Abstract
Objective
To review and meta-analyse the studies evaluating the status of serum inflammatory markers in women with Polycystic Ovary Syndrome (PCOS).
Design
Systematic review and meta-analysis of articles published in English before January 2010 and identified using the Entrez-PubMed engine.
Setting
Academic hospital
Interventions
Measurement of serum concentrations of inflammatory markers by high-sensitivity techniques.
Main Outcome Measures
Meta-analyses of the mean difference in serum C-reactive protein (CRP), interlekin-6 (IL-6) and tumor necrosis factor-α (TNF-α) concentrations among patients with PCOS and appropriate controls, applying random-effects models to limit interstudy variability, and using appropriate estimates of evidence dissemination bias.
Results
Meta-analysis of the 31 articles meeting inclusion criteria showed that circulating CRP was 96% higher in women with PCOS compared to controls (95% confidence interval 71% – 122%, z = 7.32, p < 0.0001) without evidence dissemination bias (Egger’s regression intercept 0.45, 95% confidence interval −2.30 – 3.21, P = 0.739). These findings persisted after excluding five studies with mismatches in body mass and/or frequency of obesity between women with PCOS and controls. Meta-analyses involving 10 studies of IL-6, and 9 studies of TNF-α revealed no statistically significant differences between PCOS and controls.
Conclusion
Women with PCOS exhibit elevation in circulating CRP that is independent of obesity. This finding corroborates existing molecular evidence of the chronic low-grade inflammation that may underpin the pathogenesis of this disorder.
Keywords: Chronic low-grade inflammation, adipose tissue, insulin resistance, obesity, glucose tolerance
Introduction
Chronic low-grade inflammation is involved in the pathogenesis of obesity-related diabetic syndromes. Leukocytes present in both the circulation and adipose-tissue are capable of promoting insulin resistance in obesity and type 2 diabetes (1). Polycystic Ovary Syndrome (PCOS) is also a proinflammatory state. Recent studies demonstrate that a dietary trigger such as glucose is capable of inciting an inflammatory response in mononuclear cells (MNC) of women with PCOS independent of body mass (2–5). There is also an association between inflammation at the molecular level and insulin resistance in the disorder (3–5). Elevations of a number of circulating proatherogenic inflammatory mediators have been independently reported in PCOS (6–9), and corroborated by preliminary reports of glucose-stimulated upregulation of proatherogenic molecular pathways in the disorder (10–12). It remains to be established whether the proinflammatory state in PCOS is primarily a result of inflamed adipose tissue since there is increased prevalence of abdominal adiposity in PCOS across all weight classes (13).
There is a genetic basis for the inflammation observed in PCOS (14). Variants in genes encoding several proinflammatory cytokines and their receptors associated with insulin resistance, obesity and/or diabetes have also been found to be associated with PCOS (15–21). Moreover, variants in the genes encoding tumor necrosis factor-α (TNF-α) (16), type 2 TNF receptor and interleukin-6 (IL-6) (18–20) and its signal transducer (21) have been reported in association with PCOS in European populations. These findings are in conceptual agreement with a common evolutionary background for PCOS and metabolic disorders.
There are numerous studies in the literature reporting elevations of circulating inflammatory molecules in PCOS, it remains unclear whether their elevations are related to PCOS itself, or are a function of obesity and/or abdominal adiposity. There is also controversy regarding the relevance of circulating inflammatory molecules because most proinflammatory mediators exert their effect in tissue in an autocrine and paracrine fashion. In the case of TNF-α, the metabolic effects of this known mediator of insulin resistance are typically estimated indirectly by the soluble fraction of its type 2 receptor (1,22–24). In contrast, IL-6 is an endocrine cytokine produced by MNC and adipose tissue that is directly responsible for stimulating hepatic C-reactive protein (CRP) synthesis (25–28). CRP in turn, has emerged as a major predictor of metabolic dysfunction in asymptomatic individuals, and is also produced by adipose tissue (29,30).
We have conducted a systematic review of the studies published to date addressing the status of circulating inflammatory markers in PCOS compared to non-hyperandrogenic controls. We also performed meta-analyses of studies reporting circulating CRP, TNF-α and IL-6 levels in PCOS to determine whether they reflect the chronic low-grade inflammation intrinsic to the disorder to be of clinically utility.
Materials and Methods
PubMed searches of studies published before January 2010 that addressed PCOS and inflammatory markers were conducted as described in Supplemental Data Table 1. The studies identified were considered for further review only if they fulfilled the following criteria:
Strict definition of PCOS according to National Institute of Health (NIH) criteria (31), European Society of Human Reproduction and Embryology / American Society of Reproductive Medicine (ESHRE / ASRM) Rotterdam criteria (32) or Androgen Excess and PCOS Society (AEPCOS) criteria (33).
Cross-sectional comparison of reproductive-age women with PCOS and non-hyperandrogenic controls.
Measurement of circulating inflammatory molecules concentrations by high-sensitivity methods.
Meta-analyses of CRP, IL-6 and TNF-α serum concentrations were limited to studies that included a minimum of 25 women with PCOS and a similar number of non-hyperandrogenic controls defined as the absence of androgen excess or ovulatory dysfunction. If a series of articles by the same authors was identified, the report with the larger sample size was selected for inclusion in the meta-analysis to avoid over-representation of cases.
The absolute mean concentrations and standard deviations of CRP, IL-6 and TNF-α were standardized as the percent mean in which control groups means represented 100% to adjust for the large difference in absolute means among individual studies. This large difference most likely reflects variability among assays used for measurement.
A separate random-effects model was constructed for each inflammatory marker using the DerSimonian and Laird weighting method, which incorporates between-study variability into the calculations. We selected this method because of the high likelihood that there was empirical heterogeneity resulting from the variability in the ethnicity and race of the subjects, differences among studies in the criteria used to define PCOS, and inconsistent control of confounding factors such as obesity. All calculated p-values are two-sided, and an α level of 0.05 was used to determine statistical significance. Evidence dissemination bias was estimated by funnel plot asymmetry and Egger’s regression test.
Studies reporting the status of circulating inflammatory markers in PCOS other than CRP, IL-6 and TNF-α were systematically reviewed and summarized. However, the paucity of studies (i.e. three or less) addressing each marker precluded performance of meta-analyses.
Results
Meta-analysis of serum CRP concentrations in women with PCOS and controls
Supplemental Data Figure 1 includes the QUORUM flowcharts for the separate meta-analysis of serum CRP, IL-6 and TNF-α concentrations. We identified 125 studies to screen for retrieval that were potentially relevant to serum CRP levels and PCOS (2,6,7,19,20,34–152).
Twelve review articles were immediately excluded (40,43,46,55,60,62,76,89,117,122, 128,136), and the remaining 113 studies were retrieved for a more detailed evaluation.
Twenty four studies were subsequently excluded for the following reasons: 1) Standard assays were used for measuring CRP instead of high-sensitive assays in 7 studies (34,36,37,53,59,63,146); 2) Serum CRP concentrations were not measured in 1 study (145); 3) The relatives of women with PCOS were evaluated instead of actual patients in 2 studies (83,106); 4) Patients with androgen excess disorders other than PCOS were evaluated in 4 studies (50,70,104,127); 5) Only pregnant women with PCOS were evaluated in 3 studies (41,65,108); 6) PCOS was diagnosed with criteria other than the NIH, ESHRE / ASRM or AEPCOS definitions in 7 studies (38,42,44,47,67,75,132).
