lungs, transfered Il2ra−/− CD8+ T cells failed to produce IL-10 during influenza infection (Fig. 4d). We also made a WT:Il2ra−/− mixed bone marrow chimera mice and infected these mice with influenza. Il2ra−/− CTL mounted an antigen-specific IFN-γ response which was moderately diminished compared to WT cells. In contrast, Il2ra−/− CTL failed to produce IL-10 even when we accounted for the diminished pro-inflammatory cytokine response by normalizing the IL-10 producing cells to antigen specific IFN-γ producing cells (Fig. 4e, f). On the other hand, the Il2ra−/− CTL are able to produce equivalent level of TNF and only slightly diminished level of Granzyme B compared to WT CTL in the lung (Fig. 4f and Supplementary Fig. 14). IL-21 is another important CD4+ T cell derived “helper” cytokine. However Il21r−/− CTL are able to produce IL-10 at levels comparable to WT CTL when influenza infection was carried out in the WT: Il21r−/− mixed bone marrow chimera mice (Fig. 4g). These data collectively demonstrated that IL-2 (rather than IL-21) is the major CD4+ T cell-derived molecule driving the development of IL-10-producing CTL during influenza infection. Furthermore, rIL-2 administration to influenza infected CD4+ T cell-depleted mice at the time of effector T cell infiltration into the lung i.e. d5- 6 p.i. enhanced the induction of IL-10 production by CTL (Fig. 4 h, i). Taken together, these data strongly suggest a critical role for IL-2 and IL-2R dependent signaling in stimulating IL-10 production by CTL at the site of virus infection.
Figure 4. IL-2 is required for the induction IL-10-producing CTL in vivo.
(a, b). IL-2 production by gated Thy1+ cells from influenza infected lung is measured by ICS. (a) IL-2 production by Thy1+CD4+ and Thy1+CD4− cells depicted (b) with the indicated IL-2 MFI (c) IL-2 production by cultured MLN and lung cells from influenza infected mice treated with indicated antibodies is determined by ELISA. (d) Following transfer of WT or Il2ra−/− T cells into Thy1.1 mice and influenza infection, the normalized percentages of IL-10+ cells among influenza-specific CTL (IFN-γ+) from infected lungs at d8 p.i. is determined. (e, f) WT:Il2ra−/− chimeric mice were infected with influenza. At d7 p.i., the production of IL-10, TNF and IFN-γ by CTL was measured by ICS. (f) The normalized percentages of IL-10+ or TNF+ cells among lung influenza-specific CTL (IFN-γ+) are depicted. (g) Following infection of WT:Il2ra−/− chimeric mice the normalized percentages of lung IL-10+ cells among specific IFN-γ+ CD8+ T cells at d7 p.i., are depicted. (h, i) Following α-CD4 Ab treatment and influenza infection Vert-X mice received rmIL-2 or PBS at d5 and 6 p.i.,. The expression of IL-10-eGFP by CTL was measured by flow cytometry. (i) At d7 p.i, the percentages of IL-10-eGFP+ cells among gated CTL are depicted. Numbers represent percentages of cells in gated population. *, P <= 0.05; **, P <= 0.01. Data are representative of at least two experiments.
IL-2 and IL-27 cooperate to induce IL-10+ CTL
Our analysis so far indicates that both CD4+ T- cell-derived IL-2 as well as the cytokine IL-27 are necessary for the development of IL-10 producing CTL during influenza infection. Next, we sought to determine if IL-27 and CD4+ T cell-derived IL-2 act synergistically to activate IL-10 gene expression by CTL. To this end, we again co-cultured CFSE labeled CL-4 cells with influenza-infected DC in the presence or absence of TS-1 cells. Inclusion of TS-1 cells or IL-27 alone in the culture enhanced the induction of IL-10 producing CTL (Fig. 5a). However, the presence either of TS-1 cells plus IL-27 or IL-2 plus IL-27 together in co-cultured with the CD8+ T cells had a synergistic effect on the induction of IL-10 producing CTL (Fig. 5a). Likewise, the synergy between IL-27 and CD4+ T cells was dependent primarily on the CD4+ T cell-derived IL-2 (Fig. 5a). Although CD4+ T cells are the major source of IL-2, CD8+ T cells are capable of producing a small amount of IL-2 during activation (Fig. 3c). This small amount of IL-2 produced by CD8+ T cells alone was insufficient to drive the development of IL-10 producing CTL. However, in the absence of CD4+ T cell derived IL-2, the small amount of CTL derived IL-2 is essential for enhancing the effect of IL-27 on induction of IL-10 producing CTL (Fig. 5a), suggesting that the induction of IL-10 producing CTL by IL-27 likewise requires the presence of IL- 2. The synergy between IL-27 and CD4+ T cell -derived IL-2 is also evident at the single cell level (Fig. 5b). Furthermore, IL-27 and IL-2 act synergistically to induce IL-10 producing CTL in the OT-I & OT-II culture system during the initial (primary) activation (Supplementary Fig. 15) or at the effector stage (Supplementary Fig. 16).
