Abstract
NF-κB and Akt are two main cell survival pathways that attenuate the anticancer efficacy of therapeutics. Our previous studies demonstrated that the Smac mimetic compound 3 (SMC3) specifically suppresses c-IAP1 and induces TNF-α autocrine to kill cancer cells. However, SMC3 also induces a cell survival signal through NF-κB activation. In this report, we further found that SMC3 potently activates Akt, which inhibits SMC3-induced cancer cell death. Strikingly, concurrent blocking NF-κB and Akt resulted in a significantly potentiated cytotoxicity. Because heat shock protein 90 (Hsp90) plays an important role in maintaining the integrity of both the NF-κB and Akt pathways in cancer cells, we examined if suppression of Hsp90 is able to potentiate SMC3-induced cancer cell death. The results show that targeting Hsp90 does not interfere with SMC3-induced c-IAP1 degradation and TNF-α autocrine, the key processes for SMC3-induced cancer cell apoptosis. However, Hsp90 inhibitors effectively blocked SMC3-induced NF-κB activation through degradation of RIP1 and IKKβ, two key components of the NF-κB activation pathway, and reduced both the constitutive and SMC3-induced Akt activity through degradation of the Akt protein. Consistently, with the co-treatment of SMC3 and Hsp90 inhibitors, apoptosis was markedly sensitized and a synergistic cytotoxicity was observed. The results suggest that concurrent targeting c-IAP1 and Hsp90 by combination of SMC3 and Hsp90 inhibitors is an effective approach for improving the anticancer value of SMC3.
Keywords: NF-κB, Akt, Smac mimetic, Hsp90, Cytotoxicity, Apoptosis
Introduction
Cell survival signaling blocks cancer cell death induced by chemotherapy, which underlies one of the main mechanisms of chemoresistance. It is unveiled in recent years that both constitutive and inducible cell survival signals attenuate anticancer activity of therapeutics. Importantly, multiple cell survival pathways could be simultaneously activated, resulting in more than one checkpoint to facilitate cancer cell’s escape from therapy [1]. Therefore, to understand how cell survival signaling is regulated during chemotherapy and to develop means to suppress cell survival signaling in cancer cells hold the key for improving anticancer chemotherapy.
The transcription factor nuclear factor-κB (NF-κB) is often activated in different human tumors [2–4]. Because it up-regulates expression of numerous anti-apoptotic genes, NF-κB is regarded as a main cancer cell survival signal that inhibits cytotoxicity induced by chemotherapy [5, 6]. While cancer therapeutics kill cancer cells through apoptosis, they also simultaneously stimulate NF-κB, blunting their anti-cancer efficacy [7, 8]. Akt is another important cell survival signal that contributes to cancer cell’s chemoresistance. Basal and therapeutic-induced Akt activations are found to promote cancer cell survival [9, 10]. Akt protects cancer cells partly through regulating factors involved in apoptosis and proliferation such as Bcl-2 family proteins BAD and Mcl-1, inhibitor of apoptosis protein (IAP) survivin, mammalian target of rapamycin (mTOR) and Cox-2 [11–15]. More importantly, there is crosstalk between Akt and NF-κB to further promote cell survival [7]. As in regard of anticancer therapy, blocking either NF-κB or Akt is well determined to sensitize cancer cells to therapeutic-induced cytotoxicity [7, 12, 13, 16, 17]. Furthermore, concurrent blockage of NF-κB and Akt has been shown to be more effective in enhancing the anticancer potency of genotoxic anticancer drugs and TNF-α [18, 19].
Hsp90 (heat shock protein 90) is a protein chaperon that maintains the stability and function of various signaling proteins involved in cell proliferation, growth and survival. Hsp90 inhibitors induce degradation of the Hsp90 client proteins by disrupting their interaction with Hsp90. Because many cancer cells are addicted to high Hsp90 activity for survival and proliferation, Hsp90 inhibitors are anticancer therapeutics which are tested in clinical trials [20]. Among the Hsp90 client proteins there are key components for both the NF-κB and Akt pathways. For example, RIP1 and IKKβ, two critical molecules for the NF-κB activation, are degraded when Hsp90 is inhibited in cancer cells, leading to blockage of the TNF-α-induced NF-κB activation pathway [21, 22]. The Akt protein is also an Hsp90 client, which is dramatically destabilized when Hsp90 is inhibited [20]. Therefore, the concurrent NF-κB and Akt blocking property of Hsp90 inhibitors makes these chemicals a good adjuvant for anticancer chemosensitization.
Smac (Second mitochondria-derived activator of caspases, also called direct IAP binding protein with low iso-electric point) is a mitochondrial protein that is released in the early phase of apoptosis. When relocated to the cytosol, Smac promotes apoptosis through counteracting inhibitor of apoptosis proteins, a protein family that negatively regulates apoptosis through increasing apoptotic threshold. Recently developed Smac mimetics (SMs) have shown potent anticancer activity [23–26]. One of the SMs, Smac mimetic compound 3 (SMC3), enhances apoptosis through specific elimination of c-IAP1 and induction of TNF-α autocrine [23, 27]. Our recent studies showed SMC3 activates the NF-κB pathway that blunts SMC3’s anticancer activity, and blockage of NF-κB effectively sensitizes cancer cells to SMC3-induced apoptosis [28, 29].
In this study, we demonstrate that SMC3 potently induces Akt activation, which cooperatively with NF-κB to attenuate apoptosis in different cancer cells. Strikingly, Hsp90 inhibitors concurrently block SMC3-induced activation of NF-κB and Akt while do not interfere with the apoptosis-inducing mechanisms of SMC3. When Hsp90 inhibitors and SMC3 were combined in treating cancer cells, a synergistic cytotoxicity was achieved. The results suggest that concurrently targeting c-IAP1 and Hsp90 by combination of SMC3 and Hsp90 inhibitor is a useful approach to achieve enhanced anticancer efficacy through suppressing the survival pathways NF-κB and Akt.