The remaining 89 studies were considered potentially appropriate. An additional 58 studies were excluded for the following reasons: 1) Less than 25 subjects with PCOS were included in 14 studies (2,49,54,57,67,69,78,82,107,118,121,135,141,147); 2) No controls were included in 28 studies (51,56,66,68,71,74,77,80,81,84–87,95,98,100,102,105,112,115,119,120, 123,126,139,143,148,152); 3) Patients and controls were also included in ulterior extended series by the same authors in 14 studies (7,35,58,92–94,101,110,113,116,125,131,149,150); 4) CRP concentrations were measured as a function of a polymorphism in the gene encoding the progesterone receptor, and not in PCOS and controls separately in 1 study (73); 5) It was unclear whether CRP was measured using a high sensitivity assay in 1 study, and there was no response from the corresponding author to a request for clarification (137).
The remaining 31 studies containing usable information by outcome were used to perform the meta analysis (6,19,39,45,48,52,61,64,72,79,88,90,91,94,96,97,99,103,109,111,114,124,129,130,133, 134,138,140,142,144,151).
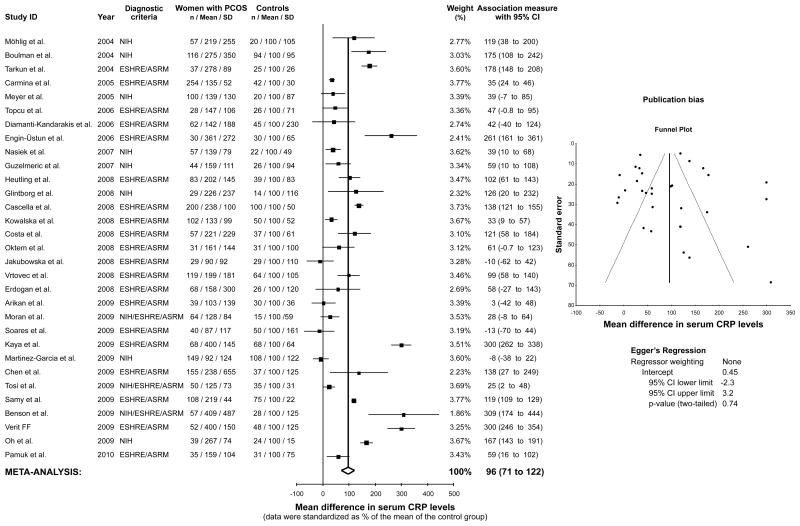
The CRP meta-analysis included 3,648 women (2,359 women with PCOS and 1,289 controls). Mean serum CRP levels were 96% higher in women with PCOS compared to controls (95% confidence interval 71% − 122%, z = 7.32, p < 0.0001, Figure 1). There was no evidence dissemination bias in the CRP meta-analysis (Egger’s regression intercept 0.45, 95% confidence interval −2.30 – 3.21, P = 0.739, Figure 1). The results were similar after excluding 5 studies (19,48,60,103,114) with mismatches in body mass and/or frequency of obesity between women with PCOS and controls (102% relative increase in PCOS compared to controls, 95% confidence interval 73% − 131%, z = 6.93, p < 0.0001; Egger’s regression intercept −0.79, 95% confidence interval −3.85 – 2.26, P = 0.598).
Figure 1.
Meta-analysis of serum C-reactive protein (CRP) levels in women with PCOS and controls. Evidence dissemination bias was assessed by funnel plot and Egger’s regression. NIH, National Institute of Health, ESHRE/ASRM, European Society of Human Reproduction and Embryology / American Society of Reproductive Medicine.
Meta-analysis of serum IL-6 concentrations in women with PCOS and controls
We identified 49 studies that were potentially relevant to serum IL-6 levels and PCOS (19,20,35,54,60,78,82,88,96,97,99,116–118,121,126,129,138,140,143,150,153–180).
Eight review articles were immediately excluded (60,117,159,165,166,170,171,177), and the remaining 41 studies were retrieved for a more detailed evaluation.
Fifteen studies were subsequently excluded for the following reasons: 1) Basal serum IL-6 levels were not reported in 6 studies (20,116,129,150,157,163); 2) A standard assay was used to measure IL-6 instead of a high-sensivity assay in 1 study (99); 3) IL-6 was only measured in follicular fluid in 2 studies (153,156); 4) IL-6 was only measured in cell culture supernatants in 3 studies (169,173,179); 5) IL-6 was measured in animals in 2 studies (155,175); 6) Subjects who did not have PCOS were evaluated in 1 study (162).
The remaining 26 studies were considered potentially appropriate. An additional 16 studies were excluded for the following reasons: 1) Less than 25 PCOS patients were included in 8 studies (54,78,82,118,121,158,174,176); 2) No controls were included in 6 studies (126,143,154,160, 161,164); 3) Patients and controls were also included in ulterior extended series by the same authors in 1 study (178); 4) Serum IL-6 was measured in only 8 controls in 1 study (180).
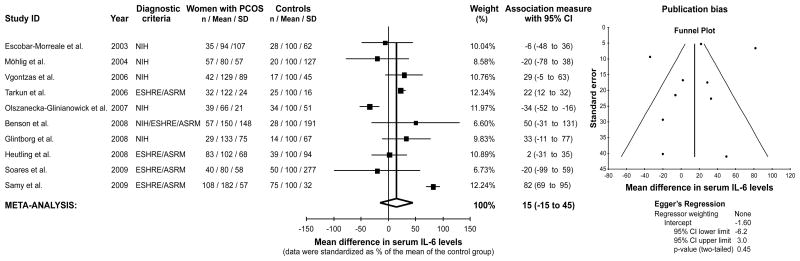
The remaining 10 studies containing usable information by outcome were used to perform the IL-6 meta analysis (19,35,88,96,97,138,140,167,168,172). This meta-analysis included 852 women (522 women with PCOS and 330 controls). There was no significant difference in serum IL-6 levels in women with PCOS compared to controls, with a 15% relative difference in group means (95% confidence interval −15% – 45%, z = 0.97, P = 0.331, Figure 2). There was no evidence dissemination bias in the IL-6 meta-analysis (Egger’s regression intercept −1.62, 95% confidence interval −6.27 – 3.02, P = 0.443, Figure 2). The results were similar after excluding one study (19) with body mass mismatches between women with PCOS and controls (18% relative increase in PCOS compared to controls, 95% confidence interval −13% – 50%, z = 1.13, p = 0.257; Egger’s regression intercept −1.49, 95% confidence interval −6.98 – 4.00, P = 0.541).
Figure 2.
Meta-analysis of serum interleukin-6 (IL-6) levels in women with PCOS and controls. Evidence dissemination bias was assessed by funnel plot and Egger’s regression. NIH, National Institute of Health, ESHRE/ASRM, European Society of Human Reproduction and Embryology / American Society of Reproductive Medicine.
Meta-analysis of serum TNF-α concentrations in women with PCOS and controls
We identified 52 studies that were potentially relevant to serum TNF-α levels and PCOS (2,5,17,35,40,54,78,82,99,107,111,129,138,140,153,155,157–159,165–169,171–174,176,177, 179,181–201).