Figure 5. IL-2 and IL-27 synergistically induce IL-10 production by both murine and human CTL.
(a, b) CFSE labeled CD8+ CL-4 cells were stimulated with influenza-infected DC in the presence or absence of CD4+ TS-1 cells for 4d. The cultured cells were treated with indicated conditions. Then the production of IL-10 and IFN-γ by CL-4 cells were measured through ICS. (b) The MFI of IL-10 in IL-10+ cells is depicted. (c) WT mice were infected with influenza and various cell types of the infected lungs were sorted out as described in methods. The expression of IL-27 EBI-3 and p28 subunits was measured through quantitative RT-PCR. (d) WT mice were injected with Rat IgG or α-CD4 Ab and infected with influenza. At d6 p.i., the levels of IL-27 p28 in BALF were measured through ELISA. (e) Purified human CD8+ T cells were stimulated with α-CD3 plus α-CD28 with indicated conditions for 3d. Then the levels of IL-10 in the medium were measured by ELISA. Numbers are the percentages of cells in gated population. (a-d) Data are representative of at least three separate experiments. (e) Data are representative of at least two separate experiments employing an additional donor.
Having established the contribution of CD4+ T cells acting via IL-2 to stimulate IL-10 expression by CD8+ T cells we wanted to probe the source(s) of the IL-27 in vivo. Innate immune cells in the infected lungs are the main source of IL-27 (Fig. 5c). In this regard it is noteworthy that neutrophils may be a major source of IL-27. Interestingly, consistent with the finding that production of IL-10 by CTL is largely restricted to the site of infection, potential IL-27 producing cells including neutrophils and monocytes/macrophages as well as IL-27 mRNA were enriched at the infection site compared to the draining MLN (Supplementary Fig. 17). Furthermore, CD4+ cell depletion did not affect IL-27 production in vivo (Fig. 5d). This confirms that the impaired induction of IL-10 producing CTL in CD4+ T cell-depleted mice is not due to the lack of IL-27. IL-27 deficiency also did not impair IL-2 production by T cells (Supplementary Fig. 18), suggesting that the impaired induction of IL-10 producing CTL in IL-27-deficient mice is not due to a lack of IL-2 production. Taken together, these data demonstrated that innate cell-derived IL-27 and CD4+ T cell derived IL-2 are independently regulated but act in a coordinate manner to induce IL-10 production by CTL in the respiratory tract during influenza infection.
The unexpected finding of cooperation between these two cytokines in the murine system prompted us to investigate whether IL-2 and IL-27 likewise act co-operatively to induce IL-10 production by human CD8+ T cells. IL-2 and IL-27 co-operatively induces both IL-10 mRNA expression and IL-10 protein production by CD8+ T cells (Supplementary Fig. 19 and Fig. 5e).