Materials and methods
Reagents and antibodies
SMC3 was a generous gift from Dr. Xiaodong Wang, University of Texas Southwest Medical Center. Hsp90 inhibitors, 17AAG, CCT018159 and rifabutin were purchased from Sigma (St. Louis, MO) and Calbiochem (La Jolla, CA), respectively. IKK inhibitor II and LY294002 were from Calbiochem. The following antibodies were used for Western blot: anti-caspase-8 and anti-caspase-3 (Pharmingen, San Diego, CA), anti-PARP (BioSource, Camarillo, CA), anti-Bcl-xL, anti-Akt, anti-phospho-Akt (Ser473) (Cell Signaling, Beverly, MA), anti-MnSOD (BD Biosciences, San Diego, CA), anti-RIP1, anti-IKKβ, anti-β-tubulin (Sigma, St. Louis, MO), anti-cIAP1, anti-cIAP2 (Santa Cruz, CA). The human TNF-α detection ELISA kit was purchased from eBioscience (San Diego, CA). Short interfering RNAs (siGENOME SMARTpool) for RelA and Akt (pooled siRNAs against Akt1, Akt2 and Akt3) were purchased from Dharmacon (Lafayette, CO). Non-targeting siRNA (Silencer® negative control #1 siRNA) were obtained from Ambion (Austin, TX).
Cell culture
The human lung cancer cell line H23, and human hepatoma cell line HepG2 were obtained from American Type Culture Collection (Manassas, VA). H23 cells were cultured in RPMI 1640 with 10% fetal bovine serum, 1 mmol/l glutamate, 100 units/ml penicillin, and 100 μg/ml streptomycin. HepG2 cells were cultured in DMEM with 4.5 g/l glucose, 10% fetal bovine serum, 1 mmol/l glutamate, 100 units/ml penicillin, and 100 μg/ml streptomycin. HBEC-1 and HBEC-2 cells, human bronchial epithelial cells immortalized by insertion of cyclin-dependent kinase 4 and human telomerase reverse transcriptase [30], were provided by Drs. Jerry Shay and John Minna (University of Texas Southwestern Medical Center) and cultured in keratinocyte serum-free medium on collagen-coated plates.
Cytotoxicity assay
Cytotoxicity was determined using a lactate dehydrogenase (LDH) release-base cytotoxicity detection kit (Promega, Madison, WI). Cells were seeded in 48-well plates at 70–80% confluence. After culture overnight, cells were treated as indicated in each figure legend. LDH release was measured and cell death was calculated as described previously [12, 31]. For measuring apoptotic cell death, H23 cells were treated as described in the figure legend. The cells were collected and stained with propidium iodide (100 μg/ml) for 30 min and detected by flow cytometry with FACSCalibur (BD Biosciences). Cell distribution at Sub-G1, which was regarded as apoptotic cells, was analyzed with the CellQuest program (BD Biosciences).
Measurement of autocrine TNF-α secretion by ELISA
Cells were seeded in 12-well plates at 70–80% confluence. After overnight culture, the cells were treated as described in the figure legends. The culture media were collected and the concentration of TNF-α was detected by ELISA analysis with the human TNF-α ELISA kit following the instructions of the manufacturer (eBioscience Inc.) [29].
Western blot
Cells were harvested and lysed in M2 buffer (20 mM Tris–HCl, pH 7.6; 0.5% NP-40; 250 mM NaCl; 3 mM EGTA; 3 mM EDTA; 2 mM DTT; 0.5 mM phenylmethylsulfonyl fluoride; 20 mM β-glycerophosphate; 1 mM sodium vanadate; and 1 μg/ml leupeptin). Equal amounts of protein extracts were resolved in 12% SDS-PAGE and the proteins of interest were probed by Western blot and visualized by enhanced chemiluminescence according manufacturer’s instructions (Amersham, Piscataway, NJ) [18, 32].
Transfection and luciferase report assay
Cells grown in 24-well plates were transfected with p5×κB-Luc and pRSV-LacZ with FuGENE 6 according to manufacturer’s instruction (Roche, Indianapolis, IN). Twenty-four hours after transfection, cells were treated as indicated in each figure legend. Luciferase activity was measured using a luciferase assay kit (Promega) and normalized to β-galactosidase activity [33]. siRNA was transfected with INTERFERin ™ (PolyPlus-transfection). Forty-eight hours after transfection, cells were treated with SMC3 as described in figure legends and then followed by Western blot or cell death assay.
Statistical analysis
Data are expressed as means ± standard deviation (SD). Statistical significance was examined by one-way analysis of variance (ANOVA) pairwise comparison. To assess the potential for interactions between factors, we included interaction terms in the models. When tests indicated an interaction, 95% confidence intervals for the differences in means were obtained to assess the magnitude of the interaction. In all analyses, P < 0.05 was considered statistically significant [28, 29].