Thirteen review articles were immediately excluded (40,159,165,166,171,177,181,185,188,191,193,194,199), and the remaining 39 studies were retrieved for a more detailed evaluation.
Eighteen studies were subsequently excluded for the following reasons: 1) Basal serum TNF-α levels were not reported in 5 studies (17,129,157,186,190); 2) TNF-α was only measured in follicular fluid in 1 study (153); 3) TNF-α was only measured in cell culture supernatants in 6 studies (2,169,173,179,182,189); 4) TNF-α was measured in animals in 4 studies (155,187,196,198); 5) Subjects who did not have PCOS were evaluated in 1 study (200); 6) PCOS was not defined by strict criteria in 1 study (183).
The remaining 21 studies were considered potentially appropriate. An additional 12 studies were excluded for the following reasons: 1) Less than 25 women with PCOS were included in 11 studies (5,54,78,82,107,158,174,176,192,197,201); 2) No controls were included in 1 study (195).
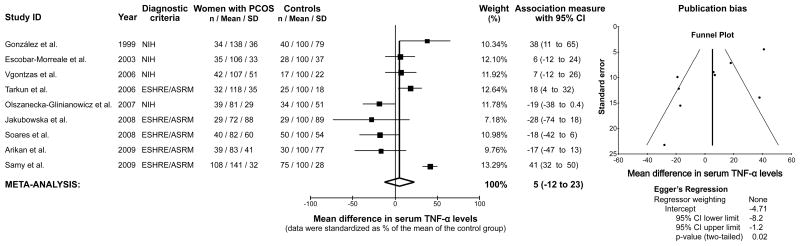
The remaining 9 studies containing usable information by outcome were used to perform the TNF-α meta-analysis (35,99,111,138,140,167,168,172,184). This meta-analysis included 726 women (398 women with PCOS and 328 controls). There was no significant difference in serum TNF-α levels in women with PCOS compared to controls, with a 5% relative difference in group means (95% confidence interval −12% – 23%, z = 0.58, P = 0.561, Figure 3). There was also evidence of a significant publication bias favoring studies underestimating the differences in TNF-α levels between women with PCOS and controls (Egger’s regression intercept −4.71, 95% confidence interval −8.18 – −1.25, P = 0.015, Figure 3). The results were similar after excluding one study (184) with body mass mismatches between women with PCOS and controls (1% relative increase in PCOS compared to controls, 95% confidence interval -18% - 20%, z = 0.14, p = 0.887; Egger’s regression intercept -5.78, 95% confidence interval -8.56 -3.00, P = 0.002).
Figure 3.
Meta-analysis of serum tumor necrosis factor- α (TNF- α) levels in women with PCOS and controls. Evidence dissemination bias was assessed by funnel plot and Egger’s regression. NIH, National Institute of Health, ESHRE/ASRM, European Society of Human Reproduction and Embryology / American Society of Reproductive Medicine.
Systematic review of other serum inflammatory markers in PCOS patients and controls
Table 1 describes the few studies about serum inflammatory markers other than CRP, IL-6 and TNF-α that included more than 25 women with PCOS and an appropriate number of controls. PCOS and obesity appeared to independently and consistently contribute to serum IL-18 elevations (150,202,203). Circulating sICAM-1 was increased in women with PCOS in 2 of 3 studies, although obesity played a contributing role in this increase (6,35,204). The white blood cell count (WBC) was also higher in women with PCOS, compared to controls with obesity contributing to the elevation in the smaller of the 2 studies (53,205). sTNF receptor levels measured in 2 studies yielded conflicting results regarding the circulating status, and the effects of PCOS and obesity (35,172). Finally, single studies reported elevated levels of sVCAM-1, MMP-9, MCP-1, MIP-1α, TIMP-1, sE-selectin, sCD40L and soluble CD36, and decreased levels of neutrophil gelatinase-associated lipocalin and its complex with MMP-9 in women with PCOS (6,92,96,134,206,207).
Table 1.
Serum inflammatory markers other than C-reactive protein, interleukin-6 and tumor necrosis factor-α in women with PCOS compared to non-hyperandrogenic controls
| Authors, Year | Marker | Diagnostic criteria | Women with PCOS (n) | Controls(n) | Effects of PCOS | Effect of obesity | Comments |
|---|---|---|---|---|---|---|---|
| Escobar-Morreale et al., 2004 (202) | IL-18 | NIH | 60 | 34 | ↑ | ↑ | BMI-matched, age- corrected |
| Zhang et al., 2006 (203) | IL-18 | ESHRE/ASRM | 42 | 38 | ↑ | ↑ | BMI- and glucose- matched |
| Kaya et al., 2010 (150) | IL-18 | ESHRE/ASRM | 60 | 60 | ↑ | ↑ | Overall ↑ due to normal weight PCOS |
| Glintborg et al., 2009 (207) | MCP-1, MIP-1α | NIH | 30 | 63 | ↑ | Directly correlated with BMI | All overweight or obese PCOS |
| Diamanti-Kandarakis et al.,2008 (92) | MMP-9,NGAL | NIH | 40 | 40 | ↓in normal- and overweight PCOS | None | BMI- and age-matched |
| Liu et al., 2008 (206) | MMP-9, TIMP-1 | ESHRE/ASRM | 42 | 40 | ↑ | BMI- and age-matched | |
| Glintborg et al., 2008 (96) | sCD36 | NIH | 30 | 14 | ↑ | Not assessed | Weight- and age-matched |
| Oktem et al., 2009 (134) | sCD40L | ESHRE/ASRM | 31 | 31 | ↑ | None | BMI- and age-matched |
| Diamanti-Kandarakis et al., 2006 (6) | sE-selectin | ESHRE/ASRM | 62 | 45 | ↑ | Directly correlated with BMI | |
| Escobar-Morreale et al., 2003 (35) | sICAM-1 | NIH | 35 | 28 | None | None | BMI-matched, age- corrected |
| Nasiek et al., 2004 (204) | sICAM-1 | NIH | 57 | 22 | ↑ in obese PCOS | None | BMI- and age-matched |
| Diamanti-Kandarakis et al., 2006 (6) | sICAM-1 | ESHRE/ASRM | 62 | 45 | ↑ | Directly correlated with BMI | |
| Peral et al., 2002 (17) | sTNFR2 | NIH | 42 | 36 | None | ↑ | BMI-matched, age- corrected |
| Olszanecka-Glinianowicz et al., 2007 (172) | sTNFR 1&2 | NIH | 39 | 34 | ↑ | Not assesed | All obese subjects, age- matched |
| Diamanti-Kandarakis et al., 2006 (6) | sVCAM-1 | ESHRE/ASRM | 62 | 45 | None | ||
| Orio et al., 2005 (53) | WBC | NIH | 150 | 150 | ↑ | None | BMI- and age-matched |
| Kebapcilar et al., 2009 (205) | WBC | ESHRE/ASRM | 48 | 30 | ↑ | ↑ | BMI- and age-matched |
Symbols: ↑, increased.