Induction of IL-10 producing CTL require Blimp-1
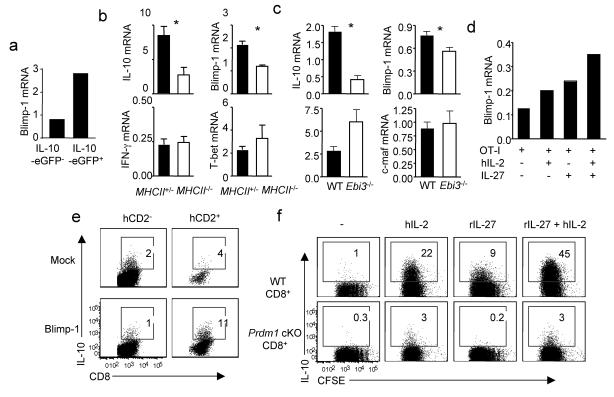
To begin to define the signaling pathways within CTL controlling the induction of IL-10 gene expression, we investigated transcription factors potentially implicated in controlling IL-10 production by CTL. IL-10+ cells expressed higher levels of the transcriptional regulator Blimp-1 than IL-10− CD8+ T cells (Fig. 6a). Blimp-1 expression is known to be induced by IL-2 and Blimp-1 deficient CD4+ T cells show comparable production of IFN-γ and IL-4 but diminished IL-10 in response to TCR stimulation25,26. We therefore investigated the role of Blimp-1 in the production of IL-10 by CTL. CD8+ T cells from infected lungs of MhcII−/− mice express significantly lower levels of Blimp-1 and IL-10 (but not IFN-γ or T-bet) than T cells isolated from littermate control (Fig. 6b). Similarly, T cells from Ebi3−/− mice display lower levels of Blimp-1 and IL-10 than controls but comparable levels of IFN-γ and c-maf (Fig. 6c). Furthermore, exposure to either IL-2 or IL-27 can enhance Blimp-1 expression in CTL but simultaneous treatment of CTL with both cytokines act synergistically to enhance Blimp-1 expression (Fig. 6d). We then ectopically expressed Blimp-1 in CD8+ T cells by transducing CD8+ T cells with a Blimp-1 expressing retrovirus. Ectopic expression of Blimp-1 enhanced IL-10 production by CTL (Fig. 6e), although it did not induce IL-10 production by CTL as effectively as IL-2 plus IL-27 (data not shown), suggesting additional factors may contribute to maximal IL-10 production by CTL. We next wished to determine if IL-2 or IL-27 induced IL-10 production by CTL requires Blimp-1 expression by CTL. To this end, we isolated CD8+ T cells from WT mice or mice with Prdm1 (the gene encoding Blimp-1) selectively disrupted in T cells (CD4-Cre-Prdm1fl/fl) and stimulated them with DC plus soluble anti-CD3 in the absence or presence of IL-27, IL-2 or the combination of the two cytokines. CD8+ T cells lacking Blimp-1 proliferated normally but failed to produce IL-10 in response to IL-2, IL-27 or IL-2 plus IL-27 (Fig. 6f). Thus, IL-2-IL-27 mediated induction of IL-10 production by CTL in vitro is Blimp-1 dependent.
Figure 6. Induction of IL-10 producing CTL by IL-2 and IL-27 is Blimp-1 dependent.
(a) Vert-X mice were infected with influenza. At d7 p.i., lung CD8+ CD44hiIL-10-eGFP− cells and CD8+ CD44hiIL-10-eGFP+ cells were FACS sorted. The expression of IL-10 and Blimp-1 were measured by quantitative RT-PCR. (b) MhcII+/− or MhcII−/− mice were infected with influenza. At d7 p.i., lung CD8+ cells were isolated and the expression of IL-10, Blimp-1, IFN-γ and T-bet was determined by quantitative RT-PCR. (c) WT or Ebi3−/− mice were infected with influenza. At d7 p.i., lung CD8+ cells were isolated and the expression of IL-10, Blimp-1, IFN-γ and T-bet was determined by quantitative RT-PCR. (d) OT-I cells were cultured with influenza-OVA infected DC in the absence or presence of hIL-2, IL-27 or hIL-2 plus IL-27 for 4d. The expression of Blimp-1 was measured by quantitative RT-PCR. (e) OT-I cells were transduced with control vector (mock) or Blimp-1 expressing retrovirus (Blimp-1). Then cells were cultured for additional 3 days and IL-10 production by OT-I cells measured by ICS. hCD2− (untransduced cells), hCD2+ (transduced cells). (f) CFSE labeled CD8+ T cells from WT or CD4-Cre Prdmfl/fl (Prdm1 cKO) mice were stimulated with DC plus soluble α-CD3 for 4 days under indicated conditions and IL-10 production by CTL then measured by ICS. Numbers are the percentages of cells in the gated population. Data are representative of at least two independent experiments.