Results
SMC3-induced Akt activation protects cancer cells against cytotoxicity
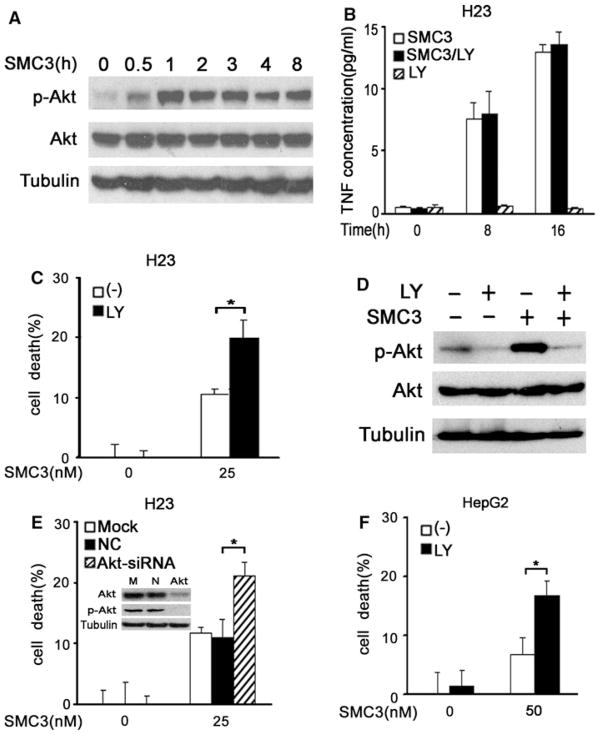
Our previous studies found SMC3 activates NF-κB through autocrine TNF-α, which blunts apoptosis [28, 29]. However, it is unclear if SMC3 activates Akt. To address this question, H23 cells were treated with SMC3 for different time periods, and phosphorylated Akt (at Ser473), an active form of Akt, was detected by Western blot. As shown in Fig. 1a, Akt activation was rapidly induced by SMC3, starting at 30 min, peaking at 1 h and stayed at a high level for at least 8 h. Akt activation by SMC3 was also observed in HepG2 cells (Fig. 3c and data not shown). To see if Akt is required for SMC3-induced TNF-α secretion, a key process for SMC3’s cytotoxicity in cancer cells [27], the specific inhibitor LY294002 for the Akt upstream kinase PI3K was used to suppress Akt activity. Akt is unlikely involved in SMC3-induced TNF-α secretion because LY294002 had no detectable effect on this action of SMC3 (Fig. 1b). Similar observation was made when another Akt inhibitor quercetin was used (data not shown). We then examined if blocking Akt with LY294002 impacts SMC3-induced cell death. There was a limited cytotoxicity when the cells were treated with a moderate concentration of SMC3 or LY294002 individually. However, the co-treatment of LY294002 and SMC3 resulted in a potentiated cell death (Fig. 1c). LY294002 was confirmed to effectively suppress both the basal and SMC3-induced Akt activity (Fig. 1d). To further substantiate the role of Akt in regulating SMC3-induced cytotoxicity, Akt-siRNA was used to specifically knockdown Akt protein expression in H23 cells. Consistent with the results with LY294002, efficient knockdown of Akt expression and activity significantly enhanced SMC3-induced cell death (Fig. 1e. Similar role of Akt activation in regulating SMC3-induced cell death was also detected in HepG2 cells (Figs. 1f, 2e). These results indicate that SMC3 activates Akt, which attenuates cytotoxicity induced by this Samc mimetic.
Fig. 1.
SMC3-induced Akt activation protects cancer cells from cytotoxicity. a H23 cells were treated with SMC3 (25 nM) for the indicated times. Akt and phosphor-Akt (Ser473) were detected by Western blot. β-tubulin was detected as an input control. b H23 cells were pretreated with LY294002 (10 μM) for 1 h followed by SMC3 (25 nM) treatment for the indicated times or left untreated. The concentrations of TNF-α in culture media were measured by ELISA and expressed as mean ± SD (n = 3). c H23 cells were pretreated with LY294002 (10 μM) for 1 h followed by SMC3 (25 nM) treatment or left untreated for 30 h. Cell death was measured by LDH leakage assay (n = 3). * P < 0.05. d H23 cells were pretreated with LY294002 (10 μM) for 1 h followed by SMC3 (25 nM) treatment for additional 1 h. Akt and phosphor-Akt (Ser473) were detected by Western blot. β-tubulin was detected as an input control. e H23 cells were mock transfected or transfected with 5 nM pooled siRNA against Akt1, Akt2, and Akt3 or negative control siRNA. Forty-eight hours after transfection, the cells were treated with 25 nM SMC3 or left untreated for 30 h. Cell death was measured as described in c (n = 3). Inset knockdown of Akt was confirmed by Western blot in H23 cells. * P < 0.05. f HepG2 cells were pretreated with LY294002 (10 μM) for 1 h followed by SMC3 (50 nM) treatment or left untreated for 30 h. Cell death was measured as described in c (n = 3). * P < 0.05
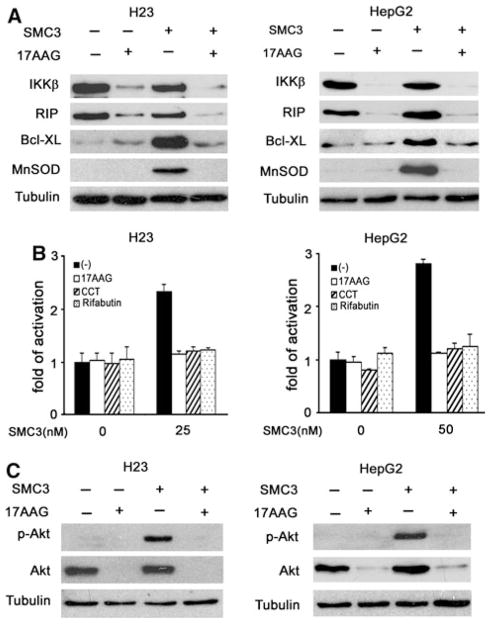
Fig. 3.
