Summary
The Gram-negative bacteria Vibrio cholerae poses significant public health concerns by causing an acute intestinal infection afflicting millions of people each year. V. cholerae motility, as well as virulence factor expression and outer membrane protein production, have been shown to be affected by bile (Childers & Klose, 2007). The current study examines the effects of bile on V. cholerae phospholipids. Bile exposure caused significant alterations to the phospholipid profile of V. cholerae but not of other enteric pathogens. These changes consisted of a quantitative increase and migratory difference in cardiolipin, decreases in phosphatidylglycerol and phosphatidylethanolamine, and the dramatic appearance of an unknown phospholipid determined to be lyso-phosphatidylethanolamine. Major components of bile were not responsible for the observed changes, but long chain polyunsaturated fatty acids, which are minor components of bile, were shown to be incorporated into phospholipids of V. cholerae. Although the bile-induced phospholipid profile was independent of the V. cholerae virulence cascade, we identified another relevant environment in which V. cholerae assimilates unique fatty acids into its membrane phospholipids—marine sediment. Our results suggest that Vibrio species possess unique machinery conferring the ability to take up a wider range of exogenous fatty acids than other enteric bacteria.
Introduction
The El Tor biotype of Vibrio cholerae is responsible for the longest and most widespread cholera pandemic in history, dating from 1961 to the present and causing outbreaks in Southeast Asia, the Asia mainland, the Middle East, Africa and Central and South America. Considered an “emerging and reemerging infection,” cholera constantly threatens developing countries, is particularly deadly for children and has been categorized as a bioterrorism watchlist agent (Department of Health and Human Services, National Institutes of Health, NIAID). A highly adaptive organism, V. cholerae normally inhabits freshwater, estuarine and oceanic environments, often associated with sea life such as oysters, plankton, crabs and copepods (Colwell, 1996, Huq et al., 2006). Recently, fish were also identified as being significant reservoirs and vectors of V. cholerae (Senderovich et al., 2010). Human consumption of contaminated food or water introduces the bacteria to the gastrointestinal tract, where it can survive the extreme acidity of the stomach and subsequently colonize the small intestine. During the incubation period (1–5 days), V. cholerae multiplication and regulated expression of virulence factors initiates the onset of the rapidly debilitating and dehydrating disease cholera.
The small intestine, specifically the duodenum, is the site where voluminous amounts (400–800ml) of bile are secreted to aid in the digestion of fats. The composition of bile is complex, mainly consisting of bile acids and other lipid molecules such as cholesterol and phospholipids. Bile acids, with their amphipathic nature, act as a detergent that not only facilitates the breakdown of ingested lipids, but also exerts potent antimicrobial activity. Bile exposure causes bacterial membrane and DNA damage, yet the exact mechanisms of action remain unknown (Prieto et al., 2004, Merritt & Donaldson, 2009). However, enteric pathogens such as E. coli and V. cholerae have evolved to resist these diverse antimicrobial effects by both preventing entrance of and extruding bile acids. In addition to possessing an outer membrane which functions as an effective hydrophobic barrier, Gram-negative bacteria also activate efflux systems for rapid removal of bile acids and Tol proteins for outer membrane stabilization (Gunn, 2000, Bina & Mekalanos, 2001, Prouty et al., 2002, Rosenberg et al., 2003). Fatty acids, although minor components of bile (Blomstrand & Ekdahl, 1960, Nakayama & Johnston, 1962, Nakayama et al., 1964, Nakayama, 1967, Mingrone et al., 1983), are prevalent within the intestinal tract and are specifically generated from triacylglycerols and phospholipids prior to absorption by the intestinal epithelia (Iqbal & Hussain, 2009). Importantly, the intestinal epithelial cell surface is the site of colonization for V. cholerae.
Studies have shown that V. cholerae can sense and respond to bile as a signal for initiation of pathogenesis-related processes. Upon exposure to the concentration of bile found in the intestinal lumen, V. cholerae enhances motility and represses expression of the toxR-dependent major virulence factors cholera toxin (CT) and the toxin-coregulated pilus (TCP) (Gupta & Chowdhury, 1997). A recent study attributed the inhibition of virulence factors to the unsaturated fatty acid content in bile, while both unsaturated fatty acids and cholesterol caused enhanced motility (Chatterjee et al., 2007). However, exposure of V. cholerae to low bile concentrations found at the mucosal surface results in the reverse phenotype, with repression of motility and increased expression of CT and TCP (Schuhmacher & Klose, 1999). Bile also activates several efflux pumps, induces biofilm formation and affects production of the outer membrane proteins OmpU and OmpT (Provenzano & Klose, 2000, Chatterjee et al., 2004, Hung et al., 2006, Cerda-Maira et al., 2008). Considering that bacteria maintain lipid homeostasis when faced with varying environmental conditions such as solvent stress and changes in temperature and pH (Zhang & Rock, 2008), we hypothesized that bile may trigger a similar type of homeoviscous adaptation that would be reflected in the V. cholerae phospholipids. We report herein that V. cholerae, as well as several other Vibrio species, significantly alters its phospholipid profile in response to bile and that these changes are partly due to differential uptake and utilization of the exogenous fatty acids available in bile. We further demonstrate that V. cholerae can also assimilate fatty acids accessible in marine sediment, a relevant environment harboring V. cholerae.
Results
Bile exposure results in an altered phospholipid profile of Vibrio species, but not E. coli or S. enterica
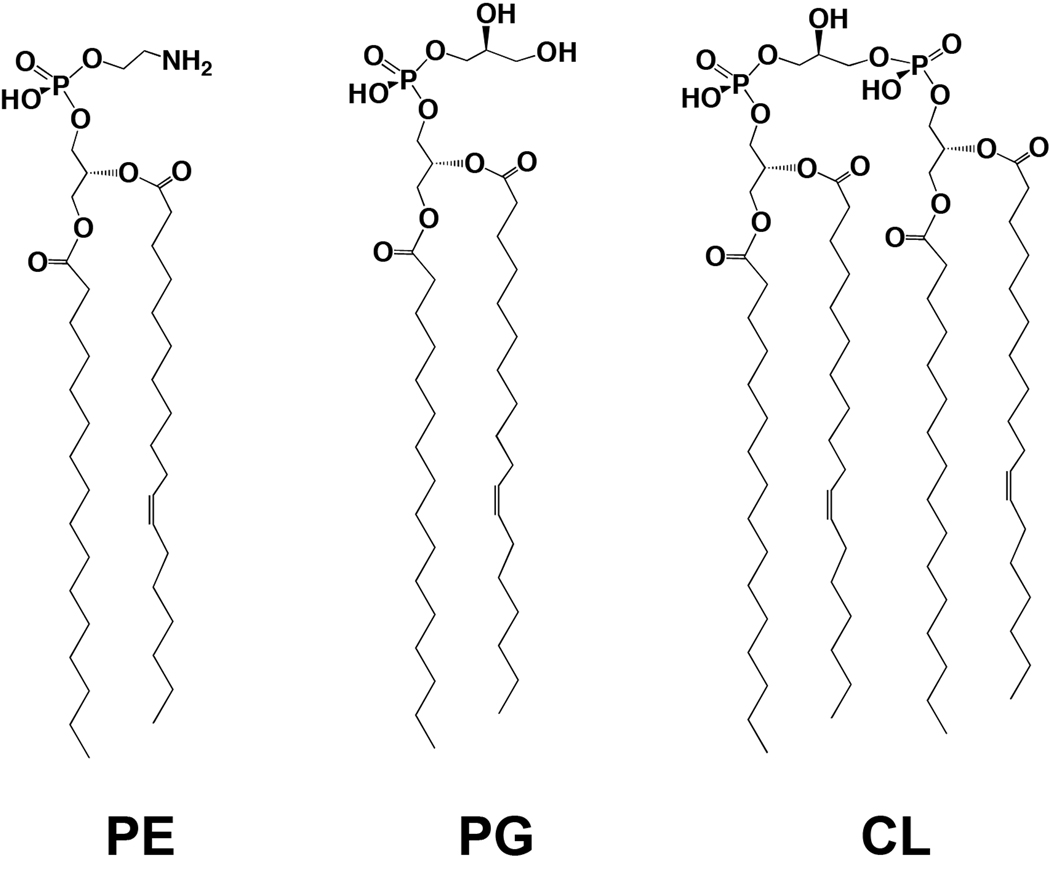
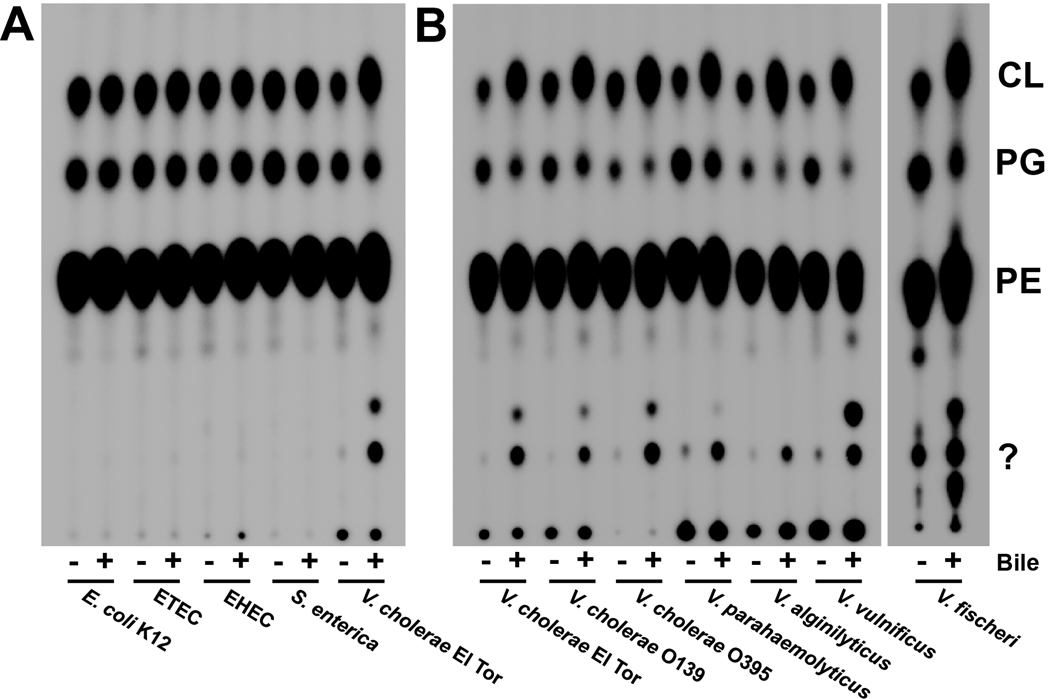
To test the effect of bile on bacterial phospholipids, bacteria (see Table S1 for list of strains) were grown in the presence and absence of 0.4% bile, which is both a concentration documented to induce virulence effects in V. cholerae (Gupta & Chowdhury, 1997, Schuhmacher & Klose, 1999, Hung et al., 2005, Hung et al., 2006) and a concentration within the range of bile in the intestines of healthy individuals (Hofmann, 1993). 32Pi was included in the growth media and the radiolabeled phospholipids were extracted and examined by thin-layer chromatography (TLC). The major phospholipids found in Vibrio, Escherichia and Salmonella species are phosphatidylethanolamine (PE), phosphatidylglycerol (PG) and cardiolipin (CL) (Fig. 1). Initial examination of the phospholipid profiles of E. coli and V. cholerae grown in the presence of 0.4% bile revealed substantial differences in both amounts of these phospholipids and Rf values following TLC separation. Fig. 2A shows a comparison of the phospholipids isolated from multiple E. coli strains, a S. enterica serovar Typhimurium and a V. cholerae O1 El Tor strain grown in the absence and presence of 0.4% bile. Densitometry analysis revealed that exposure to bile did not significantly alter the phospholipid profiles of E. coli and S. enterica. In the absence of bile, V. cholerae has 83.9% PE, 10.1% PG, 4.0% CL, and 1.9% of an unknown phospholipid; however, growth in 0.4% bile resulted in 56.9% PE, 5.0% PG, 8.7% CL and 29.6% of an unknown phospholipid (detailed densitometry data can be found in Table S2). This effect was also observed when commercially available ox, porcine, or ovine bile preparations were used (data not shown).