Abbreviations: BMI, body mass index; ESHRE/ASRM, European Society of Human Reproduction and Embryology / American Society of Reproductive Medicine; IL, interleukin; MMP, Matrix metalloproteinase; MIP-1α, Macrophage inflammatory protein-1α MCP-1, Monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocain; NIH, National Institute of Health; sE-selectin, soluble endothelial leucocyte adhesion molecule; sICAM, soluble intercellular adhesion molecule; sTNFR, soluble tumor necrosis factor receptor, sVCAM, soluble vascular cell adhesion molecule; TIMP, tissue inhibitor of matrix metalloproteinase; WBC, white blood count.
In studies including less than 25 women with PCOS, circulating levels of IL-1Ra, sICAM-1, MCP-1, MMP-2, MMP-9, MIF, neopterin, TIMP-1 and WBC were elevated in the disorder (8,54,57,121,208), although the MMP-2, MMP-9 and WBC elevations were no longer significant when controlling for indexes of fat mass (54) or obesity (8). Finally, one study reported high IL-1β levels and low IL-7 levels in obese women with PCOS (174).
Discussion
The present meta-analysis of the mean differences in CRP, IL-6 and TNF-α clearly indicates that CRP is a circulating marker of the proinflammatory state in PCOS as evidenced by the 2-fold elevation in circulating CRP in women with disorder compared to controls. There is no difference in the levels of IL-6 or TNF-α among both groups with the latter finding affected by evidence dissemination bias cautioning interpretation of the results. There are less consistent changes in other circulating inflammatory markers in studies with insufficient numbers to perform a meta-analysis, many of which are isolated reports. Nevertheless, the limited evidence to date suggests that in PCOS, elevations in serum IL-18 concentrations (150,202,203) and WBC (53,57,205) can be independent of obesity.
Elevated circulating CRP in PCOS is independent of obesity since this finding persisted after excluding all the studies with mismatches in frequency of obesity or body mass between groups from the meta-analysis. This is important because obesity is a well documented proinflammatory state independently associated with elevations in all three of these markers (209–211), and inflamed adipose tissue is a known source of IL-6 and TNF-α (26,212) the former of which stimulates CRP synthesis in the liver (28). There is a high prevalence of obesity in the women with PCOS some of the studies analyzed. The degree of elevation in circulating levels of CRP and IL-6 in PCOS is much greater when obesity is also present (39,45,79,88,90,114,133,138,144). In fact, several studies show that serum CRP elevations in women with PCOS are no longer statistically significant when controlling for indices of obesity such as body mass index (6,72), or that obesity alone is responsible for the CRP increase (19,35,103,129). Similarly, the highest circulating TNF-α levels are evident in the obese regardless of PCOS status, as demonstrated in the initial report of increased serum TNF-α in normal weight women with PCOS (184), and in the largest report to date of increased serum TNF-α in the disorder (138).
The limitations of our meta-analyses include the sole use of the PubMed Entrez search engine to identify studies, and the exclusion of studies written in languages other than English. Despite the effort to minimize interstudy variability using random-effects models, the following additional study design limitations may have influenced our interpretation:
Small sample sizes, and uncontrolled confounding variables, with inadequate matching for body mass and prevalence of obesity arising most frequently.
Lack of uniform diagnostic criteria for PCOS.
Exclusion of studies using standard assays insensitive to small differences between groups.
Time-dependent individual variation in circulating levels.
Concomitant subclinical inflammatory illnesses that confound the results by altering circulating levels.
Evaluation only in the fasting state in most studies.
The relevance of the last concern is based on the new awareness that dietary components can stimulate inflammation which may accentuate clinically relevant differences between women with PCOS and controls (2–5,121). These differences may in turn, reflect molecular inflammatory responses involved in the promotion of insulin resistance and atherogenesis.
In conclusion, our meta-analysis of the most comparable studies indicate that circulating CRP is elevated in PCOS reflective of the chronic low-grade inflammation present in the disorder. In contrast, the trend towards slightly greater circulating levels of IL-6 and TNF-α among women with PCOS is far from reaching statistical significance. Because the CRP elevation attributable to PCOS is relatively small, caution is merited in using CRP to attribute metabolic and cardiovascular risk to PCOS per se. Finally, our review brings to the forefront factors that should be considered in designing future studies. Some examples include cross-sectional comparison of patients and controls using power analyses-based sample sizes, use of quality-controlled high-sensitivity assays, proper control of confounders, repeated measurements over time, and evaluation of diet-induced changes in the circulation.
Supplementary Material
QUORUM flowcharts of the systematic review and meta-analysis of serum C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) concentrations in women with PCOS and controls.
Acknowledgments
GRANTS: Supported by Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation grant PI080944 to H.F.E.M., and National Institutes of Health grant HD048535 to F.G.. CIBERDEM is also an initiative of Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation.
Although space constraint precludes acknowledgment of individual Authors, we would like to thank all the Authors of the original articles reviewed who kindly responded to our requests for data and / or clarifications. Their assistance has greatly contributed to the quality of this work. This study was developed as part of an Androgen Excess PCOS Society (AE-PCOS) consensus task force on the cardiovascular impact of PCOS.
Footnotes
DISCLOSURE STATEMENT: The Authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- 2.González F, Minium J, Rote NS, Kirwan JP. Hyperglycemia alters tumor necrosis factor-alpha release from mononuclear cells in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5337–42. doi: 10.1210/jc.2005-0694. [DOI] [PubMed] [Google Scholar]
- 3.González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336–40. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 4.González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor κB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1508–12. doi: 10.1210/jc.2005-2327. [DOI] [PubMed] [Google Scholar]
- 5.González F, Rote NS, Minium J, Kirwan JP. In vitro evidence that hyperglycemia stimulates tumor necrosis factor-alpha release in obese women with polycystic ovary syndrome. J Endocrinol. 2006;188:521–9. doi: 10.1677/joe.1.06579. [DOI] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I, et al. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod. 2006;21:1426–31. doi: 10.1093/humrep/del003. [DOI] [PubMed] [Google Scholar]