The in vivo induction of IL-10+ CTL requires Blimp-1
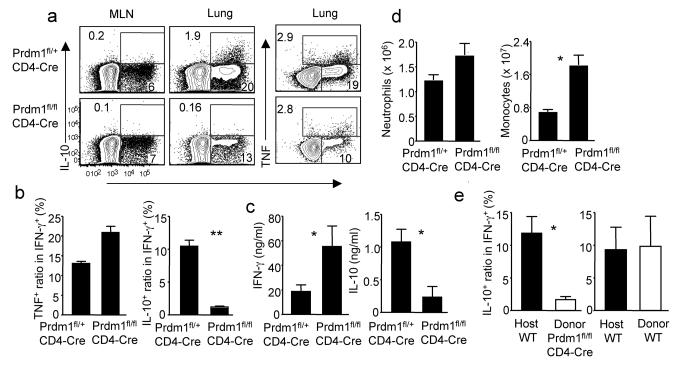
We next evaluated whether Blimp-1 is required for IL-10 production by CTL in vivo. To evaluate this, we infected CD4-Cre Prdm1fl/fl (cKO) mice with influenza and at day 7 p.i. measured IL-10 production by CTL in the MLN and infected lungs. Compared to CD8+ T cells from influenza infected control Blimp-1 sufficient (CD4-Cre Prdm1fl/+) mice, Blimp-1 deficient CD8+ T cells exhibited markedly diminished IL-10 production (Fig. 7a, b) with comparable production of IFN-γ or TNF following influenza infection (Fig. 7a, b). The selective deficit in IL-10 production was also evident in the bronchioalveolar lavage fluid (BALF) of the infected Prdm1 cKO mice (Fig. 7c). The diminished release of IL-10 into the BALF likely also reflects defective IL-10 production by CD4+ T cells since the production of IL-10 by CD4+ T cells is also impaired in Prdm1 cKO mice (data not shown). Prdm1 cKO mice also demonstrated enhanced pulmonary inflammation in response to infection characterized by increased infiltration of the infected lungs by mononuclear and granulocytic inflammatory cells and enhanced production of proinflammatory cytokines similar to the impact of IL-10R blockade (Fig. 7d and Supplementary Fig. 20)6. Next we sought to address whether the impaired IL-10 production by CD8+ T cells from Prdm1 cKO mice is a CD8+ T cell intrinsic defect. We first observed that infected Prdm1 cKO mice exhibited comparable levels of IL-27 in the respiratory tract as infected WT mice along with moderately diminished IL-2 production due to the diminished production of IL-2 by CD4+ T cells but increased production of IL-2 by CD8+ T cells respectively (Supplementary Fig. 21 and data not shown). To directly examine the role of Blimp-1 expression in regulating IL-10 production by CTL, we transferred WT or Blimp-1 deficient T cells into Thy1 mismatched mice and analyzed IFN-γ, TNF and IL-10 production by CTL following influenza infection. Transferred Blimp-1 deficient but not WT CTL failed to produce IL-10 in response to antigenic stimulation (Fig. 7e and Supplementary Fig. 22). Together these data suggest that the role of Blimp-1 in regulating IL-10 production by CTL is cell-intrinsic.
Figure 7. Blimp-1 deficiency in T cells results in diminished IL-10 production and enhanced pulmonary inflammation.
CD4-Cre Prdm1fl/+ or CD4-Cre Prdm1fl/fl mice were infected with influenza. (a, b) At d7 p.i., the production of IL-10 and IFN-γ by CTL was measured by ICS. (b) The normalized percentages of IL-10+ or TNF+ cells in influenza-specific CTL (IFN-γ+) in CD4-Cre Prdm1fl/+ or CD4-Cre Prdm1fl/fl mice are depicted. (c) At d7 p.i., the levels of IL-10 and IFN-γ in BALF were determined through ELISA. (d) At d9 p.i., the numbers of lung monocytes and neutrophils were measured through flow cytometry. Numbers are the percentages of cells in gated population. *, P <= 0.05; **, P <= 0.01. (b, c) Data are from three pooled experiments. (e) WT or CD4-Cre Prdm1fl/fl T cells were transferred into Thy1.1+ WT mice and infected with influenza. At d7 p.i., the production of IL-10 and IFN-γ by CTL was measured by ICS following restimulation with influenza-infected BMDC. The normalized percentages of IL-10+ cells in influenza-specific CTL (IFN-γ+) from infected lungs are depicted. Pooled data from two experiments are represented.
Discussion
CD4+ T-cell “help” for CD8+ T cell responses have been previously demonstrated to be required for memory CTL formation, maintenance and recall responses27-29, in CTL migration to mucosal surfaces30 and in certain experimental settings for optimum primary CTL responses to weak stimuli31-33. Strong inflammatory stimuli like influenza infection, however, trigger potent primary CTL responses independent of CD4+ T cell “help”19,20. Our results support this concept as CD4+ T cell “help” is minimally required for expression of type 1 effector cytokines such as IFN-γ and cytolytic machinery such as Granzyme B by CTL in vivo. However, without CD4+ T cell “help”, anti-virus CTL activation and differentiation is incomplete since CTL are unable to optimally produce IL-10, a critical regulatory cytokine necessary to control excess lung inflammation during immune mediated virus clearance6.