Fig. 1.
The major bacterial phospholipids found in E. coli, S. enterica and V. cholerae are phosphatidylethanolamine (PE), phosphatidylglycerol (PG), and cardiolipin (CL).
Fig. 2. Comparison of the phospholipid profiles of E. coli, S. enterica and Vibrio species grown in the presence and absence of bile.
A. V. cholerae exhibits a different phospholipid profile than three E. coli strains and S. enterica serovar Typhimurium when grown in the presence of bile. ETEC, enterotoxigenic E. coli; EHEC, enterohaemorrhagic E. coli. Density analysis (Quantity One) indicated no significant quantitative change between the levels of the three major PLs in E. coli and Salmonella, whereas the same analysis for V. cholerae El Tor revealed significant increases (two-fold) in CL, decreases in PG and increases in two minor PLs. Densitometry data can be found in Table S2.
B. The altered phospholipid profile following bile exposure is observed in several Vibrio species.
Analysis of several other Vibrio species grown with and without bile yielded similar phospholipid profiles in both migrational patterns and percentages of phospholipids (Fig. 2B). This effect on phospholipids was examined with regard to bile concentration by growing the bacteria to mid-log (A600 of ~0.5) in concentrations of bile ranging from 0.01% to 0.4% in the presence of 32Pi. The phospholipid changes were observed at a concentration as low as 0.01%, with the phenotype becoming more pronounced with increasing bile concentration (Fig. 3A, densitometry data in Table S3). To test the temporal response to bile, bacteria were grown to mid-log, pelleted and suspended in LB containing 0.4% bile and 32Pi. Samples were taken at the indicated times and lipids were extracted and analyzed. Within 15 minutes of bile exposure the migratory phenotype was observed and became stronger the longer the bacterium was grown in the presence of bile (Fig. 3B, densitometry data in Table S3). To further explore the pertinence of these observations, E. coli and V. cholerae were grown in the presence and absence of bile under several conditions: minimal media growth, microaerobic growth and anaerobic growth. Phospholipid analysis revealed that V. cholerae continues to alter its phospholipids in these environments while E. coli does not (Fig. S1). Interestingly, V. cholerae converts all PG to CL when grown in anaerobic conditions. We then used antibiotic-mediated inhibition to determine whether the changes in phospholipids are dependent upon protein and/or fatty acid synthesis. Protein synthesis was inhibited with chloramphenicol, whereas fatty acid biosynthesis was inhibited with cerulenin, which binds β-keto-acyl-ACP preventing its interaction with malonyl-CoA, thus effectively stalling the initiation stage of fatty acid biosynthesis (D'Agnolo et al., 1973). When either protein synthesis or fatty acid synthesis is inhibited, V. cholerae still adopts the bile-induced phospholipid profile (Fig. S2). In conclusion, Vibrio species, but not E. coli or S. enterica, obtain an altered phospholipid profile during growth in bile and these changes are not dependent on fatty acid or protein synthesis.
Fig. 3. The observed bile-induced phospholipid changes in V. cholerae occur at very low bile concentrations and within 15m of bile exposure.
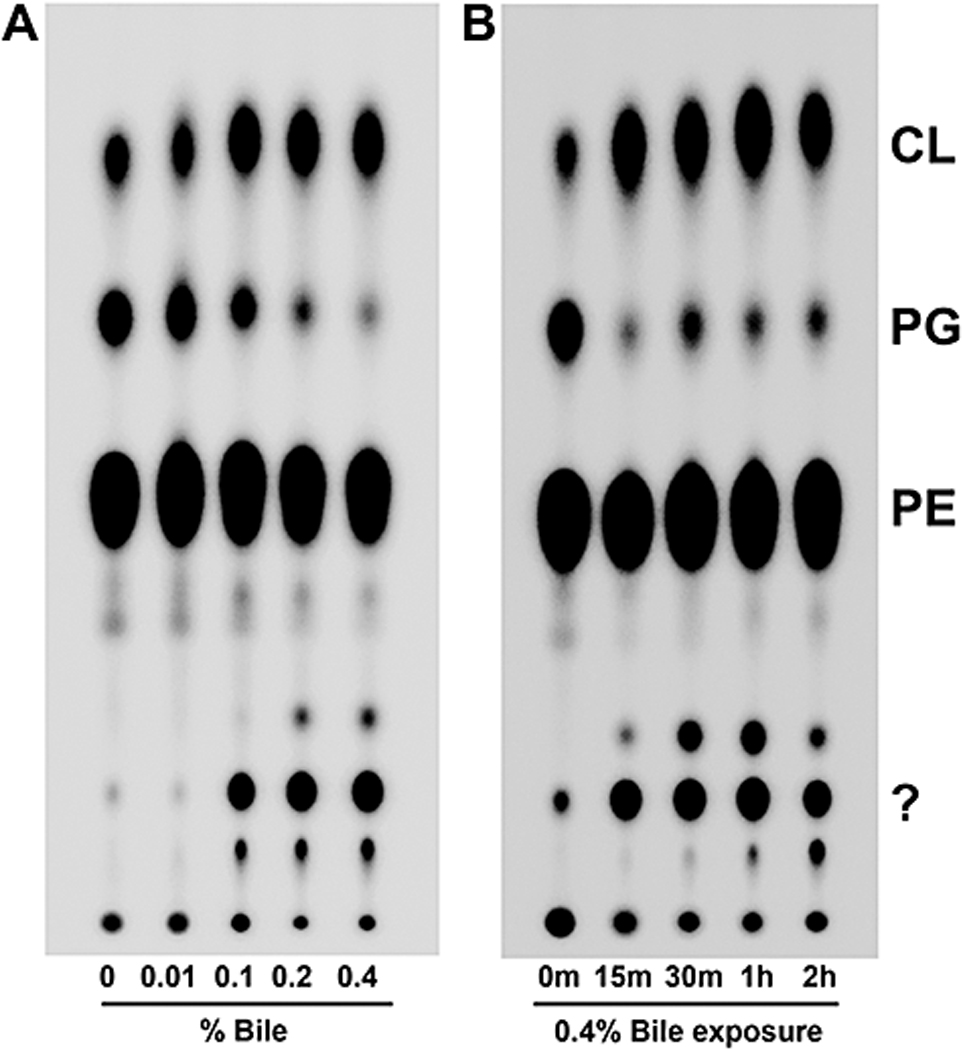
A. The migratory shift of phospholipids occurs over a range of bile concentrations. Bacteria were grown to mid-log phase in media with the indicated bile concentrations and 32Pi prior to lipid extraction. Densitometry data can be found in Table S3.
B. The altered phospholipid profile is observed just 15m after bile exposure and is sustained at least 2 hours when constitutively grown in bile. Bacteria were grown to an A600 of ~ 0.5 (0m) prior to pelleting of the bacteria and resuspension in LB supplemented with 0.4% bile containing 32Pi. Samples were taken at the indicated times post bile exposure. Densitometry data can be found in Table S3.