- 7.Diamanti-Kandarakis E, Alexandraki K, Piperi C, Protogerou A, Katsikis I, Paterakis T, et al. Inflammatory and endothelial markers in women with polycystic ovary syndrome. Eur J Clin Invest. 2006;36:691–7. doi: 10.1111/j.1365-2362.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 8.Lewandowski KC, Komororowski J, O’Callaghan CJ, Tan BK, Chen J, Prelevic GM, et al. Increased circulating levels of matrix metalloproteinase -2 & 9- in women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1173–7. doi: 10.1210/jc.2005-0648. [DOI] [PubMed] [Google Scholar]
- 9.Hu WH, Qiao J, Zhao SY, Zhang XW, Li MZ. Monocyte chemoattractant protein-1 and its correlation with lipoprotein in polycystic ovary syndrome. Beijing Da Xue Xue Bao. 2006;38:487–91. [PubMed] [Google Scholar]
- 10.González F, Rote NS, Minium J, Kirwan JP. Altered lipopolysaccharide-stimulated IL6 release from mononuclear cells of women with polycystic ovary syndrome in response to hyperglycemia-evidence of LPS tolerance. 4th Meeting of the Androgen Excess Society; 2006;Jun 23; p. 30. [Google Scholar]
- 11.González F, Rote NS, Minium J, Kirwan JP. In vitro evidence that hyperglycemia stimulates increased IL-6 release from mononuclear cells of obese women with polycystic ovary syndrome. Fertil Steril. 2006;86:S449–S50. doi: 10.1677/joe.1.06579. [DOI] [PubMed] [Google Scholar]
- 12.González F, Rote NS, Minium J, Kirwan JP. Hyperglycemia stimulates proatherogenic inflammation pathways in polycystic ovary syndrome. Fertil Steril. 2006;86:S450. [Google Scholar]
- 13.Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007;92:2500–5. doi: 10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- 14.Escobar-Morreale HF, Botella-Carretero JI, Álvarez-Blasco F, Sancho J, San Millan JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005;90:6364–9. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- 15.Escobar-Morreale HF, Luque-Ramírez M, San Millan JL. The molecular-genetic basis of functional hyperandrogenism and polycystic ovary syndrome. Endocr Rev. 2005;26:251–82. doi: 10.1210/er.2004-0004. [DOI] [PubMed] [Google Scholar]
- 16.Escobar-Morreale HF, Calvo RM, Sancho J, San Millan JL. TNF-alpha and hyperandrogenism: a clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab. 2001;86:3761–7. doi: 10.1210/jcem.86.8.7770. [DOI] [PubMed] [Google Scholar]
- 17.Peral B, San Millan JL, Castello R, Moghetti P, Escobar-Morreale HF. The methionine 196 arginine polymorphism in exon 6 of the TNF receptor 2 gene (TNFRSF1B) is associated with the polycystic ovary syndrome and hyperandrogenism. J Clin Endocrinol Metab. 2002;87:3977–83. doi: 10.1210/jcem.87.8.8715. [DOI] [PubMed] [Google Scholar]
- 18.Villuendas G, San Millan JL, Sancho J, Escobar-Morreale HF. The -597 G->A and -174 G->C polymorphisms in the promoter of the IL-6 gene are associated with hyperandrogenism. J Clin Endocrinol Metab. 2002;87:1134–41. doi: 10.1210/jcem.87.3.8309. [DOI] [PubMed] [Google Scholar]
- 19.Möhlig M, Spranger J, Osterhoff M, Ristow M, Pfeiffer AF, Schill T, et al. The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinol. 2004;150:525–32. doi: 10.1530/eje.0.1500525. [DOI] [PubMed] [Google Scholar]
- 20.Erdogan M, Karadeniz M, Berdeli A, Alper G, Caglayan O, Yilmaz C. The relationship of the interleukin-6 -174 G>C gene polymorphism with oxidative stress markers in Turkish polycystic ovary syndrome patients. J Endocrinol Invest. 2008;31:624–9. doi: 10.1007/BF03345614. [DOI] [PubMed] [Google Scholar]
- 21.Escobar-Morreale HF, Calvo RM, Villuendas G, Sancho J, San Millan JL. Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obes Res. 2003;11:987–96. doi: 10.1038/oby.2003.136. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Murray DL, Choy LN, Spielgeman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA. 1994;91:4854–8. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha supresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–8. [PubMed] [Google Scholar]
- 24.del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol. 1999;276:E849–55. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 25.Jones TH. Interleukin-6 an endocrine cytokine. Clin Endocrinol (Oxf) 1994;40:703–13. doi: 10.1111/j.1365-2265.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- 26.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 27.Purohit A, Ghilchik MW, Duncan L, Wang DY, Singh A, Walker MM, et al. Aromatase activity and interleukin-6 production by normal and malignant breast tissues. J Clin Endocrinol Metab. 1995;80:3052–8. doi: 10.1210/jcem.80.10.7559896. [DOI] [PubMed] [Google Scholar]
- 28.Moshage HJ, Roelofs HM, van Pelt JF, Hazenberg BP, van Leeuwen MA, Limburg PC, et al. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem Biophys Res Commun. 1988;155:112–7. doi: 10.1016/s0006-291x(88)81056-8. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 30.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–4. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 31.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. 4. Boston: Blackwell Scientific Publications; 1992. pp. 377–84. [Google Scholar]
- 32.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 33.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 34.Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86:2453–5. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- 35.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Sancho J, San Millan JL. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia. 2003;46:625–33. doi: 10.1007/s00125-003-1090-z. [DOI] [PubMed] [Google Scholar]
- 36.Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:123–7. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 37.Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4649–54. doi: 10.1210/jc.2002-021688. [DOI] [PubMed] [Google Scholar]
- 38.Bahceci M, Tuzcu A, Canoruc N, Tuzun Y, Kidir v, Aslan C. Serum C-reactive protein (CRP) levels and insulin resistance in non-obese women with polycystic ovarian syndrome, and effect of bicalutamide on hirsutism, CRP levels and insulin resistance. Horm Res. 2004;62:283–7. doi: 10.1159/000081973. [DOI] [PubMed] [Google Scholar]
- 39.Boulman N, Levy Y, Leiba R, Shachar S, Linn R, Zinder O, et al. Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2160–5. doi: 10.1210/jc.2003-031096. [DOI] [PubMed] [Google Scholar]
- 40.Dhindsa G, Bhatia R, Dhindsa M, Bhatia V. Insulin resistance, insulin sensitization and inflammation in polycystic ovarian syndrome. J Postgrad Med. 2004;50:140–4. [PubMed] [Google Scholar]
- 41.Sacks GP, Seyani L, Lavery S, Trew G. Maternal C-reactive protein levels are raised at 4 weeks gestation. Hum Reprod. 2004;19:1025–30. doi: 10.1093/humrep/deh179. [DOI] [PubMed] [Google Scholar]
- 42.Talbott EO, Zborowski JV, Boudreaux MY, McHugh-Pemu KP, Sutton-Tyrrell K, Guzick DS. The relationship between C-reactive protein and carotid intima-media wall thickness in middle-aged wome with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:6061–7. doi: 10.1210/jc.2003-032110. [DOI] [PubMed] [Google Scholar]