Hsp90 inhibitors simultaneously block SMC3-induced NF-κB and Akt activation. a H23 and HepG2 cells were pretreated with 17AAG (50 nM) for 10 h, followed by SMC3 treatment (25 nM for H23 and 50 nM for HepG2 cells) for 24 h, IKKβ, RIP1, Bcl-xL and MnSOD proteins were detected by Western blot. β-tubulin was detected as an input control. b H23 and HepG2 cells were co-transfected with p5×κB-Luc and pRSV-LacZ. Twenty-four hours after transfection, the cells were pretreated with 17AAG (50 nM), CCT018159 (10 μM) or rifabutin (10 μM) for 10 h followed by SMC3 treatment for 24 h or left untreated. Luciferase activity was detected and normalized to β-galactosidase activity (n = 3). c H23 and HepG2 cells were pretreated with 17AAG (50 nM) for 10 h, followed by SMC3 treatment (25 nM for H23 and 50 nM for HepG2 cells) for 4 h. Phosphor-Akt (Ser473) and Akt were detected by Western blot. β-tubulin was detected as an input control
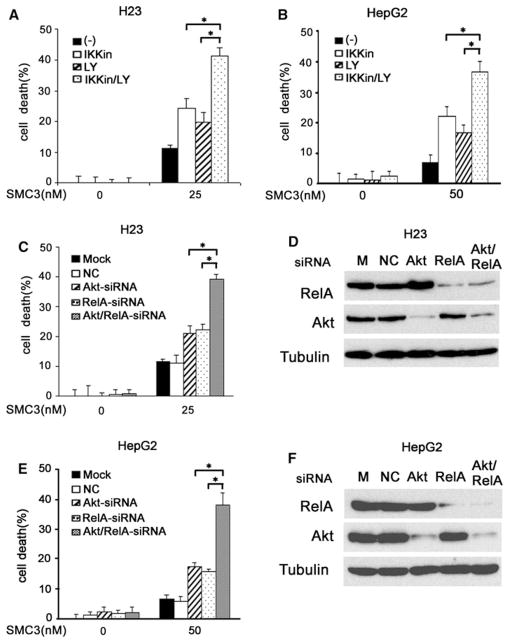
Fig. 2.
NF-κB and Akt cooperatively attenuate SMC3-induced cytotoxicity. a H23 cells were pretreated with IKK inhibitor II (10 μM) or LY294004 (10 μM) alone or both for 1 h then treated with SMC3 (25 nM) or left untreated for 30 h. Cell death was measured by a LDH release array (n = 3). * P <0.05. b HepG2 cells were pretreated with IKK inhibitor II (10 μM) or LY294004 (10 μM) alone or both for 1 h then treated with SMC3 (50 nM) or left untreated for 30 h. Cell death was measured by a LDH release array (n = 3). * P < 0.05. c H23 cells were mock transfected or transfected with indicated siRNAs or negative control siRNA. Forty-eight hours after transfection, the cells were treated with SMC3 or left untreated for 30 h. Cell death was measured as described in (a) (n = 3). * P < 0.05. d H23 cells were transfected as described in c and the indicated proteins were detected by Western blot. β-tubulin was detected as an input control. e HepG2 cells were transfected and treated, and cell death was detected as described in (c) (n = 3). * P < 0.05. f. Confirmation of knockdown of protein expression as described in (d)
NF-κB and Akt cooperatively attenuate SMC3-induced cytotoxicity
Our previous and present studies demonstrate both NF-κB and Akt are activated to inhibit SMC3-induced cell death, promoting us to investigate whether concurrent blockage of these cell survival pathways cooperatively potentiates SMC3-induced cytotoxicity in cancer cells. To this end, IKK inhibitor II that suppresses the NF-κB pathway and LY294002 that inhibits the Akt pathway were used to treat the cells individually or in combination before SMC3 exposure. IKK inhibitor II was confirmed to effectively suppress the canonical NF-κB activation pathway (Fig. S1). While the IKK inhibitor or LY294002 individually slightly increased cell death, the combination of both caused significantly higher SMC3-inuced cytotoxicity in H23 and HepG2 cells (Fig. 2a, b). To validate the results, Akt-siRNA and RelA-siRNA were used to suppress Akt and RelA expression, respectively in H23 and HepG2 cells. As expected, while the control siRNA had little effect, and RelA-siRNA and Akt-siRNA slightly increased cytotoxicity, combination of RelA-siRNA and Akt-siRNA significantly augmented SMC3-induced cell death (Fig. 2c, e). The efficiency of knockdown of RelA or Akt expression was confirmed by Western blot (Fig. 2d, f). These results strongly suggest that NF-κB and Akt cooperatively attenuate SMC3-induced cell death, and concurrently blocking these two pathways potently sensitizes cancer cells to cytotoxicity induced by SMC3.
Hsp90 inhibitors simultaneously block SMC3-induced NF-κB and Akt activation
Hsp90 inhibitors are able to suppress both the NF-κB and Akt pathways activated by different inducers [20]. We then examined whether Hsp90 inhibitors block SMC3-induced NF-κB and Akt activation. In both H23 and HepG2 cells, the Hsp90 inhibitor 17AAG caused dramatic degradation of RIP1 and IKKβ, two critical components for TNF-α-induced NF-κB activation, regardless of the presence of SMC3. The induction of the two NF-κB targets Bcl-xL and MnSOD by SMC3 was effectively blocked by 17AAG, suggesting targeting Hsp90 by 17AAG effectively blocks SMC3-induced NF-κB activation (Fig. 3a). Effective suppression of SMC3-induced NF-κB activation by 17AAG and other two Hsp90 inhibitors rifabutin and CCT018159 was also detected in H23 and HepG2 cells by a luciferase reporter assay (Fig. 3b). These results are consistent with previous reports [21], and indicate that SMC3 does not interfere with the function of the Hsp90 inhibitor. Suppression of Hsp90 by 17AAG also caused decrease in Akt protein expression levels and blocked basal and SMC3- induced Akt activation in H23 and HepG2 cells (Fig. 3c). These results suggest that Hsp90 inhibitors are able to concurrently suppress SMC3-induced NF-κB and Akt activation in cancer cells.