The bile-induced phospholipid profile is not dependent on the V. cholerae virulence cascade
Virulence in V. cholerae is triggered by environmental signals, such as bile, and mediates virulence factor expression conducive for colonization of the intestine. Expression of toxin coregulated pilus and cholera toxin is controlled by the ToxR regulon, which consists of a set of over 20 genes (Childers & Klose, 2007). ToxR is a transmembrane DNA binding protein that controls virulence gene expression by activating ToxT, a second transcriptional activator that directly activates transcription of the virulence genes tcpH, encoding TCP, and ctxAB, encoding CTX (Shakhnovich et al., 2007). In vitro activation of the ToxR regulon in strains of the El Tor biotype is accomplished by growing the bacteria at 37°C in a bicarbonate-containing medium (AKI medium) (Iwanaga & Yamamoto, 1985). To test whether the altered phospholipid profile is dependent upon this major virulence pathway, both gene deletion and chemical inhibition were used to disable the virulence cascade during growth with and without bile. Virstatin is a small molecule inhibitor that blocks dimerization of ToxT, thus disallowing ToxR-dependent transcription of virulence factors (Hung et al., 2005). Following TLC analysis of isolated phospholipids, neither a toxR knockout nor chemical inhibition of ToxT abrogated the change in phospholipids (Fig. S3). Taken together, this suggests that the bile-induced phospholipid profile is not dependent on the V. cholerae virulence cascade.
The altered phospholipid profile cannot be attributed to individual major bile acids, lecithin or bilirubin
To elucidate what, specifically, in bile caused the effect on phospholipids, we grew both E. coli and V. cholerae in the presence of the major individual components of bile. The six major bile acids present in humans (chenodeoxycholic acid, cholic acid, deoxycholic acid, lithocholic acid, taurocholic acid and glycocholic acid) and lecithin (L-α-phosphatidylcholine) were added individually to the growth medium at a concentration (500µM) known to be both subinhibitory and physiologically relevant (Hofmann, 1993, Cerda-Maira et al., 2008). Bilirubin was added at the physiological concentration of 0.4mg/dL. E. coli phospholipids were identical regardless of growth with individual bile components (Fig. S4-A). Likewise, V. cholerae phospholipids displayed a similar profile among the individual components (Fig. S4-B); PE and PG remained relatively constant throughout, whereas the only significant difference was an increase (50% by densitometry) in CL for V. cholerae grown in the presence of chenodeoxycholic acid, cholic acid and deoxycholic acid. Similar results were obtained in experiments in which the concentration of individual bile acids was increased to 2mM (data not shown). It was concluded that none of these individual bile components significantly contributed to the altered migratory phenotype seen in the phospholipid profile of V. cholerae grown, which was chosen for continued study.
V. cholerae grown in bile produces new phospholipid species containing long chain polyunsaturated fatty acids
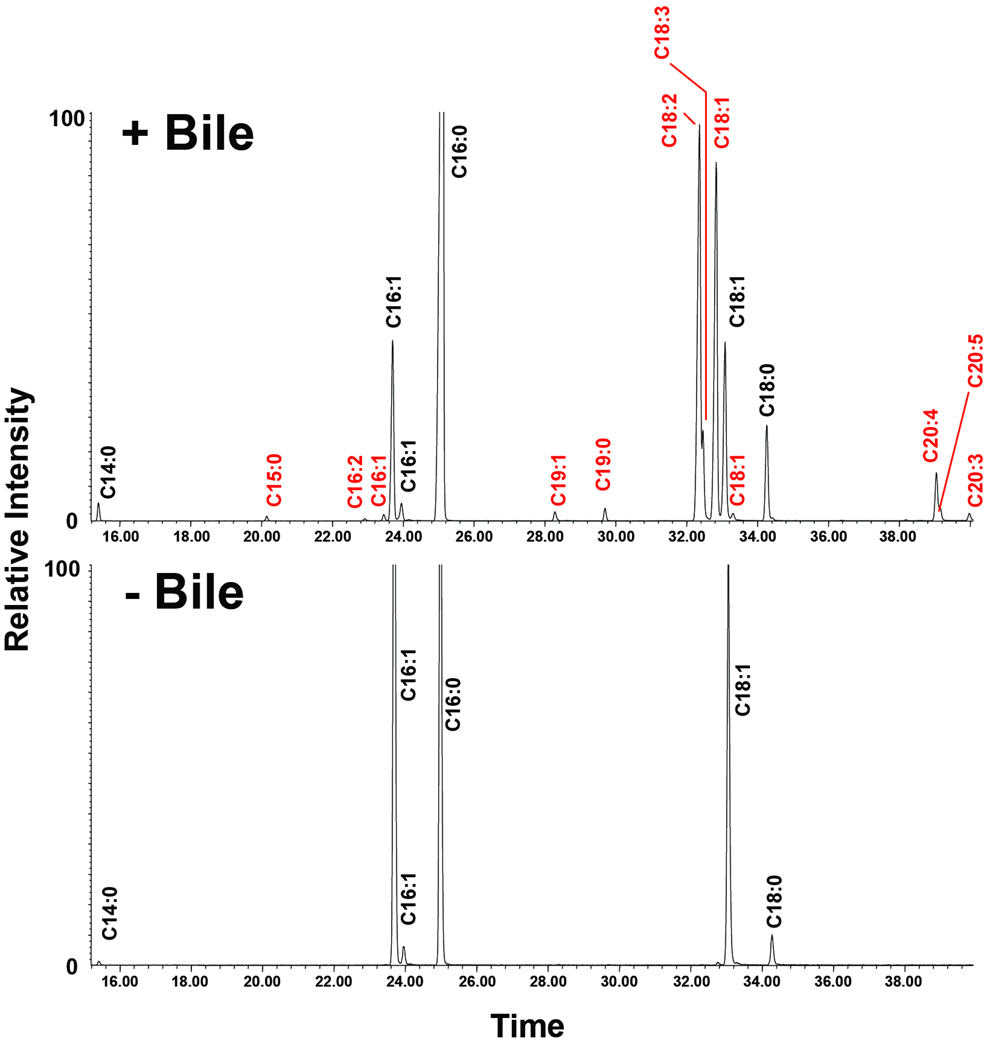
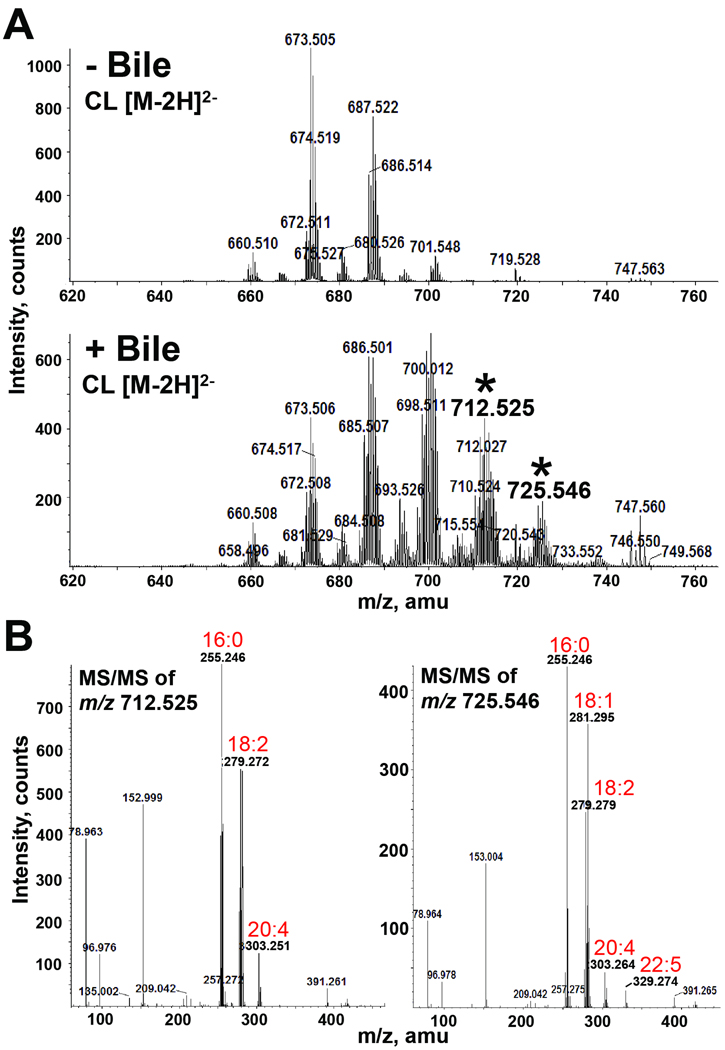
The observed migratory differences in phospholipids could be explained by either modification to the head group and/or changes in the fatty acyl chains. For determination of these structural changes, we performed both GC/MS and LC/ESI-MS/MS on CL species that were isolated by preparative TLC following bacterial growth in the presence and absence of bile. Both analyses identified new fatty acid constituents in the bile-grown sample. The GC/MS chromatograms shown in Fig. 4 indicate that palmitic acid (C16:0), palmitoleic acid (C16:1) and vaccenic acid (C18:1) represent the predominant fatty acids of cardiolipin species, which corresponds to the normal fatty acid makeup of E. coli and V. cholerae (Raziuddin, 1976, Yokota et al., 1980). Growth in bile resulted in a significant decrease in palmitoleic acid (C16:1) and the appearance of several new fatty acids including linoleic acid (C18:2), arachidonic acid (20:4), and others (Fig. 4). Some of the additional fatty acids detected by GC/MS were identified by MS/MS in CL species unique to the bile-grown sample. The two most predominant [M-2H]2− ion peaks (m/z 712.525 and 725.546) within each newly identified cluster were chosen for MS/MS analysis (Fig. 5A). The carboxylic anions of the released fatty acids from the dissociation of the m/z 712.525 ion revealed CL species containing acyl chains of C16:0/C16:0/C20:4/C18:2 (predicted [M-2H]2− at m/z 712.45 as determined by using the cardiolipin structure generation tool on the Lipid Maps website: http://www.lipidmaps.org (Fig. 5B). The MS/MS spectrum of the [M-2H]2− ion at m/z 725.546 predicted two CL species with the fatty acyl compositions of: C16:0/C16:0/C18:2/C22:5 (predicted [M-2H]2− at m/z 725.47) and C16:0/C18:1/C18:2/C20:4 (predicted [M-2H]2− at m/z 725.47) (Fig. 5B). Although we only selected two peaks, it is expected that analysis of other peaks unique to the spectra of CL from growth in bile would reveal additional fatty acids detected by GC/MS. Since we expected that these fatty acids originated from bile, we performed ESI-MS on crude bile. Indeed, several of the unique fatty acids present in bile-exposed V. cholerae CL were reflected by the fatty acids detected in crude bile (Fig. 6).