IL-2 has been shown to promote CTL memory and to support terminal effector cell differentiation by up regulating cytolytic molecules e.g. Granzyme B in secondary lymphoid organs23,34,35. Our findings on the impact of defective IL-2 signaling on Granzyme B expression by proliferating and/or differentiating CTL in the draining MLN support these earlier findings23,34,35. However, within the inflammatory milieu of the infected lungs, other signals can replace IL- 2 in the induction of CTL Granzyme B. Proinflammatory molecules such as type I interferons which can induce antigen-independent Granzyme B expression in CTL within the respiratory tract36, may replace IL-2. By contrast we find that IL-2 has a critical non-redundant role in promoting the induction of IL-10 producing CTL.
Here we also demonstrated a synergistic role for IL-27 in the CD4+ T cell mediated induction of IL-10 by CTL. Although recognized as a stimulator of TH1 responses, IL-27 has more recently been appreciated as an important IL-10 independent and dependent regulator of host immune responses37 e.g. induction of IL-10 expression by CD4+ Tr1 and CD4+ effector cells in vivo10,14,15. Our findings suggest that anti-viral CTL within the virally-infected lungs are also targets of IL-27 in vivo. While IL-27 is generally believed to be the product of activated macrophages and DC38, our results suggest that neutrophils may also be an important source of IL-27 during acute respiratory viral infections. This interplay between inflammatory myeloid cell-derived IL-27 and CTL-derived IL-10 suggests a novel regulatory loop where influx of inflammatory myeloid cells into the infected lungs in response to infection augments IL-10 production by CTL responding to infection which in turn dampens both the activation state of the innate immune cells and additional infiltration of these myeloid inflammatory cells6.
Blimp-1 is a transcriptional repressor that plays a role in B cell differentiation, as well as T cell homeostasis, T effector cell differentiation and migration39. As previously reported 40, Blimp-1deficient CTL demonstrated a modest deficit in migration into the influenza infected lungs but the Blimp-1 deficient CTL within the infected lungs exhibit no deficit in pro-inflammatory effector cytokine production. Blimp-1 deficient CTL were, however, drastically impaired in IL-10 production, suggesting stringent requirements for Blimp-1 expression by IL-10 producing CTL in vivo in the infected lungs. Consistent with this hypothesis, IL-2- and/or IL-27- dependent IL-10 production by CTL in vitro required their expression of Blimp-1. Of note both IL-10 non-producing (IL-10−) and IL-10+ CTL express Blimp-1 but the IL-10+ CTL express 2-3 fold higher levels of Blimp-1. Thus a modest difference in the level of expression of this transcriptional regulator appears to markedly influence the ability of the activated CTL to express the IL-10 gene, a result consistent with recently published evidence relating Blimp-1 gene dosage to CD8+ T cell exhaustion41. While our findings indicate a likely non-redundant requirement for both Blimp-1 and IL-2 to generate IL-10 producing CTL, both IL-10+ and IL-10−CTL express Blimp-1 and are exposed to IL-2 and IL-27 in the infected lungs. Undoubtedly other, as yet unidentified factors, also play a part in controlling IL-10 gene expression by CTL in the infected respiratory tract.
In summary, we have described the cellular and molecular mechanisms controlling a regulatory cytokine production by anti-viral CTL. Our data reveals a novel co-ordination between innate cell-derived signal and TH cell-derived signal in fine-tuning the anti-viral CTL responses during acute respiratory virus infection. Our findings may provide the groundwork for future studies in manipulating IL-10 production in anti-viral T cells to control excessive host inflammation during acute respiratory virus infection.