Hsp90 inhibitors do not interfere with SMC3-induced c-IAP1 degradation and TNF-α secretion in cancer cells
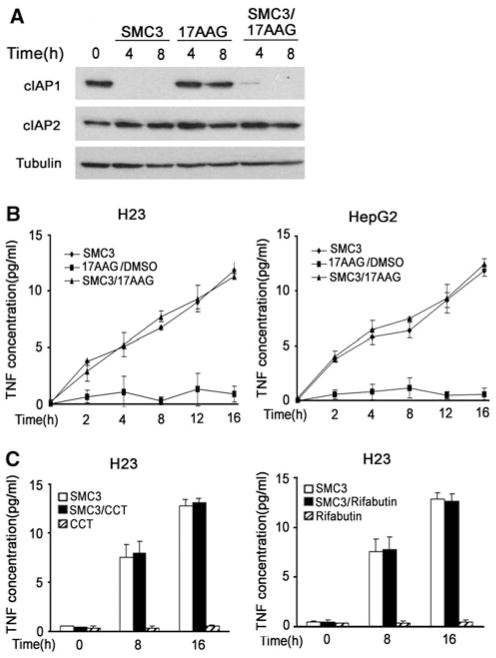
Having confirmed that Hsp90 inhibitors effectively block SMC3-induced NF-κB and Akt activation in cancer cells, we then performed experiments to determine if these inhibitors impact SMC3-induced c-IAP1 degradation and TNF-α secretion, two critical processes for SMC3’s cancer cell killing activity [23, 27]. Indeed, regardless of the presence of 17AAG, SMC3 effectively triggered c-IAP1 degradation (Fig. 4a). As reported previously [27, 29], SMC3 had marginal effect on c-IAP2 expression. There was no detectable effect on c-IAP1 and c-IAP2 expression by 17AAG alone (Fig. 4a). In addition, induction of TNF-α secretion by SMC3 was not affected by 17AAG, CCT018159 or Rifabutin in H23 and HepG2 cells (Fig. 4b, c, and data not shown). Altogether, these data demonstrate that Hsp90 inhibition has no effect on c-IAP1 degradation and TNF-α autocrine induced by SMC3, and thus, is unlikely to interfere with the apoptosis pathway activated by SMC3.
Fig. 4.
No effect of Hsp90 inhibitors on SMC3-induced c-IAP1 degradation and TNF-α secretion. a H23 cells were pre-treated with 17AAG for 10 h followed by SMC3 for the indicated times. c-IAP1 and c-IAP2 were detected by Western blot. β-tubulin was detected as an input control. b H23 or HepG2 cells were pretreated with 17AAG (50 nM) for 10 h followed by SMC3 treatment (50 nM) for the indicated times. The concentration of TNF in cell culture media was measured by ELISA. c H23 cells were pretreated with CCT018159 (10 μM) or rifabutin (10 μM) for 10 h followed by SMC3 treatment (50 nM) for the indicated times. The concentration of TNF in cell culture media was measured by ELISA
Inhibiting Hsp90 enhances SMC3-induced apoptosis
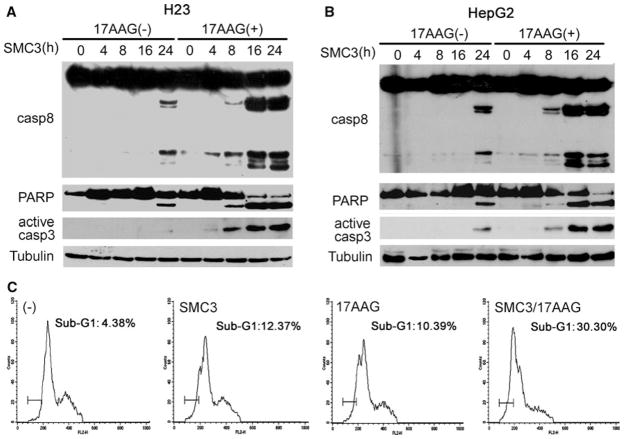
After establishing that Hsp90 inhibitors suppress SMC3-stimulated NF-κB and Akt activation while do not interfere with SMC3-induced c-IAP1 degradation and TNF-α auto-crine, we next examined whether co-treatment with SMC3 and Hsp90 inhibitors results in enhanced apoptosis. Consistent with previous report that SMC3 kills cancer cells through autocrine TNF-α-mediated activation of the extrinsic apoptosis pathway, SMC3 alone moderately activated caspase-8, the initiator caspase for the extrinsic apoptosis pathway [27, 29], which was detected at a late time point (24 h) (Fig. 5a, b). The activation of caspase 3 and cleavage of PARP was weakly detected at 24 h post treatment by SMC3 alone. Strikingly, when cells were co-treated with 17AAG and SMC3, the activation of caspase-8 was strongly potentiated and occurred much earlier (8 h), suggesting that the SMC3-induced extrinsic apoptosis pathway was sensitized by 17AAG. Consistently, activation of caspase-3 and cleavage of PARP were much stronger and occurred earlier (Fig. 5a, b). In addition, we examined sub-G1 distribution, another approach to detect apoptosis, by flow cytometry. Combination of 17AAG and SMC3 significantly increased the sub-G1 population, compared to the cells treated with 17AAG or SMC3 alone (Fig. 5c). In the samples with 17AAG plus SMC3 treatment, dead cells showed typical apoptotic morphological features (data not shown). Collectively, these data suggest that inhibiting Hsp90 sensitizes SMC3-incuced apoptosis in cancer cells.