Fig. 4. GC/MS analysis of the methyl esters of the fatty acids hydrolyzed from CL in V. cholerae grown in the presence and absence of bile.
The CL of V. cholerae grown in bile is composed of different percentages and a wider variety of fatty acids than those found in the absence of bile. Fatty acids unique to the bile-adapted CL are labeled in red.
Fig. 5. Mass spectrometric analysis of CL extracted from V. cholerae grown in the presence and absence of bile.
A. Negative-ion ESI-MS of [M-2H]2− ions of V. cholerae CL isolated from cultures grown in the presence and absence of bile. Selected peaks (*) found only in the presence of bile were subsequently analyzed by MS/MS.
B. Negative-ion MS/MS analysis of the [M-2H]2− ions at m/z 712.525 and 725.546 detected only in the presence of bile. The product-ion spectra show that these CL species contain longer, polyunsaturated fatty acyl chains (18:2, 20:4 and 22:5).
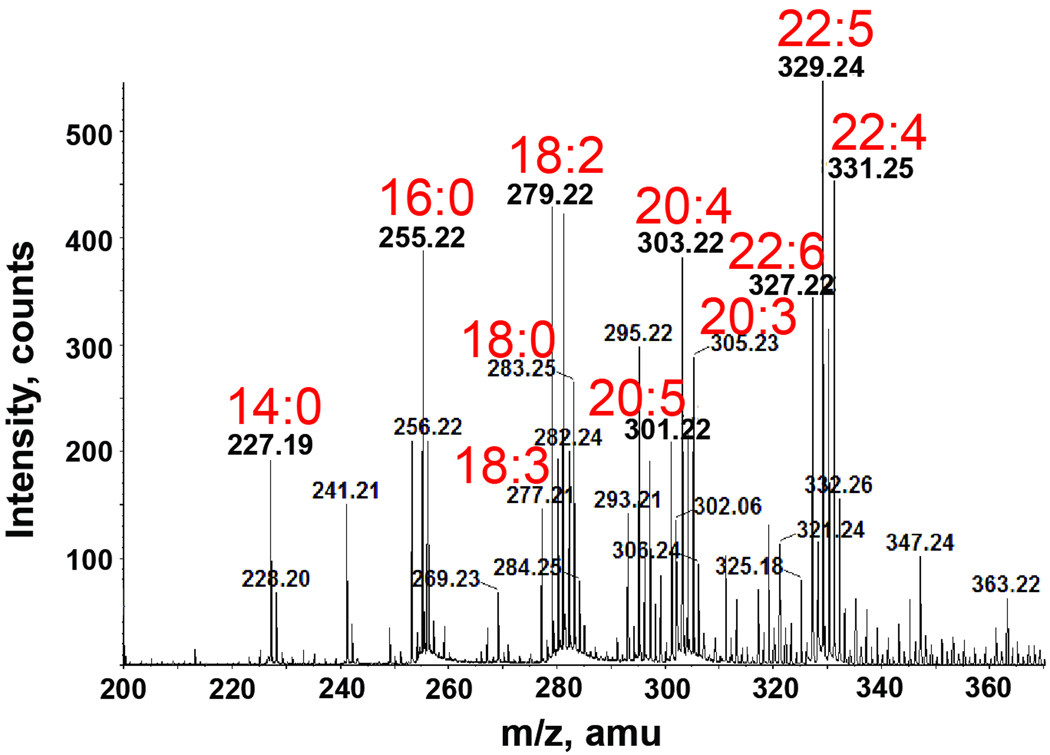
Fig. 6. Negative-ion ESI-MS analysis of the fatty acid content in crude bile.
Various long chain polyunsaturated fatty acids (18:2, 18:3, 20:3, 20:4, 20:5, 22:4, 22:5 and 22:6) were identified as constituents of bile.
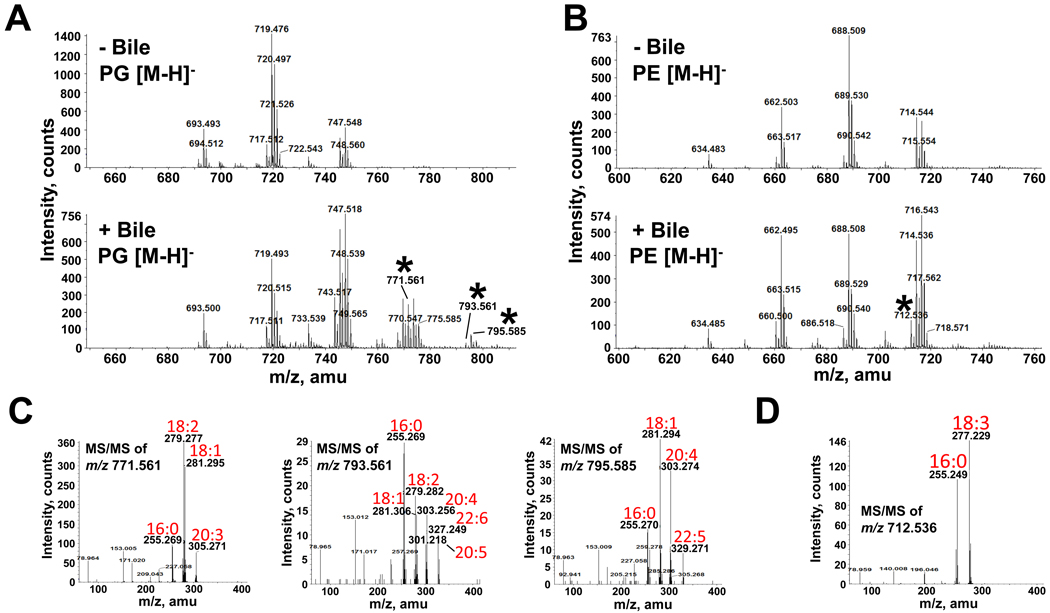
Incorporation of these longer chain polyunsaturated fatty acids was not limited to CL, since ESI-MS of both PE and PG from bile-exposed V. cholerae also identified unique species (Fig. 7A and B). Fragmentation of newly detected [M-H]− ions from bile-exposed V. cholerae PG revealed species containing the bile-specific fatty acids C20:3, C20:4, C20:5, C22:5 and C22:6 (Fig. 7C). MS/MS of the PG [M-H]− ion peak at m/z 771.561 reveals that it is a mixture of PG (C16:0/C20:3) and PG (C18:1/C18:2) (predicted m/z of 772.53). MS/MS of the PG [M-H]− ion peak at m/z 793.561 predicts three PG species: C16:0/C22:6, C18:2/C20:4 and C18:1/C20:5 (predicted m/z of 794.51). MS/MS of the PG [M-H]− ion peak at m/z 795.561 shows that it is a mixture of PG (C16:0/C22:5) and PG (C18:1/C20:4) (predicted m/z of 796.53). MS/MS of the PE [M-H]− ion peak at m/z 712.536 predicted a PE species containing C16:0/C18:3 (predicted m/z of 713.50) (Fig. 7D), identifying yet another newly incorporated fatty acid, C18:3. Some of these new fatty acyl constituents were also found in the precursor to phospholipid synthesis, phosphatidic acid (PA) (data not shown). In summary, V. cholerae acquires fatty acids from bile and assimilates them into its phospholipids. Importantly, when the same LC-MS/MS analysis was employed for E. coli, no new phospholipid species were identified (Fig. S5).
Fig. 7. Mass spectrometric analysis of PG and PE extracted from V. cholerae grown in the presence and absence of bile.
A and B. Negative-ion ESI-MS of [M-H]− ions of V. cholerae PG (A) and PE (B) isolated from cultures grown in the presence and absence of bile. Selected peaks (*) found only in the presence of bile were subsequently analyzed by MS/MS.
C. Negative-ion MS/MS of the PG [M-H]− ions at m/z 771.561, 793.561 and 795.585 detected only in the presence of bile. These product-ion spectra revealed the presence of numerous long chain polyunsaturated fatty acids (18:2, 20:3, 20:4, 22:5 and 22:6) as constituent of PG.
D. Negative-ion MS/MS of the PE [M-H]− ion at m/z 712.536 detected only in the presence of bile. This product-ion spectrum revealed the presence of a fatty acid (18:3) unique to bile.