Online Methods
Mouse and infection
C57/BL6, BALB/c, Rag2−/−, Ebi3−/−, Ifng−/−, p35−/−, OT-II, Prdm1fl/fl, MhcII−/−, CD4-Cre transgenic mice were purchased from Taconic Farm or The Jackson Laboratories. Vert-X mice 21 were from Dr. Christopher Karp at Cincinnati Children Hospital Medical Center, Il21r−/− mice were from Dr. Warren Leonard at NIH, Yeti mice 22 were from Dr. Markus Mohrs at Trudeau Institute. CL-4, TS-1, Il2ra−/−, Il2−/−, MhcII−/−-Vert-x and Vert-X-OT-I mice were bred in house. All mice were housed in a specific pathogen-free environment and all animal experiments were performed in accordance with protocols approved by the University of Virginia Animal Care and Use Committee. For virus infection, mice were infected with sub-lethal dose of A/PR/8-34 in serum free Iscove's media intranasally following anesthesia with ketamine and xylazine.
Quantitative RT-PCR
Lung cell suspensions were prepared as described42. CD8+ cells were purified through MACS-beads (Miltenyi Biotech). mRNA isolation, reverse transcription and realtime PCR were performed as previously described6. Data were generated with the Delta CT method by normalizing to hypoxanthine phosphoribosyltransferase (HPRT).
Influenza-specific CD8+ T cell restimulation by BMDC
BMDC were harvested on day 6-7 post culture with GM-CSF and infected with influenza at approximated 100 M.O.I. overnight. Then BMDC were counted and mixed with lung or MLN cells at a 1.5 to 1 ratio in the presence of Golgi-Stop (BD Biosciences) and hIL-2 (40U/ml) for additional 6-7 h. The surface staining of cell surface markers, intracellular staining of cytokines were performed as described6.
DC/T cell co-culture
CD4+ and CD8+ transgenic T cells were isolated from spleen and lymph nodes of indicated mice through MACS-beads (Miltenyi Biotech). Splenic DC were isolated from spleen of WT mice through MACS-beads (Miltenyi Biotech). Then DC were infected with approximately with 100 M.O.I. of virus (For CL-4/TS-1 experiments, DC were infected with influenza A/PR8; for OT-I/OT-II experiments, DC were infected with recombinant influenza A/PR8-OT-I plus A/PR8-OT-II virus). Then, DC were mixed with CFSE labeled CD8+ T cells (5×104) at the ratio of 1 DC : 10 T cells in round-bottom 96 wells. In some wells, we included the same number of CD4+ T cells. The conditions of the culture are indicated in the text. For experiments cultured with WT or Blimp-1 deficient CD8+ T cells. We stimulated T cells with soluble α-CD3 (0.05 μg/ml) with influenza infected BMDC at the ratio of 1 DC : 10 T cells. The T cells were cultured for 4 d and restimulated with either cognate peptide (1 μg/ml) or PMA (100ng/ml) plus ionomycin (1 μg/ml) in the presence of Golgi-Stop (1 μl/ml). For the secondary culture with activated effector T cells. V-OT-I or OT-II cells were activated separately as described above. Then 5×104 V-OT-I cells or V-OT-I (5×104) plus OT-II cells (5×104) were left unstimulated or stimulated with plate-bound α-CD3 (coated with 50μl 1μg/ml in PBS for 3-4h in 37 °C) for 2d. All blocking Abs are used at the concentration of 20 μg/ml. recombinant human IL-2 and mouse IL-27 were used as 300 U/ml and 10 ng/ml respectively.
Human CD8+ T cell culture
CD8+ T cells were isolated from periphery blood of healthy individuals via MACS. 5×104 CD8+ T cells were then stimulated with plate bound α-CD3 plus α-CD28 (50μl 4μg/ml in PBS for 3-4h in 37 °C) for 3d in the presence or absence of hIL-2 (300 U/ml) and/or rhIL-27 (10 ng/ml). Then the culture supernatant were harvested for human IL-10 ELISA and cells were harvested for quantitative RT-PCR analysis for IL-10 expression.
Bone marrow Chimera
To generate WT and Il2ra−/− mixed bone marrow chimera, we lethally irradiated (1020 Rads) WT mice and reconstituted the irradiated mice with Thy1.1+ WT bone marrow mixed with Thy1.2+ Il2ra−/− bone marrow. To generate WT and Il21r−/− mixed bone marrow chimera, we lethally irradiated (1020 Rads) WT mice and reconstituted the irradiated mice with CD45.1+ WT bone marrow mixed with CD45.2+ Il21r−/− bone marrow. After 8-10 weeks, the reconstituted mice were then infected influenza.