Fig. 5.
Inhibiting Hsp90 enhances SMC-3 induced apoptosis. H23 (a) or HepG2 (b) cells were pretreated with 17AAG (50 nM) for 10 h or left untreated and followed by SMC3 treatment (25 nM for H23 and 50 nM for HepG2 cells) for the indicated time periods. Caspase-8, PARP, and activated caspase-3 were detected by Western blot. β-tubulin was detected as an input control. c H23 cells were pretreated with 17AAG (50 nM) for 10 h or left untreated and followed by SMC3 treatment (25 nM) for 30 h. The cells were stained with propidium iodide (100 μg/ml) for 30 min and analyzed by flow cytometry
Combination of Hsp90 inhibitors and SMC3 causes synergistic cytotoxicity in cancer cells
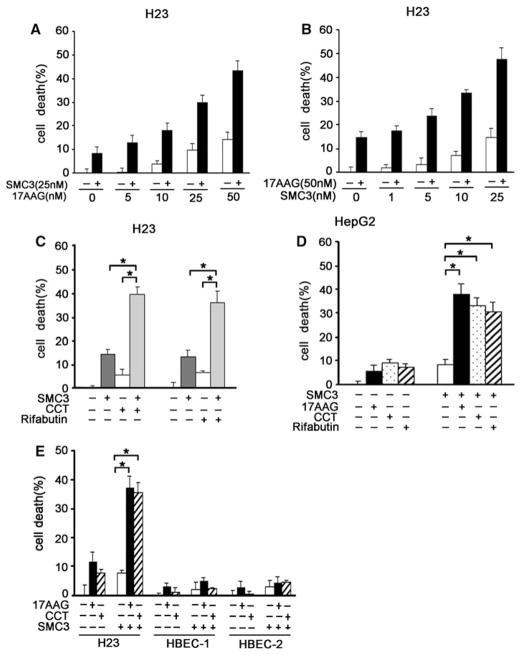
We then examined whether Hsp90 inhibitor and SMC3 cooperatively kill cancer cells. In H23 cells, potentiation of cell death in a dose-dependent manner was detected with increasing concentrations of 17AAG plus a fixed concentration of SMC3; and vise versa (Fig. 6a, b). Similar effects were observed when other Hsp90 inhibitors, CCT018159 and rifabutin, were combined with SMC3 in treating H23 and HepG2 cells (Fig. 6c, d). The cell killing effect of 17AAG and SMC3 combination is much greater than the sum of the effects of treatment with the two agents individually (P < 0.01), suggesting a synergy in cytotoxicity was achieved in the drug combination (Fig. 6a, d). Consistently, the synergistic cytotoxicity by SMC3 plus 17AAG was dependent on TNF-α, suggesting that the synergistic cytotoxicity caused by SMC3 plus Hsp90 inhibitors involves the TNF-α autocrine-mediated extrinsic apoptosis pathway (Fig. S2). It is noteworthy that the combination of Hsp90 inhibitors and SMC3 had marginal effect in non-transformed human normal bronchial epithelial cells (HBEC-1 and -2), suggesting that this anticancer approach is non-toxic in normal bronchial epithelial cells (Fig. 6e). Together with the results that inhibiting Hsp90 concurrently blocks SMC3-induced NF-κB and Akt activation, these data suggest that Hsp90 inhibitors sensitize cancer cells to SMC3-induced cytotoxicity at least partly through blocking these two cell survival pathways.
Fig. 6.
Combination of Hsp90 inhibitors and SMC3 causes synergistic cytotoxicity in cancer cells. a H23 cells were pretreated with 17AAG (5–50 nM) for 10 h or remained untreated and followed by SMC3 treatment (25 nM) for 30 h. Cell death was measured by LDH release assay (n = 3). b H23 cells were pretreated with 17AAG (50 nM) for 10 h or left untreated and followed by SMC3 treatment (1–25 nM) for 30 h. Cell death was measured as described in (a)(n = 3). c H23 cells were pretreated CCT018159 (10 μM) or rifabutin (10 μM) or left untreated for 10 h then followed by SMC3 (25 nM) treatment for 30 h. Cell death was measured by LDH release assay. (n = 3). * P < 0.05. d HepG2 cells were pretreated with 17AAG (50 nM), CCT018159 (10 μM) or rifabutin (10 μM) for 10 h or left untreated followed by 30 h of treatment with 50 nM of SMC3. Cell death was measured as described in (a). (n = 3). * P < 0.05. e H23, HBEC-1 or HBEC-2 cells were pretreated 17AAG (50 nM) or CCT018159 (10 μM) or left untreated for 10 h then followed by SMC3 (25 nM) treatment for 30 h. Cell death was measured by LDH release assay (n = 3). * P < 0.05
Discussion
In this report, we provide evidence showing that in addition to NF-κB, the specific c-IAP1 inhibitor SMC3 also potently activates Akt, which blunts SMC3’s anticancer activity. Concurrent blocking NF-κB and Akt significantly sensitizes cancer cells to SMC3-induced cytotoxicity. We further show that inhibition of Hsp90 effectively suppresses SMC3-induced NF-κB and Akt activation while retains the SMC3-induced apoptosis pathway intact. Strikingly, combination of SMC3 and Hsp90 inhibitors achieved a synergistic anticancer activity in cancer cells while had little effect on non-transformed cell’s viability. These results suggest that concurrent targeting c-IAP1 and Hsp90 by combination of SMC3 and Hsp90 inhibitors is an effective approach to achieve an enhanced anticancer efficacy. Although other effects by Hsp90 inhibition may be involved, we believe the potentiated cytotoxicity in cancer cells is achieved at least partly through blocking SMC3-induced NF-κB and Akt activation.