Identification of the unknown phospholipid
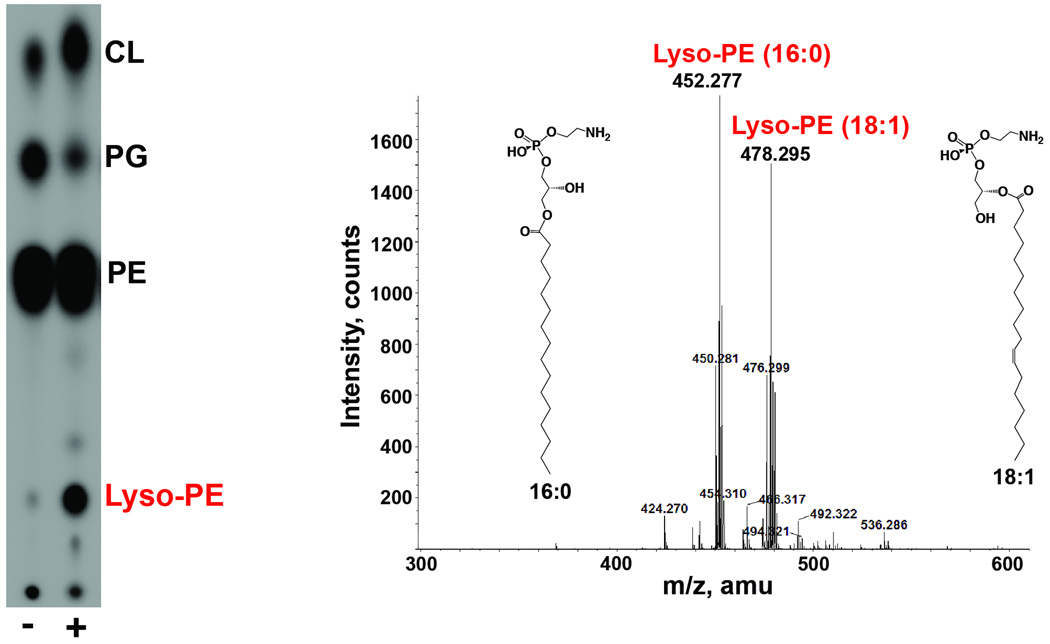
Although several minor unknown phospholipids were apparent following growth in bile, we chose to further analyze the unknown phospholipid that appeared most frequently and most abundantly among Vibrio species. This unknown V. cholerae phospholipid was isolated by preparative TLC and analyzed by ESI-MS/MS. The phospholipid was determined to be two species of lyso-phosphatidylethanolamine (lyso-PE), one harboring C18:1 and the other containing C16:0 (Fig. 8). Neither of these acyl chains represents fatty acids detected only in bile. None of the individual bile components used in this study induced the production of lyso-PE to the level observed with crude bile (Fig. S4). However, regardless of growth media or conditions, lyso-PE was consistently produced to 25–30% of total lipids when 0.4% bile was included.
Fig. 8. Mass spectrometry identification of the unknown phospholipids.
The unknown phospholipid was purified by preparative TLC. The lipid was identified as lyso-phosphatidylethanolamine by ESI-MS, revealing two different acyl chain constituents, 16:0 and 18:1. The structures displayed are speculative since the acyl chain could be at either the sn-1 or sn-2 position.
V. cholerae also acquires fatty acids from the sediment
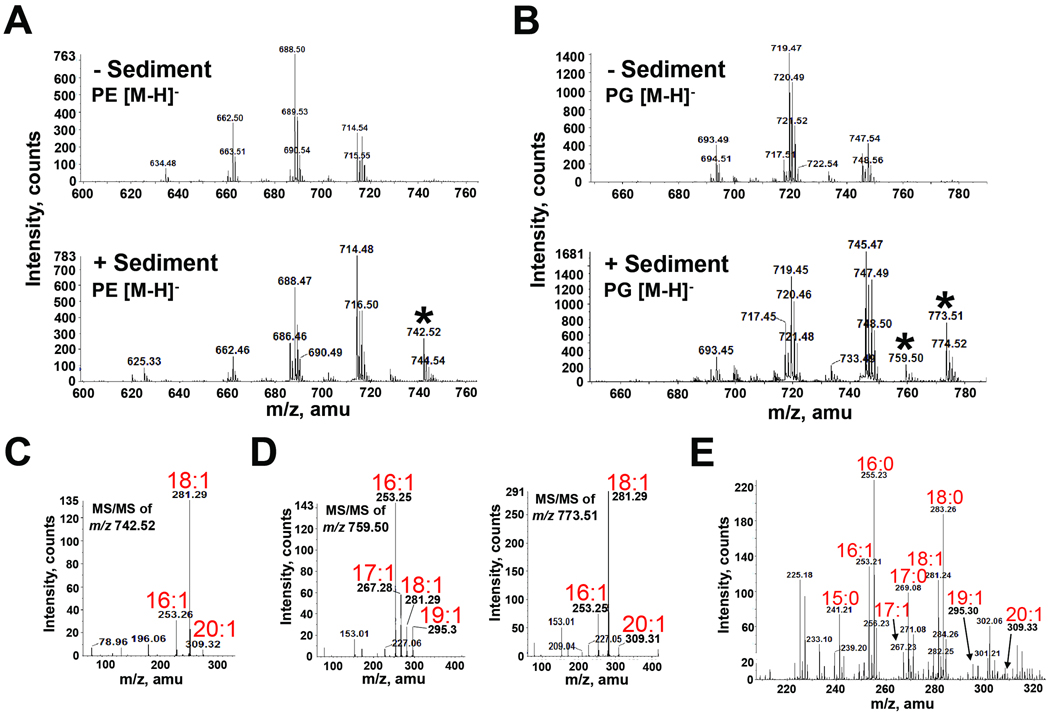
Known to inhabit the pelagic zone, V. cholerae was also recently found naturally occurring in the benthic zone, particularly in the upper surface layer (sediment) of the ocean (Vezzulli et al., 2009). To determine if this utilization of unique fatty acids translates to the aquatic environment, V. cholerae was grown in the presence of sediment obtained from a salt marsh in the Gulf of Mexico. After growth with sediment, LC-MS/MS analysis of V. cholerae phospholipids uncovered new species of PE and PG not seen with growth in LB alone or in bile (Fig. 9A–D). These species were shown to contain C17:1, C19:1 and C20:1, unique fatty acids not identified in bile. MS/MS of the PE [M-H]− ion peak at m/z 742.52 predicts PE species of C16:1/C20:1 and C18:1/C18:1 (predicted m/z of 743.55). MS/MS of the PG [M-H]− ion peak at m/z 759.50 predicts PG species of C17:1/C18:1 and C16:1/C19:1 (predicted m/z of 760.53). MS/MS of the PG [M-H]− ion peak at m/z 773.51 predicts PG species of C16:1/C20:1 and C18:1/C18:1 (predicted m/z of 774.54), the same fatty acid constituents as identified in PE. Eicosenoic acid (C20:1) and odd chain C17:1 and C19:1 represent monounsaturated fatty acids not present in V. cholerae phospholipids grown under normal conditions. Fatty acid analysis confirmed sediment as the source of these fatty acids (Fig. 9E). CL was also found to contain the new fatty acids (Fig. S6). Thus, sediment serves as another environmentally relevant source of fatty acids that V. cholerae acquires and uses in its own phospholipids. Growth in sediment also caused V. cholerae to produce the same two species of lyso-PE identified in Fig. 7 (data not shown).
Fig. 9. Mass spectrometric analysis of PG and PE extracted from V. cholerae grown in the presence and absence of sediment indicates assimilation of fatty acids present in ocean sediment.
A and B. Negative-ion ESI mass spectra of [M-H]− ions of V. cholerae PE (A) and PG (B) isolated from cultures grown in the presence and absence of sediment. Selected peaks (*) found only in the presence of sediment were subsequently analyzed by MS/MS.
C. Negative-ion MS/MS of the PE [M-H]− ion at m/z 742.52 detected only in the presence of sediment. This product-ion spectra revealed the presence of the monounsaturated fatty acid 20:1 as a constituent of PE.
D. Negative-ion MS/MS analysis of the product-ion spectra of the PG [M-H]− ions at m/z 759.5 and 773.51 detected only in the presence of sediment. These [M-H]− ions revealed the presence of the monounsaturated fatty acids 17:1, 19:1 and 20:1 as constituents of PG.
E. Negative-ion ESI-MS analysis of the fatty acid content in sediment identified unique fatty acids (17:1, 19:1 and 20:1) found in V. cholerae PLs following growth in sediment.