Cell transfer and infection
For T cells transferring into WT mice: cells were isolated from Thy1.2+ WT, Il2ra−/− or CD4-Cre Prdm1fl/fl spleen and lymph nodes. A total of 50 million cells were then transferred into Thy1.1+ mice. 24h later, the recipient mice were infected with influenza. For CD4+ and CD8+ T cell transferring into Rag2−/− mice: 15 million purified WT CD8+ T cells were either alone or mixed with 30 million purified WT CD4+ T cells or Il2−/− CD4+ T cells and then transferred into Rag2−/− mice. 24h later, recipient mice were infected with influenza. At d9 p.i., lung cells were collected and IL-10/IFN-γ production by CD8+ T cells were determined.
Cell sorting
For experiments to measure IL-27 expression, WT mice were infected with influenza and at different cell populations were sorted based on following markers at d5 p.i.: Neutrophils: CD11b+Ly6G+; DCs, MHCIIhiCD11chi; Monocytes/macrophages, CD11b+MHCII+CD11c− or low; T cells, Thy1.2+, lung resident cells, CD45−. For effector T cells sorting from infected Vert-X mice, we sorted CD44hiIL-10-eGFP+ or CD44hiIL-10-eGFP− CD8+ T cells from d7 infected lungs.
Retroviral transduction
Control pMI retroviral vector and Blimp-1 containing pMI retroviral vector were a gift from Dr. Thomas R. Malek at the University of Miami 25. Retroviral supernatants were generated by transient transfection of HEK-293T cells with TransIT-LT1 reagent (Mirus) in the presence of pCL-Eco Retrovirus packaging plasmid. Retrovirus-containing supernatants were collected 48 h after transfection and used for spin infection (2,500 rpm, 2 h) of pre-activated OT-I cells (BMDC plus 1 μg/ml of OVA peptide for 24h). Then cells were washed and placed into culture with influenza-OVA infected DC for additional 3d.
Flow cytometry analysis
Antibodies were purchased from BD Biosciences or eBioscience. Cells were acquired through a 6-color FACS-Canto system (BD Biosciences). Data were then analyzed by FlowJo software (Treestar).
Statistical analysis
Data are mean ± s.e.m. Two-tailed Student's t-test was used. *, P values <= 0.05. **, P values <= 0.01.
Supplementary Material
Acknowledgments
We thank the rest of Braciale Lab for critical comments and B. Small for excellent technical assistance. We thank C. L. Karp, M. Mohrs, W. Leonard and T. R. Malek for critical reagents. This work was supported by the US National Institutes of Health (grants AI-15608, HL-33391, AI-37293 and U19 AI-083024 to T. J. B.), UVA-Center for Immunity, Inflammation and Regenerative Medicine Start-up Funds to R.S. and an American Lung Association Postdoctoral Fellowship (RN-123000) to J.S.
Footnotes
Author Contributions
J.S. designed the project, performed most of the experimental work, analyzed the data and wrote the manuscript. H.D. and E. K. M. performed some of the quantitative RT-PCR and ELISA experiments. R.S. contributed to critical reagents and suggestions. T.J.B. supervised the project, analyzed the data and wrote the manuscript.
Competing financial interests
The authors declare that they have no competing financial interests.
Reference
- 1.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 2.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 3.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–97. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankovic D, et al. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–83. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009 doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–8. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 8.Barrat FJ, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maynard CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–41. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 10.Pot C, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi R, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 14.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 15.Jankovic D, Trinchieri G. IL-10 or not IL-10: that is the question. Nat Immunol. 2007;8:1281–3. doi: 10.1038/ni1207-1281. [DOI] [PubMed] [Google Scholar]
- 16.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–67. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saraiva M, et al. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–19. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 19.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76:12388–93. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson S, et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity. 2009;30:218–27. doi: 10.1016/j.immuni.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madan R, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–20. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 24.Belz GT, Masson F. Interleukin-2 tickles T cell memory. Immunity. 32:7–9. doi: 10.1016/j.immuni.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–52. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 26.Martins GA, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–65. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 27.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–33. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–82. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Zhang H, Zhao J. The role of CD4 T cell help for CD8 CTL activation. Biochem Biophys Res Commun. 2009;384:405–8. doi: 10.1016/j.bbrc.2009.04.134. [DOI] [PubMed] [Google Scholar]
- 34.Pipkin ME, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalia V, et al. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–30. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: a double-edged sword for offense and defense. J Leukoc Biol. 2009;86:1295–303. doi: 10.1189/jlb.0609445. [DOI] [PubMed] [Google Scholar]
- 39.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 11:114–20. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–95. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–20. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–18. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.