Anticancer chemotherapeutics kill cancer cells mainly by activating cell death pathways such as apoptosis. While DNA damage drugs activate the mitochondrial (intrinsic) apoptosis pathway [34], the recently developed SMC3 activates the extrinsic apoptosis pathway through autocrine TNF-α [23, 27]. As a potential mechanism for cancer cells’ response to therapeutic stress and acquired chemoresistance, cell survival pathways are also activated when the cells are exposed to therapeutics. Therefore, shifting the balance between pro-death and pro-survival to the side of death through either enhancing apoptosis signals or blocking survival pathways holds the key for improving anticancer efficacy and preventing chemoresistance. Our previous reports have determined that SMC3 induces the canonical NF-κB activation depending on TNF-α auto-crine, which attenuates apoptosis [28, 29]. Results from this study demonstrate that SMC3 also simultaneously induces Akt, which is another brake for SMC3’s anti-tumor activity. It is unlikely that SMC3 activates Akt through NF-κB as observed in NIH3T3 cells [35], because effective blocking NF-κB had no detectable effect on SMC3-induced Akt activation (Fig. S3). In addition, SMC3 exerted no effect on phosphorylation of the PI3K p85 subunit (Fig. S3). Therefore, how Akt is activated by SMC3 deserves further studies. While individually blocking NF-κB or Akt slightly increased SMC3-induced cytotoxicity, concurrent suppression of these two survival pathways potentiated anticancer effect of SMC3 in a much higher extent. Consistently, a recent report clearly showed that both NF-κB and Akt are involved in SMC3-resistance in cancer cells [36]. These observations suggest that blocking multiple cell survival pathways activated by chemotherapy would more effectively increase therapeutic efficacy. Consistent with this view, other chemotherapeutics such as cisplatin, etoposide and TNF-α activate both NF-κB and Akt, and concurrently blocking both pathways potently improves their anticancer efficacy [18, 19].
Aiming to concurrently block NF-κB and Akt to sensitize SMC3’s anticancer activity, we chose Hsp90 inhibitors because inhibiting Hsp90 is able to simultaneously turn off these two cell survival pathways [20–22]. Indeed, Hsp90 is often utilized for survival by various human cancer cells, and Hsp90 inhibitors are potential anticancer agents tested in preclinical studies or clinical trials [37, 38]. As expected, inhibiting Hsp90 decreased the expression of RIP1 and IKKβ, two key mediators for the TNF-α-activated NF-κB pathway, which consequently blocked SMC3-induced NF-κB activation. The protein level and activity of Akt were also simultaneously suppressed in Hsp90-inhibited cells. These results show that Hsp90 blocks SMC3-induced NF-κB and Akt activation. On the other hand, Hsp90 inhibitors do not affect SMC3-induced c-IAP1 degradation and TNF-α autocrine, two critical processes for SMC3-induced cancer cell apoptosis. Therefore, SMC3 and the Hsp90 inhibitors do not interfere with each other’s anti-cancer function while the combination of them can effectively block the unwanted survival signals, making the combination of these two types of anticancer agents an ideal approach for cancer therapy.
It should be noted that Hsp90 regulates a wide variety of proteins and pathways such as EGFR, Her2 and HIF-1α that are involved in cancer cell survival and proliferation [20]. Our results do not exclude involvement of other Hsp90 client proteins in the synergistic cytotoxicity achieved by combining SMC3 and Hsp90 inhibitors. Nevertheless, our studies clearly show that the combination of these two anticancer agents potently increases anticancer activity. The application of this combination could reduce the doses of each drug so that to limit adverse effects and make it more tolerable in patients. Additionally, because activation of cell survival pathways contributes to chemo-resistance [7], the combination of Hsp90 inhibitors with SMC3 to block NF-κB and Akt may prevent the development of acquired resistance to SMC3.
Taken together, based on the observations that combination of Hsp90 inhibitors and SMC3 has a synergy in killing cancer cells partly through blocking NF-κB and Akt, our results suggest a new regimen that combines these anticancer agents for cancer therapy. Further in vivo studies are warranted to verify the anticancer efficacy and side effect of this regimen. It would be also interesting to determine whether this combination therapy limits acquired chemoresistance.
Supplementary Material
Acknowledgments
This study was partly supported by a grant from NIEHS/NIH (R01ES017328) and Department of Energy Low Dose Radiation Research Program (DE-SC0001173).
Abbreviations
- IAP
Inhibitor of apoptosis protein
- NF-κB
Nuclear factor κB
- Smac
Second mitochondria-derived activator of caspase
- SMC3
Smac mimetic compound 3
- TNF-α
Tumor necrosis factor alpha
- TNFR1
TNF-α receptor 1
- Hsp90
Heat shock protein 90
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10495-010-0542-4) contains supplementary material, which is available to authorized users.
Contributor Information
Lang Bai, Molecular Biology and Lung Cancer Program, Lovelace Respiratory Research Institute, 2425 Ridgecrest Dr. SE, Albuquerque, NM 87108, USA. Infectious Disease Center, West China Hospital, Sichuan University, 37 Guoxuexiang, Chengdu 610041, Sichuan, China.
Shanling Xu, Molecular Biology and Lung Cancer Program, Lovelace Respiratory Research Institute, 2425 Ridgecrest Dr. SE, Albuquerque, NM 87108, USA.
Wenshu Chen, Molecular Biology and Lung Cancer Program, Lovelace Respiratory Research Institute, 2425 Ridgecrest Dr. SE, Albuquerque, NM 87108, USA.