- 43.Talbott EO, Zborowskii JV, Boudraux MY. Do women with polycystic ovary syndrome have an increased risk of cardiovascular disease? Minerva Ginecol. 2004;56:27–39. [PubMed] [Google Scholar]
- 44.Taponen S, Martikainen H, Järvelin M-R, Sovio U, Laitinen J, Pouta A, et al. Metabolic cardiovascular disease risk factors in women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. J Clin Endocrinol Metab. 2004;89:2114–8. doi: 10.1210/jc.2003-031720. [DOI] [PubMed] [Google Scholar]
- 45.Tarkun I, Arslan BC, Cantürk Z, Türemen E, Sahin T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89:5592–6. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 46.Bhatia V. Insulin resistance in polycystic ovarian disease. South Med J. 2005;98:903–10. doi: 10.1097/01.smj.0000177251.15366.85. [DOI] [PubMed] [Google Scholar]
- 47.Bickerton AS, Clark N, Meeking D, Shaw KM, Crook M, Lumb P, et al. Cardiovascular risk in women with polycystic ovarian syndrome (PCOS) J Clin Pathol. 2005;58:151–4. doi: 10.1136/jcp.2003.015271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmina E, Chu MC, Longo RA, Rini GB, Lobo RA. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular parameters. J Clin Endocrinol Metab. 2005;85:995–1000. doi: 10.1210/jc.2004-2279. [DOI] [PubMed] [Google Scholar]
- 49.Cho LW, Jayagopal v, Kilpatrick ES, Atkin SL. The biological variation of C-reactive protein in polycystic ovarian syndrome. Clin Chem. 2005;51:1905–7. doi: 10.1373/clinchem.2005.052753. [DOI] [PubMed] [Google Scholar]
- 50.Creatsas G, Christodoulakos G, Lambrinoudaki I. Cardiovascular disease: screening and management of the asymptomatic high-risk post-menopausal woman. Maturitas. 2005;52:532–7. doi: 10.1016/j.maturitas.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Hahn S, Tan S, Elsenbruch S, Quadbeck B, Herrmann BL, Mann K, et al. Clinical and biochemical characterization of women with polycystic ovary syndrome in North Rhine-Westphalia. Horm Metab Res. 2005;37:438–44. doi: 10.1055/s-2005-870236. [DOI] [PubMed] [Google Scholar]
- 52.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005;90:5711–6. doi: 10.1210/jc.2005-0011. [DOI] [PubMed] [Google Scholar]
- 53.Orio F, Palomba S, Cascella T, Di Biase S, Manguso F, Táuchmanova L, et al. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:2–5. doi: 10.1210/jc.2004-0628. [DOI] [PubMed] [Google Scholar]
- 54.Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Müller B. Central fat excess in polycystic ovary syndrome: relation to low-grade inflammation and insulin resistance. J Clin Endocrinol Metab. 2005;90:6014–21. doi: 10.1210/jc.2005-1002. [DOI] [PubMed] [Google Scholar]
- 55.Tan S, Hahn S, Janssen OE. Insulin resistance syndrome and polycystic ovary syndrome: implications for diagnosis and treatment. Panminerva Med. 2005;47:211–7. [PubMed] [Google Scholar]
- 56.Tarkun I, Çetinarslan B, Türemen E, Sahin T, Cantürk Z, Komsuoglu B. Effect of rosiglitazone on insulin resistance, c-reactive protein and endothelial function in non-obese women with polycystic ovary syndrome. Eur J Endocrinol. 2005;153:115–21. doi: 10.1530/eje.1.01948. [DOI] [PubMed] [Google Scholar]
- 57.Barutcuoglu B, Bozdemir AE, Dereli D, Parildar Z, Mutaf MI, Ozmen D, et al. Increased serum neopterin levels in women with polycystic ovary syndrome. Ann Clin Lab Sci. 2006;36:267–72. [PubMed] [Google Scholar]
- 58.Brinkworth GD, Noakes M, Moran LJ, Norman R, Clifton PM. Flow-mediated dilatation in overweight and obese women with polycystic ovary syndrome. BJOG. 2006;113:1308–14. doi: 10.1111/j.1471-0528.2006.01090.x. [DOI] [PubMed] [Google Scholar]
- 59.Cascella T, Palomba S, Tauchmanova L, Manguso F, Di Biase S, Labella D, et al. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2006;91:4395–400. doi: 10.1210/jc.2006-0399. [DOI] [PubMed] [Google Scholar]
- 60.Diamanti-Kandarakis E, Paterakis T, Kandarakis HA. Indices of low-grade inflammation in polycystic ovary syndrome. Ann N Y Acad Sci. 2006;1092:175–86. doi: 10.1196/annals.1365.015. [DOI] [PubMed] [Google Scholar]
- 61.Engin-Ustün Y, Ustün Y, Meydanli MM, Kafkasli A, Yetkin G. Are polycystic ovaries associated with cardiovascular disease risk as polycystic ovary syndrome? Gynecol Endocrinol. 2006;22:324–8. doi: 10.1080/09513590600630447. [DOI] [PubMed] [Google Scholar]
- 62.Kaaja RJ, Pöyhönen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens. 2006;24:131–41. doi: 10.1097/01.hjh.0000194121.19851.e5. [DOI] [PubMed] [Google Scholar]
- 63.Orio FJ, Giallauria F, Palomba S, Cascella T, Manguso F, Vuolo L, et al. Cardiopulmonary impairment in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:2967–71. doi: 10.1210/jc.2006-0216. [DOI] [PubMed] [Google Scholar]
- 64.Topcu S, Caliskan M, Ozcimen EE, Tok D, Uckuyu A, Erdogan D, et al. Do young women with polycystic ovary syndrome show early evidence of preclinical coronary artery disease? Hum Reprod. 2006;21:930–5. doi: 10.1093/humrep/dei431. [DOI] [PubMed] [Google Scholar]
- 65.Vanky E, Salvesen KA, Hjorth-Hansen H, Bjerve K, Carlsen SM. Beneficial effect of metformin on pregnancy outcome in women with polycystic ovary syndrome is not associated with major changes in C-reactive protein levels or indices of coagulation. Fertil Steril. 2006;85:770–4. doi: 10.1016/j.fertnstert.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 66.Walch K, Grimm C, Nagele F, Huber J, Wölfler M, Vytiska-Binstorfer E, et al. Impaired glucose tolerance is associated with changes in clinical and biochemical parameters in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2006;85:869–73. doi: 10.1080/00016340500342938. [DOI] [PubMed] [Google Scholar]
- 67.Bahceci M, Aydemir M, Tuzcu A. Effects of oral fat and glucose tolerance test on serum lipid profile, apolipoprotein, and CRP concentration, and insulin resistance in patients with polycystic ovary syndrome. Fertil Steril. 2007;87:1363–8. doi: 10.1016/j.fertnstert.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 68.Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: prospective, randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:456–61. doi: 10.1210/jc.2006-1988. [DOI] [PubMed] [Google Scholar]
- 69.Beckmann JA, Goldfine AB, Dunaif A, Gerhard-Herman M, Creager MA. Endothelial function varies according to insulin resistance type. Diabetes Care. 2007;30:1226–32. doi: 10.2337/dc06-2142. [DOI] [PubMed] [Google Scholar]
- 70.Bell RJ, Davison SL, Papalia MA, McKenzie DP, Davis SR. Endogenous androgen levels and cardiovascular risk profile in women across the adult life span. Menopause. 2007;14:630–8. doi: 10.1097/GME.0b013e31802b6cb1. [DOI] [PubMed] [Google Scholar]
- 71.Carmina E, Lobo RA. Prevalence and metabolic characteristics of adrenal androgen excess in hyperandrogenic women with different phenotypes. J Endocrinol Invest. 2007;30:111–6. doi: 10.1007/BF03347408. [DOI] [PubMed] [Google Scholar]
- 72.Guzelmeric K, Alkan N, Pirimoglu M, Unal O, Turan C. Chronic inflammation and elevated homocysteine levels are associated with increased body mass index in women with polycystic ovary syndrome. Gynecol Endocrinol. 2007;23:505–10. doi: 10.1080/09513590701554306. [DOI] [PubMed] [Google Scholar]