V. cholerae can assimilate a variety of long chain polyunsaturated fatty acids
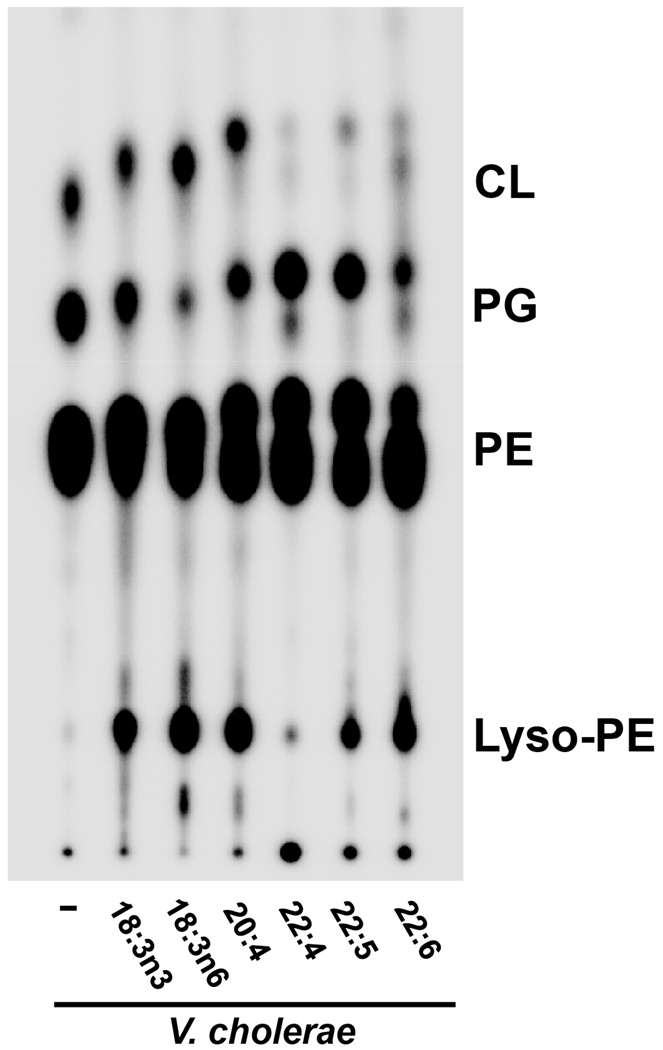
Having identified fatty acids in bile that were subsequently incorporated into V. cholerae, but not E. coli, phospholipids, we sought to examine the exogenous utilization of these fatty acids when supplemented individually. The unique, long chain polyunsaturated fatty acids C18:3, C20:4, C22:4, C22:5 and C22:6 were added individually to LB at a final concentration of 500µM prior to inoculation with V. cholerae. TLC analysis of isolated phospholipids revealed that V. cholerae phospholipids migrated faster, implying the presence of more hydrophobic species and therefore incorporation of the exogenously added long chain polyunsaturated fatty acids (Fig. 10). The V. cholerae phospholipid profile also reveals the presence of two distinct species of PE, PG and CL, suggesting that the bacteria is producing both a de novo species and an exogenously-derived species. Furthermore, all of these fatty acids except for adrenic acid (22:4) induced formation of lyso-PE in V. cholerae.
Fig. 10. Phospholipid profiles of V. cholerae grown in media supplemented with individual long chain PUFAs.
Long chain PUFAs are incorporated into V. cholerae phospholipids that reflect incorporation of each exogenous fatty acid. In some profiles, two distinct species of phospholipids can be distinguished, indicating usage of both endogenous and exogenous pools of fatty acids for phospholipid synthesis. 18:3n3, α-linolenic acid; 18:3n6, γ-linolenic acid; 20:4, arachidonic acid; 22:4, adrenic acid; 22:5, Docosapentaenoic acid; 22:6, Docosahexaenoic acid.
Discussion
The current study clearly demonstrates that V. cholerae assimilates fatty acids from the surrounding environment. It is unknown what advantage this observed remodeling of V. cholerae phospholipids confers. Bacteria modify their membrane lipid composition upon exposure to environmental changes, such as temperature, osmolarity and salinity (Cronan & Gelmann, 1975, Denich et al., 2003, Janmey & Kinnunen, 2006, Zhang & Rock, 2008). Our results indicate that incorporation of exogenous fatty acids may represent another environmental factor that can induce homeoviscous adaptation in V. cholerae. Although the ratio of anionic to zwitterionic lipids remained constant in bile-exposed V. cholerae, the observed increase in CL, decrease in PG, decrease in PE, increase in lyso-PE and adoption of long chain polyunsaturated fatty acids could bestow varied characteristics for the bacterial membrane bilayer.
In E. coli, osmotic stress and growth in high salinity result in an increased CL to PE ratio (Romantsov et al., 2009). However, this adaptation was not observed when V. cholerae was grown in varying osmotic and salt conditions (data not shown), indicating a bile-specific response with regard to phospholipid fluctuations. An intriguing property of CL is its ability to interact with a wide variety of proteins in the energy-transducing membranes of both eukaryotes and prokaryotes (Mileykovskaya et al., 2005, Mileykovskaya & Dowhan, 2009). Bacterial proteins that interact with CL include the Paracoccus denitrificans cytochrome c oxidase, the Rhodobacter sphaeroides reaction center, and both the E. coli succinate dehydrogenase and osmosensory transporter ProP (Iwata et al., 1995, McAuley et al., 1999, Yankovskaya et al., 2003, Romantsov et al., 2007, Romantsov et al., 2008). In V. cholerae, CL binds the EpsE/cyto-EpsL complex, a type II secretion complex that supports secretion of cholera toxin (Camberg et al., 2007). Although bile (0.2–0.4%) has been shown to decrease production of cholera toxin, the bile-induced increase in CL may support secretion of cholera toxin following colonization at the intestinal mucosae, where bile concentration is expected to be much lower (Hofmann, 1993).
The production of lyso-PE at such high an amount (~30%) is somewhat perplexing, especially considering that V. cholerae does not possess a homolog for outer membrane phospholipase A (OMPLA), a widespread protein among Gram-negative bacteria involved in organic solvent tolerance and bacteriocin secretion (Pugsley & Schwartz, 1984, Pedrotta & Witholt, 1999, Dekker, 2000). Production of the two lyso-PE species (Fig. 8) could be a result of the following: the activity of a single phospholipase that is active at both the sn-1 and sn-2 position, the activity of a single phospholipase that is active on two species of PE, or the participation of two separate enzymes. Owing to the aquatic nature of Vibrio, the significant presence of lyso-PE may not be surprising considering that this lysophospholipid has been identified in significant quantities among numerous Gram-negative marine bacteria (Oliver & Colwell, 1973, Bhakoo & Herbert, 1979). Lyso-PE was documented as the third most abundant phospholipid found in several Vibrio species, including V. cholerae, grown in artificial seawater medium, mirroring the results obtained during growth in bile (Oliver & Colwell, 1973).
The bacterial utilization of fatty acids has been extensively, yet incompletely, studied. As defined in E. coli, long-chain fatty acids are bound and transported through the outer membrane by FadL, ferried through the periplasm by an unknown mechanism, traverse the inner membrane via a proton electrochemical gradient-dependent process, and are converted to acyl-CoA thioesters by FadD at the inner membrane (DiRusso & Black, 1999). The long-chain fatty acyl-CoA is then targeted for β-oxidation, phospholipid synthesis or participates in transcriptional regulation. Using the clusters of orthologous groups (COG) and basic local alignment search tool (BLAST) databases, V. cholerae was found to encode 3 homologs for FadL and 3 predicted genes encoding FadD, whereas E. coli only has one copy of each. Furthermore, V. cholerae possesses homologs to all of the known Gram-negative and Gram-positive acyltransferases (PlsB, PlsC, PlsX and PlsY) that are involved in forming precursors to phospholipids from exogenous and/or de novo fatty acids. The possession of such machinery not only may explain the broader capability of V. cholerae to take up and assimilate the PUFAs, but also may introduce a more efficient mechanism that confers versatility for surviving in marine and host environments. Our data suggests that V. cholerae converts exogenous fatty acids into acyl-CoAs that are used by acyltransferases for incorporation into PA, the precursor for phospholipid synthesis. Accordingly, we observed these exogenous fatty acids in each of the major bacterial phospholipids. The assimilation of exogenous linoleic and arachidonic acid was recently demonstrated in S. agalactiae, illustrating that Gram-positive bacteria possess a mechanism for uptake and utilization of diverse fatty acids (Brinster et al., 2009). Interestingly, V. cholerae possesses a homolog for the cis-trans isomerase, an enzyme found only in some aquatic organisms and Pseudomonas sp. (Pedrotta & Witholt, 1999). This enzyme would be critical for the insertion of PUFAs into the membrane since the cis conformation would introduce significant kinks, ergo more fluidity, into the membrane.
PUFAs, particularly eicosapentaenoic (EPA) (C22:5) and docosahexaenoic acid (DHA) (22:6), are ubiquitous in marine environments (Berge & Barnathan, 2005). EPA and DHA are synthesized by some marine bacteria and have been shown to provide antioxidative properties, even when incorporated into the membrane of a genetically modified mutant of E. coli (Nichols et al., 1997, Methe et al., 2005, Nishida et al., 2006, Okuyama et al., 2008). Oxidative stress in the ocean originates from the prevalence of hydrogen peroxide (H2O2) and other reactive oxygen species produced photochemically by the adsorption of solar radiation by dissolved organic matter in seawater. H2O2 is relatively stable in seawater and can pass readily through biological membranes, thus making it a constant threat to marine microorganisms (Lesser, 2006). Nitric oxide (NO), another source of oxidative stress, is produced during human V. cholerae infection, and both LPS and cholera toxin have been implicated in NO release in animal models (Janoff et al., 1997, Paulovicova et al., 2010). The incorporation of PUFAs may allow V. cholerae to resist toxic reactive oxygen species encountered in both aquatic and host environments.
Based on our results, V. cholerae may adopt a phospholipid profile that reflects the fatty acid composition of its surrounding environment, perhaps as a mechanism of homeoviscous adaptation to survive membrane stress. Interestingly, bile phospholipids contain fair amounts of PUFAs as compared with other mammalian lipids (Hay et al., 1993, Tazuma et al., 1994, Braun et al., 2009). In the host environment, V. cholerae may utilize PUFAs that are intrinsically present or generated in the intestine. V. cholerae has been shown to secrete a lecithinase that hydrolyzes lecithin (L-α-phosphatidylcholine), a major component of bile, which would liberate a fatty acid from lecithin or lysolecithin (Esselmann & Liu, 1961, Fiore et al., 1997). In the aquatic environment, V. fischeri has been shown to adopt different fatty acid profiles depending upon its symbiotic location within the squid (Wier et al., 2010). Our results strengthen the potential importance of exogenous fatty acid utilization for Vibrio species, especially between environmental reservoirs. Vibrio may have evolved this adaptation as an efficient means for both maintenance of the membrane and carbon source acquisition. The exogenous fatty acid utilization machinery and possible membrane attributes conferred by the inherited phospholipid profile are under current investigation.