Zi Li, Molecular Biology and Lung Cancer Program, Lovelace Respiratory Research Institute, 2425 Ridgecrest Dr. SE, Albuquerque, NM 87108, USA.
Xia Wang, Laboratory of Molecular and Translational Medicine, West China Second University Hospital, Sichuan University, 3-17 Renminnanlu, Chengdu 610041, China.
Hong Tang, Infectious Disease Center, West China Hospital, Sichuan University, 37 Guoxuexiang, Chengdu 610041, Sichuan, China.
Yong Lin, Email: ylin@lrri.org, Molecular Biology and Lung Cancer Program, Lovelace Respiratory Research Institute, 2425 Ridgecrest Dr. SE, Albuquerque, NM 87108, USA.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Jin X, Wang Z, Qiu L, et al. Potential biomarkers involving IKK/RelA signal in early stage non-small cell lung cancer. Cancer Sci. 2008;99:582–589. doi: 10.1111/j.1349-7006.2007.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Jia H, Xie L, et al. Association of constitutive nuclear factor-kappaB activation with aggressive aspects and poor prognosis in cervical cancer. Int J Gynecol Cancer. 2009;19:1421–1426. doi: 10.1111/IGC.0b013e3181b70445. [DOI] [PubMed] [Google Scholar]
- 5.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 6.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14:45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–7492. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 11.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Wang X, Zhuang J, Zhang L, Lin Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis. 2007;28:2114–2121. doi: 10.1093/carcin/bgm133. [DOI] [PubMed] [Google Scholar]
- 13.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Bai L, Wang X, Xu S, Belinsky SA, Lin Y. Acquired activation of the Akt/cyclooxygenase-2/Mcl-1 pathway renders lung cancer cells resistant to apoptosis. Mol Pharmacol. 2010;77:416–423. doi: 10.1124/mol.109.061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Chen W, Zeng W, et al. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Mol Cancer Ther. 2008;7:1156–1163. doi: 10.1158/1535-7163.MCT-07-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Wang X, Bai L, Liang X, Zhuang J, Lin Y. Blockage of NF-kappaB by IKKbeta- or RelA-siRNA rather than the NF-kappaB super-suppressor IkappaBalpha mutant potentiates adriamycin-induced cytotoxicity in lung cancer cells. J Cell Biochem. 2008;105:554–561. doi: 10.1002/jcb.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Chen W, Lin Y. Sensitization of TNF-induced cytotoxicity in lung cancer cells by concurrent suppression of the NF-kappaB and Akt pathways. Biochem Biophys Res Commun. 2007;355:807–812. doi: 10.1016/j.bbrc.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 19.He HN, Wang X, Zheng XL, et al. Concurrent blockade of the NF-kappaB and Akt pathways potently sensitizes cancer cells to chemotherapeutic-induced cytotoxicity. Cancer Lett. 2010;295:38–43. doi: 10.1016/j.canlet.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Ju W, Renouard J, Aden J, Belinsky SA, Lin Y. 17-allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Res. 2006;66:1089–1095. doi: 10.1158/0008-5472.CAN-05-2698. [DOI] [PubMed] [Google Scholar]
- 22.Lewis J, Devin A, Miller A, et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem. 2000;275:10519–10526. doi: 10.1074/jbc.275.14.10519. [DOI] [PubMed] [Google Scholar]
- 23.Petersen SL, Wang L, Yalcin-Chin A, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Vince JE, Wong WW, Khan N, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Bertrand MJ, Milutinovic S, Dickson KM, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Bai L, Chen W, Wang X, Ju W, Xu S, Lin Y. Attenuating Smac mimetic compound 3-induced NF-kappaB activation by luteolin leads to synergistic cytotoxicity in cancer cells. J Cell Biochem. 2009;108:1125–1131. doi: 10.1002/jcb.22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai L, Chen W, Wang X, Tang H, Lin Y. IKKβ-mediated nuclear factor-κB activation attenuates smac mimetic-induced apoptosis in cancer cells. Mol Cancer Ther. 2009;8:1636–1645. doi: 10.1158/1535-7163.MCT-09-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez RD, Sheridan S, Girard L, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 31.Ju W, Wang X, Shi H, Chen W, Belinsky SA, Lin Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol. 2007;71:1381–1388. doi: 10.1124/mol.106.032185. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Yang Q, Wang X, Liu ZG. The essential role of the death domain kinase receptor-interacting protein in insulin growth factor-I-induced c-Jun N-terminal kinase activation. J Biol Chem. 2006;281:23525–23532. doi: 10.1074/jbc.M601487200. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Ryan J, Lewis J, Wani MA, Lingrel JB, Liu ZG. TRAF2 exerts its antiapoptotic effect by regulating the expression of Kruppel-like factor LKLF. Mol Cell Biol. 2003;23:5849–5856. doi: 10.1128/MCB.23.16.5849-5856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene. 2004;23:2797–2808. doi: 10.1038/sj.onc.1207532. [DOI] [PubMed] [Google Scholar]
- 35.Meng F, Liu L, Chin PC, D’Mello SR. Akt is a downstream target of NF-kappa B. J Biol Chem. 2002;277:29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- 36.Petersen SL, Peyton M, Minna JD, Wang X. Overcoming cancer cell resistance to Smac mimetic induced apoptosis by modulating cIAP-2 expression. Proc Natl Acad Sci USA. 2010;107:11936–11941. doi: 10.1073/pnas.1005667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramanathan RK, Trump DL, Eiseman JL, et al. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res. 2005;11:3385–3391. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 38.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics—an update. Expert Opin Emerg Drugs. 2005;10:137–149. doi: 10.1517/14728214.10.1.137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.