- 73.Karadeniz M, Erdogan M, Berdeli A, Tamsel S, Saygili F, Yilmaz C. The progesterone receptor PROGINS polymorphism is not related to oxidative stress factors in women with polycystic ovary syndrome. Cardiovasc Diabetol. 2007;6:29. doi: 10.1186/1475-2840-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koo YA, Shin SY, Yoon BK, Choi D. Pioglitazone for treating polycystic ovary syndrome in non-obese women of reproductive age with different clinical presentations. Gynecol Endocrinol. 2007;23:461–7. doi: 10.1080/09513590701492689. [DOI] [PubMed] [Google Scholar]
- 75.Meden-Vrtovec H, Vrtovec B, Osredkar J. Metabolic and cardiovascular changes in women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2007;99:87–90. doi: 10.1016/j.ijgo.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 76.Mina A, Favaloro FJ, Koutts J. Hemostatic dysfunction associated with endocrine disorders as a major risk factor and cause of human morbidity and mortality: a comprehesive meta-review. Semin Thromb Hemost. 2007;33:798–809. doi: 10.1055/s-2007-1000372. [DOI] [PubMed] [Google Scholar]
- 77.Moran LJ, Noakes M, Clifton PM, Norman RJ. The use of anti-mullerian hormone in predicting menstrual response after weight loss in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:3796–802. doi: 10.1210/jc.2007-1188. [DOI] [PubMed] [Google Scholar]
- 78.Moran LJ, Noakes M, Clifton PM, Wittert GA, Belobrajdic P, Norman RJ. C-reactive protein before and after weight loss in overweight women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2944–51. doi: 10.1210/jc.2006-2336. [DOI] [PubMed] [Google Scholar]
- 79.Nasiek M, Kos-Kudla B, Ostrowska Z, Marek B, Kudla M, Sieminska L, et al. Acute phase proteins: C-reactive protein and fibrinogen in young women with polycystic ovary syndrome. Pathophysiology. 2007;14:23–8. doi: 10.1016/j.pathophys.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 80.Orio F, Manguso F, Di Biase S, Falbo A, Giallauria F, Labella D, et al. Metformin administration improves leukocyte count in women with polycystic ovary syndrome: a 6-month prospective study. Eur J Endocrinol. 2007;157:69–73. doi: 10.1530/EJE-07-0133. [DOI] [PubMed] [Google Scholar]
- 81.Rautio K, Tapanainen JS, Ruokonen A, Morin-Papunen LC. Rosiglitazone treatment alleviates inflammation and improves liver function in overweight women with polycystic ovary syndrome: a randomized placebo-controlled study. Fertil Steril. 2007;87:202–6. doi: 10.1016/j.fertnstert.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 82.Shorff R, Kerchner A, Maifeld M, Van Beek EJR, Jagasia D, Dokras A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab. 2007;92:4609–14. doi: 10.1210/jc.2007-1343. [DOI] [PubMed] [Google Scholar]
- 83.Sir-Petermann T, Maliqueo M, Codner E, Echiburú B, Crisosto N, Pérez V, et al. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4637–42. doi: 10.1210/jc.2007-1036. [DOI] [PubMed] [Google Scholar]
- 84.Velija-Asimi Z. C-reactive protein in obese PCOS women and the effect of metformin therapy. Bosn J Basic Med Sci. 2007;7:90–3. doi: 10.17305/bjbms.2007.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vigorito C, Giallauria F, Palomba S, Cascella T, Manguso F, Lucci R, et al. Beneficial effects of a three-month structure exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:1379–84. doi: 10.1210/jc.2006-2794. [DOI] [PubMed] [Google Scholar]
- 86.Xita N, Papassotiriou I, Georgiou I, Vounatsou M, Margeli A, Tsatsoulis A. The adiponectin-to-leptin ratio in women with polycystic ovary syndrome: relation to insulin resistance and proinflammatory markers. Metabolism. 2007;56:766–71. doi: 10.1016/j.metabol.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Aramwit P, Pruksananonda K, Kasettratat N, Jammeechai K. Risk factors for ovarian hyperstimulation syndrome in Thai patients using gonadotropins for in vitro fertilization. Am J Health Syst Pharm. 2008;65:1148–53. doi: 10.2146/ajhp070566. [DOI] [PubMed] [Google Scholar]
- 88.Benson S, Janssen OE, Hahn S, Tan S, Dietz T, Mann K, et al. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav Immun. 2008;22:177–84. doi: 10.1016/j.bbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Bhardwaj S, Misra A, Khurana L, Gulati S, Shah P, Vikram NK. Childhood obesity in Asian Indians: a burgeoning cause of insulin resistance, diabetes and sub-clinical inflammation. Asia Pac j Clin Nutr. 2008;17:172–5. [PubMed] [Google Scholar]
- 90.Cascella T, Palomba S, De Sio I, Manguso F, Giallauria F, De Simone B, et al. Visceral fat is associated with cardiovascular risk in women with polycystic ovary syndrome. Hum Reprod. 2008;23:153–9. doi: 10.1093/humrep/dem356. [DOI] [PubMed] [Google Scholar]
- 91.Costa LO, dos Santos MP, Oliveira M, Viana A. Low-grade chronic inflammation is not accompanied by structural arterial injury in polycystic ovary syndrome. Diabetes Res Clin Pract. 2008;81:179–83. doi: 10.1016/j.diabres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Diamanti-Kandarakis E, Livadas S, Kandarakis SA, Margeli A, Papassotiriou I. Serum concentrations of atherogenic proteins neutrophil gelatinase-associated lipocalin and its complex with matrix metalloproteinase-9 are significantly lower in women with polycystic ovary syndrome: hint of a protective mechanism? Eur J Endocrinol. 2008;158:525–31. doi: 10.1530/EJE-07-0822. [DOI] [PubMed] [Google Scholar]
- 93.Diamanti-Kandarakis E, Livadas S, Kandarakis SA, Papassotiriou I, Margeli A. Low free plasma levels of retinol-binding protein 4 in insulin-resistant subjects with polycystic ovary syndrome. J Endocrinol Invest. 2008;31:950–5. doi: 10.1007/BF03345631. [DOI] [PubMed] [Google Scholar]
- 94.Erdogan M, Karadeniz M, Alper GE, Tamsel S, Uluer H, Caglayan O, et al. Thrombin-activatable fibrinolysis inhibitor and cardiovascular risk factors in polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2008;116:143–7. doi: 10.1055/s-2007-992118. [DOI] [PubMed] [Google Scholar]
- 95.Giallauria F, Palomba S, Maresca L, Vuolo L, Tafuri D, Lombardi G, et al. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2008;69:792–8. doi: 10.1111/j.1365-2265.2008.03305.x. [DOI] [PubMed] [Google Scholar]
- 96.Glintborg D, Hojlund K, Andersen M, Henriksen JE, Beck-Nielsen H, Handberg A. Soluble CD36 and risk markers of insulin resistance and atherosclerosis are elevated in polycystic ovary syndrome and significantly reduced during pioglitazone treatment. Diabetes Care. 2008;31:328–34. doi: 10.2337/dc07-1424. [DOI] [PubMed] [Google Scholar]
- 97.Heutling D, Schulz H, Nickel I, Kleinstein J, Kaltwasser P, Westphal S, et al. Asymmetrical dimethylarginine, inflammatory and metabolic parameters in women with polycystic ovary syndrome before and after metformin treatment. J Clin Endocrinol Metab. 2008;93:82–90. doi: 10.1210/jc.2007-0842. [DOI] [PubMed] [Google Scholar]
- 98.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jakubowska J, Bohdanowicz-Pawlak A, Milewicz A, Szymczak J, Bednarek-Tupikowska G, Demissie M. Plasma cytokines in obese women with polycystic ovary syndrome, before and after metformin treatment. Gynecol Endocrinol. 2008;24:378–84. doi: 10.1080/09513590802128968. [DOI] [PubMed] [Google Scholar]