Experimental procedures
Bacterial strains and growth conditions
The bacterial strains used in this study are summarized in Table S1. Bacteria were typically grown at 37°C in Luria-Bertani broth (EMD Chemicals). For V. parahaemolyticus, the media was supplemented with 3% NaCl. V. fischeri was grown in marine broth (Becton Dickinson). Some experiments were performed at 37°C in AKI medium as described previously (Iwanaga & Yamamoto, 1985). G56 minimal media (pH 7.5) with 0.3mM potassium phosphate was used (Ganong et al., 1980). Growth was also achieved within chambers containing microaerobic atmosphere (5% O2, 10% CO2, and 85% N2) or anaerobic atmosphere (2% H2) (Coy Laboratory Products). Inhibition of protein and fatty acid synthesis was achieved by administering the appropriate inhibitor (chloramphenicol: 150ug/ml; cerulenin: 20ug/ml) prior to addition of the 32Pi and subsequent incubation for 10 minutes.
Isolation and analysis of phospholipid species from 32Pi-labeled cells
Cultures were labeled uniformly with 2.5 µCi/ml 32Pi and grown to an OD of 0.7–1.0 prior to harvesting using a clinical centrifuge and washing with 5 ml of phosphate-buffered saline (pH 7.4). Lipids were extracted by the method of Bligh and Dyer (Bligh & Dyer, 1959) and spotted onto a Silica Gel 60 TLC plate (25,000 or 50,000 cpm/lane). Lipids were separated using a solvent system consisting of chloroform, methanol, and acetic acid (65:25:10, vol/vol). The TLC plates were exposed overnight to a PhosphorImager screen, and product formation was detected and analyzed using a Bio-Rad Molecular Imager PhosphorImager equipped with Quantity One software. Densitometry was calculated for the major phospholipids observed by TLC. For percentage calculations, the density of CL was halved for normalization to the other single phosphate-containing lipids.
Gas Chromatography/Mass Spectrometry
Fatty acid methyl esters were prepared from mild acid treatment by methanolysis in 1M HCl in methanol at 80°C (16 hours). GC/MS analysis of the fatty acid methyl esters was performed on an AT 6890N GC interfaced to a 5975B MSD, using a Supelco EC-1 fused silica capillary column (30m × 0.25 mm ID). Relative percentages were calculated by measuring the peak areas of each fatty acid, except for instances of multiple C18:1 and C16:1, of which the peak areas were combined.
Liquid Chromatography/ESI-Mass Spectrometry
Normal phase LC-ESI/MS of lipids was performed using an Agilent 1200 Quaternary LC system coupled to a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems, Foster City, CA). An Ascentis® Si HPLC column (5 µm, 25 cm × 2.1 mm) was used. Mobile phase A consisted of chloroform/methanol/aqueous ammonium hydroxide (800:195:5, v/v/v). Mobile phase B consisted of chloroform/methanol/water/aqueous ammonium hydroxide (600:340:50:5, v/v/v/v). Mobile phase C consisted of chloroform/methanol/water/aqueous ammonium hydroxide (450:450:95:5, v/v/v/v). The elution program consisted of the following: 100% mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 11 min. The LC gradient was then changed to 100% mobile phase C over 3 min and held at 100% C for 3 min, and finally returned to 100% A over 0.5 min and held at 100% A for 5 min. The total LC flow rate was 3 µl/min. The post-column splitter diverted ~10% of the LC flow to the ESI source of the Q-Star XL mass spectrometer, with MS settings as follows: IS = −4500 V, CUR = 20 psi, GS1 = 20 psi, DP = −55 V, and FP = −150 V. Nitrogen was used as the collision gas for MS/MS experiments. Data acquisition and analysis were performed using the instrument’s Analyst QS software.
Purification of cardiolipin and the unknown phospholipids
200ml cultures were grown with and without 0.4% bile to an A600 of ~1.0 prior to harvesting and washing with PBS. Large-scale lipid extraction by the method of Bligh and Dyer (Bligh & Dyer, 1959) was followed by spotting the total phospholipid fraction onto Silica Gel 60 TLC plates and separation using a solvent system consisting of chloroform, methanol and acetic acid (65:25:10, v/v). The plates were sprayed with water to visualize the lipid bands and the silica containing CL and the unknown phospholipid was scraped from the plate. Pure lipid was extracted from the silica by using the method of Bligh and Dyer (Bligh & Dyer, 1959). The individual lipids were dried under nitrogen and weighed prior to subsequent analyses.
Materials
Bovine bile, ox bile, porcine bile, bovine/ovine bile, bile acids, lecithin and bilirubin were obtained from Sigma. Also purchased from Sigma were cerulenin, γ-linolenic acid, arachidonic acid and docosahexaenoic acid. α-linolenic acid, adrenic acid and docosapentaenoic acid were obtained from Cayman Chemical Company. 32Pi was purchased from PerkinElmer Life Sciences and Silica gel 60 (0.25 mm) thin layer plates were purchased from EM Separation Technology (Merck). Virstatin was purchased from Alexis Biochemicals. All other chemicals were reagent grade and were purchased from Fisher. The 2-inch plug of sediment used in this study was taken from a salt marsh that is part of the Wetlands Education Center at the University of Texas Marine Science Institute in Port Aransas, TX.
Supplementary Material
Acknowledgments
This work was supported by NIH grant AI76322 to M.S.T. and by the LIPID MAPS Large Scale Collaborative Grant GM06933 to Duke University Medical Center. We thank other members of the Trent laboratory for critical reading of the manuscript.
References
- Berge JP, Barnathan G. Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv Biochem Eng Biotechnol. 2005;96:49–125. doi: 10.1007/b135782. [DOI] [PubMed] [Google Scholar]
- Bhakoo M, Herbert RA. The Effects of Temperature on the Fatty Acid and Phospholipid Composition of Four Obligately Psychrophilic. Vibrio Spp. Arch Microbiol. 1979;121:121–127. [Google Scholar]
- Bina JE, Mekalanos JJ. Vibrio cholerae tolC is required for bile resistance and colonization. Infect Immun. 2001;69:4681–4685. doi: 10.1128/IAI.69.7.4681-4685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blomstrand R, Ekdahl P. Fatty acid pattern of human bile under normal and pathological conditions. Proc Soc Exp Biol Med. 1960;104:205–209. doi: 10.3181/00379727-104-25780. [DOI] [PubMed] [Google Scholar]
- Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G, Lehmann WD, Fuellekrug J, Stremmel W, Ehehalt R. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis. 2009;15:1705–1720. doi: 10.1002/ibd.20993. [DOI] [PubMed] [Google Scholar]
- Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature. 2009;458:83–86. doi: 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- Camberg JL, Johnson TL, Patrick M, Abendroth J, Hol WG, Sandkvist M. Synergistic stimulation of EpsE ATP hydrolysis by EpsL and acidic phospholipids. EMBO J. 2007;26:19–27. doi: 10.1038/sj.emboj.7601481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda-Maira FA, Ringelberg CS, Taylor RK. The bile response repressor BreR regulates expression of the Vibrio cholerae breAB efflux system operon. J Bacteriol. 2008;190:7441–7452. doi: 10.1128/JB.00584-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Chaudhuri S, Saha G, Gupta S, Chowdhury R. Effect of bile on the cell surface permeability barrier and efflux system of Vibrio cholerae. J Bacteriol. 2004;186:6809–6814. doi: 10.1128/JB.186.20.6809-6814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Dutta PK, Chowdhury R. Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect Immun. 2007;75:1946–1953. doi: 10.1128/IAI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers BM, Klose KE. Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol. 2007;2:335–344. doi: 10.2217/17460913.2.3.335. [DOI] [PubMed] [Google Scholar]
- Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- Cronan JE, Jr, Gelmann EP. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975;39:232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agnolo G, Rosenfeld IS, Awaya J, Omura S, Vagelos PR. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973;326:155–156. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- Dekker N. Outer-membrane phospholipase A: known structure, unknown biological function. Mol Microbiol. 2000;35:711–717. doi: 10.1046/j.1365-2958.2000.01775.x. [DOI] [PubMed] [Google Scholar]
- Denich TJ, Beaudette LA, Lee H, Trevors JT. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J Microbiol Methods. 2003;52:149–182. doi: 10.1016/s0167-7012(02)00155-0. [DOI] [PubMed] [Google Scholar]
- DiRusso CC, Black PN. Long-chain fatty acid transport in bacteria and yeast. Paradigms for defining the mechanism underlying this protein-mediated process. Mol Cell Biochem. 1999;192:41–52. [PubMed] [Google Scholar]
- Esselmann MT, Liu PV. Lecithinase production by gramnegative bacteria. J Bacteriol. 1961;81:939–945. doi: 10.1128/jb.81.6.939-945.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore AE, Michalski JM, Russell RG, Sears CL, Kaper JB. Cloning, characterization, and chromosomal mapping of a phospholipase (lecithinase) produced by Vibrio cholerae. Infect Immun. 1997;65:3112–3117. doi: 10.1128/iai.65.8.3112-3117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganong BR, Leonard JM, Raetz CR. Phosphatidic acid accumulation in the membranes of Escherichia coli mutants defective in CDP-diglyceride synthetase. J Biol Chem. 1980;255:1623–1629. [PubMed] [Google Scholar]
- Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000;2:907–913. doi: 10.1016/s1286-4579(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Gupta S, Chowdhury R. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun. 1997;65:1131–1134. doi: 10.1128/iai.65.3.1131-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DW, Cahalane MJ, Timofeyeva N, Carey MC. Molecular species of lecithins in human gallbladder bile. J Lipid Res. 1993;34:759–768. [PubMed] [Google Scholar]
- Hofmann AF. The enterohepatic circulation of bile acids in health and disease. In: Sleisenger MH, Fordtran JS, editors. Gastrointestinal Disease: Pathophysiology, Diagnosis, Management. 5th ed. Philadelphia: Saunders; 1993. pp. 127–150. [Google Scholar]
- Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science. 2005;310:670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- Hung DT, Zhu J, Sturtevant D, Mekalanos JJ. Bile acids stimulate biofilm formation in Vibrio cholerae. Mol Microbiol. 2006;59:193–201. doi: 10.1111/j.1365-2958.2005.04846.x. [DOI] [PubMed] [Google Scholar]
- Huq A, Grim C, Colwell RR, Nair GB. Chapter 6. Detection, isolation, and identification of Vibrio cholerae from the environment. Curr Protoc Microbiol. 2006;(Unit6A 5) doi: 10.1002/9780471729259.mc06a05s02. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M, Yamamoto K. New medium for the production of cholera toxin by Vibrio cholerae O1 biotype El Tor. J Clin Microbiol. 1985;22:405–408. doi: 10.1128/jcm.22.3.405-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Kinnunen PK. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006;16:538–546. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Janoff EN, Hayakawa H, Taylor DN, Fasching CE, Kenner JR, Jaimes E, Raij L. Nitric oxide production during Vibrio cholerae infection. Am J Physiol. 1997;273:G1160–G1167. doi: 10.1152/ajpgi.1997.273.5.G1160. [DOI] [PubMed] [Google Scholar]
- Lesser MP. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- McAuley KE, Fyfe PK, Ridge JP, Isaacs NW, Cogdell RJ, Jones MR. Structural details of an interaction between cardiolipin and an integral membrane protein. Proc Natl Acad Sci U S A. 1999;96:14706–14711. doi: 10.1073/pnas.96.26.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt ME, Donaldson JR. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol. 2009;58:1533–1541. doi: 10.1099/jmm.0.014092-0. [DOI] [PubMed] [Google Scholar]
- Methe BA, Nelson KE, Deming JW, Momen B, Melamud E, Zhang X, Moult J, Madupu R, Nelson WC, Dodson RJ, Brinkac LM, Daugherty SC, Durkin AS, DeBoy RT, Kolonay JF, Sullivan SA, Zhou L, Davidsen TM, Wu M, Huston AL, Lewis M, Weaver B, Weidman JF, Khouri H, Utterback TR, Feldblyum TV, Fraser CM. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc Natl Acad Sci U S A. 2005;102:10913–10918. doi: 10.1073/pnas.0504766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta. 2009;1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E, Zhang M, Dowhan W. Cardiolipin in energy transducing membranes. Biochemistry (Mosc) 2005;70:154–158. doi: 10.1007/s10541-005-0095-2. [DOI] [PubMed] [Google Scholar]
- Mingrone G, Greco AV, Passi S. The possible role of free fatty acids in the pathogenesis of cholesterol gallstones in man. Biochim Biophys Acta. 1983;751:138–144. doi: 10.1016/0005-2760(83)90167-4. [DOI] [PubMed] [Google Scholar]
- Nakayama F. Quantitative microanalysis of bile. J Lab Clin Med. 1967;69:594–609. [PubMed] [Google Scholar]
- Nakayama F, Johnston CG. Fractionation of bile lipids with silicic acid column chromatography. J Lab Clin Med. 1962;59:364–370. [PubMed] [Google Scholar]
- Nakayama F, Oishi M, Sakaguchi N, Miyake H. Thin-Layer Chromatography of Bile Lipids. Clin Chim Acta. 1964;10:544–548. doi: 10.1016/0009-8981(64)90192-5. [DOI] [PubMed] [Google Scholar]
- Nichols DS, Nichols PD, Russell NJ, Davies NW, McMeekin TA. Polyunsaturated fatty acids in the psychrophilic bacterium Shewanella gelidimarina ACAM 456T: molecular species analysis of major phospholipids and biosynthesis of eicosapentaenoic acid. Biochim Biophys Acta. 1997;1347:164–176. doi: 10.1016/s0005-2760(97)00068-4. [DOI] [PubMed] [Google Scholar]
- Nishida T, Orikasa Y, Ito Y, Yu R, Yamada A, Watanabe K, Okuyama H. Escherichia coli engineered to produce eicosapentaenoic acid becomes resistant against oxidative damages. FEBS Lett. 2006;580:2731–2735. doi: 10.1016/j.febslet.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Okuyama H, Orikasa Y, Nishida T. Significance of antioxidative functions of eicosapentaenoic and docosahexaenoic acids in marine microorganisms. Appl Environ Microbiol. 2008;74:570–574. doi: 10.1128/AEM.02256-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD, Colwell RR. Extractable lipids of gram-negative marine bacteria: phospholipid composition. J Bacteriol. 1973;114:897–908. doi: 10.1128/jb.114.3.897-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovicova E, Kovacova E, Bystricky S. Vibrio cholerae O1 Ogawa detoxified lipopolysaccharide structures as inducers of cytokines and oxidative species in macrophages. J Med Microbiol. 2010;59:158–164. doi: 10.1099/jmm.0.013599-0. [DOI] [PubMed] [Google Scholar]
- Pedrotta V, Witholt B. Isolation and characterization of the cis-trans-unsaturated fatty acid isomerase of Pseudomonas oleovorans GPo12. J Bacteriol. 1999;181:3256–3261. doi: 10.1128/jb.181.10.3256-3261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto AI, Ramos-Morales F, Casadesus J. Bile-induced DNA damage in Salmonella enterica. Genetics. 2004;168:1787–1794. doi: 10.1534/genetics.104.031062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty AM, Van Velkinburgh JC, Gunn JS. Salmonella enterica serovar typhimurium resistance to bile: identification and characterization of the tolQRA cluster. J Bacteriol. 2002;184:1270–1276. doi: 10.1128/JB.184.5.1270-1276.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano D, Klose KE. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc Natl Acad Sci U S A. 2000;97:10220–10224. doi: 10.1073/pnas.170219997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley AP, Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984;3:2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raziuddin SR. Effect of growth temperature and culture age on the lipid composition of Vibrio cholerae 569B (Inaba) J Gen Microbiol. 1976;94:367–372. doi: 10.1099/00221287-94-2-367. [DOI] [PubMed] [Google Scholar]
- Romantsov T, Guan Z, Wood JM. Cardiolipin and the osmotic stress responses of bacteria. Biochim Biophys Acta. 2009;1788:2092–2100. doi: 10.1016/j.bbamem.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romantsov T, Helbig S, Culham DE, Gill C, Stalker L, Wood JM. Cardiolipin promotes polar localization of osmosensory transporter ProP in Escherichia coli. Mol Microbiol. 2007;64:1455–1465. doi: 10.1111/j.1365-2958.2007.05727.x. [DOI] [PubMed] [Google Scholar]
- Romantsov T, Stalker L, Culham DE, Wood JM. Cardiolipin controls the osmotic stress response and the subcellular location of transporter ProP in Escherichia coli. J Biol Chem. 2008;283:12314–12323. doi: 10.1074/jbc.M709871200. [DOI] [PubMed] [Google Scholar]
- Rosenberg EY, Bertenthal D, Nilles ML, Bertrand KP, Nikaido H. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol Microbiol. 2003;48:1609–1619. doi: 10.1046/j.1365-2958.2003.03531.x. [DOI] [PubMed] [Google Scholar]
- Schuhmacher DA, Klose KE. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J Bacteriol. 1999;181:1508–1514. doi: 10.1128/jb.181.5.1508-1514.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderovich Y, Izhaki I, Halpern M. Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE. 2010;5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci U S A. 2007;104:2372–2377. doi: 10.1073/pnas.0611643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazuma S, Hatsushika S, Yamashita G, Aihara N, Sasaki M, Horikawa K, Yamashita Y, Teramen K, Ochi H, Hirano N, et al. Simultaneous microanalysis of biliary cholesterol, bile acids and fatty acids in lecithin using capillary column gas chromatography: an advantage to assess bile lithogenecity. J Chromatogr B Biomed Appl. 1994;653:1–7. doi: 10.1016/0378-4347(93)e0422-m. [DOI] [PubMed] [Google Scholar]
- Vezzulli L, Pezzati E, Moreno M, Fabiano M, Pane L, Pruzzo C. Benthic ecology of Vibrio spp. and pathogenic Vibrio species in a coastal Mediterranean environment (La Spezia Gulf, Italy) Microb Ecol. 2009;58:808–818. doi: 10.1007/s00248-009-9542-8. [DOI] [PubMed] [Google Scholar]
- Wier AM, Nyholm SV, Mandel MJ, Massengo-Tiasse RP, Schaefer AL, Koroleva I, Splinter-Bondurant S, Brown B, Manzella L, Snir E, Almabrazi H, Scheetz TE, Bonaldo Mde F, Casavant TL, Soares MB, Cronan JE, Reed JL, Ruby EG, McFall-Ngai MJ. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc Natl Acad Sci U S A. 2010;107:2259–2264. doi: 10.1073/pnas.0909712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- Yokota K, Kanamoto R, Kito M. Composition of cardiolipin molecular species in Escherichia coli. J Bacteriol. 1980;141:1047–1051. doi: 10.1128/jb.141.3.1047-1051.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.