- 100.Jensterle M, Sebestjen M, Janez A, Prezelj J, Kocjan T, Keber I, et al. Improvement of endothelial function with metformin and rosiglitazone treatment in women with polycystic ovary syndrome. Eur J Endocrinol. 2008;159:399–406. doi: 10.1530/EJE-08-0507. [DOI] [PubMed] [Google Scholar]
- 101.Karadeniz M, Erdogan D, Tamsel S, Zengi A, Alper GE, Caglayan O, et al. Oxidative stress markers in young patients with polycystic ovary syndrome, the relationship between insulin resistances. Exp Clin Endocrinol Diabetes. 2008;116:231–5. doi: 10.1055/s-2007-992154. [DOI] [PubMed] [Google Scholar]
- 102.Kojotrod SB, Romundstad P, von Düring V, Sunde A, Carlsen SM. C-reactive protein levels are unaffected by metformin during pretreatment and an IVF cycle in women with polycystic ovary syndrome. Fertil Steril. 2008;89:635–41. doi: 10.1016/j.fertnstert.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 103.Kowalska I, Fernandez-Real JM, Straczkowski M, Kozlowska A, Adamska A, Ortega F, et al. Insulin resistance is associated with decreased circulating mannan-binding lectin concentrations in women with polycystic ovary syndrome. Diabetes Care. 2008;31:e20. doi: 10.2337/dc07-1872. [DOI] [PubMed] [Google Scholar]
- 104.Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57:961–5. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 105.Preiss D, Sattar N, Harborne L, Norman J, Fleming R. The effects of 8 months of metformin on circulating GGT and ALT levels in obese women with polycystic ovarian syndrome. Int J Clin Pract. 2008;62:1337–43. doi: 10.1111/j.1742-1241.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 106.Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburú B, Codner E, et al. Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:1820–6. doi: 10.1210/jc.2007-2256. [DOI] [PubMed] [Google Scholar]
- 107.Thomann R, Rossinelli N, Keller U, Tirri BF, De Geyter C, Ruiz J, et al. Differences in low-grade chronic inflammation and insulin resistance in women with previous gestational diabetes and women with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:199–206. doi: 10.1080/09513590801893398. [DOI] [PubMed] [Google Scholar]
- 108.Vanky E, Salvesen KA, Asberg A, Carlsen SM. Haemoglobin, C-reactive protein and androgen levels in uncomplicated and complicated pregnancies of women with polycystic ovary syndrome. Scand J Clin Lab Invest. 2008;68:421–6. doi: 10.1080/00365510701810613. [DOI] [PubMed] [Google Scholar]
- 109.Vrtovec B, Meden-Vrtovec H, Jensterle M, Radovancevic B. Testosterone-related shortening of QTc interval in women with polycystic ovary syndrome. J Endocrinol Invest. 2008;31:653–5. doi: 10.1007/BF03345619. [DOI] [PubMed] [Google Scholar]
- 110.Alvarez-Blasco F, Martínez-García MA, Luque-Ramírez M, Parraza N, San Millán JL, Escobar-Morreale HF. Role of haptoglobin in polycystic ovary syndrome (PCOS), obesity and disorders of glucose tolerance in premenopausal women. PLoS One. 2009;4:e5606. doi: 10.1371/journal.pone.0005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arikan S, Akay H, Bahceci M, Tuzcu A, Gokalp D. The evaluation of endothelial function with flow-mediated dilatation and carotid intima media thickness in young nonobese polycystic ovary syndrome patients; existence of insulin resistance alone may not represent an adequate condition for deterioration of endothelial function. Fertil Steril. 2009;91:450–5. doi: 10.1016/j.fertnstert.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 112.Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Simvastatin and metformin in treatment of polycystic ovary syndrome: prospective randomized trial. J Clin Endocrinol Metab. 2009;94:4938–45. doi: 10.1210/jc.2009-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carmina E, Bucchieri S, Mansueto P, Rini G, Ferin M, Lobo RA. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil Steril. 2009;91:1332–5. doi: 10.1016/j.fertnstert.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Chen MJ, Chen HF, Chen SU, Ho HN, Yang YS, Yang WS. The relationship between follistatin and chronic low-grade inflammation in women with polycystic ovary syndrome. Fertil Steril. 2009;92:2041–4. doi: 10.1016/j.fertnstert.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 115.Duleba AJ, Ahmed IM. Predictors of urinary albumin excretion in women with polycystic ovary syndrome. Fertil Steril. 2010;93:2285–90. doi: 10.1016/j.fertnstert.2008.12.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Erdogan M, Karadeniz M, Berdeli A, Tamsel S, Yilmaz C. The relationship of the interleukin-6 -174 G>C gene polymorphism with cardiovascular risk factors in Turkish polycystic ovary syndrome patients. Int J Immunogenet. 2009;36:283–8. doi: 10.1111/j.1744-313X.2009.00867.x. [DOI] [PubMed] [Google Scholar]
- 117.Eyzaguirre F, Mericq V. Insulin resistance markers in children. Horm Res. 2009;71:65–74. doi: 10.1159/000183894. [DOI] [PubMed] [Google Scholar]
- 118.Gen R, Akbay E, Muslu N, Sezer K, Cayan F. Plasma visfatin level in lean women with PCOS: relation to proinflammatory markers and insulin resistance. Gynecol Endocrinol. 2009;25:241–5. doi: 10.1080/09513590802585613. [DOI] [PubMed] [Google Scholar]
- 119.Giallauria F, Orio F, Lombardi G, Colao A, Vigorito C, Tarufi MG, et al. Relationship between heart rate recovery and inflammatory markers in patients with polycystic ovary syndrome: a cross-sectional study. J Ovarian Res. 2009;2:3. doi: 10.1186/1757-2215-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giallauria F, Palomba S, De Sio I, Maresca L, Vuolo L, Savastano S, et al. Inflammatory markers and visceral fat are inversely associated with maximal oxygen consumption in women with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2009;70:394–400. doi: 10.1111/j.1365-2265.2008.03336.x. [DOI] [PubMed] [Google Scholar]
- 121.González F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism. 2009;58:954–62. doi: 10.1016/j.metabol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoeger KM. Polycystic ovary syndrome, inflammation, and statins: do we have the right target? J Clin Endocrinol Metab. 2009;94:35–7. doi: 10.1210/jc.2008-2376. [DOI] [PubMed] [Google Scholar]
- 123.Huang S, Qiao J, Li R, Wang L, Li M. Can serum apolipoprotein C-I demostrate metabolic abnormality early in women with polycystic ovary syndrome? Fertil Steril. 2010;94:205–10. doi: 10.1016/j.fertnstert.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Kaya C, Akgül E, Pabuccu R. C-reactive protein and homocysteine levels are associated with abnormal heart rate recovery in women with polycystic ovary syndrome. Fertil Steril. 2010;94:230–5. doi: 10.1016/j.fertnstert.2009.02.076. [DOI] [PubMed] [Google Scholar]
- 125.Kaya C, Erkan AF, Cengiz SD, Dünder I, Demirel OE, Bilgihan A. Advanced oxidation protein products are increased in women with polycystic ovary syndrome: relation ship with traditional and nontraditional cardiovascular risk factors in patients with polycystic ovary syndrome. Fertil Steril. 2009;92:1372–7. doi: 10.1016/j.fertnstert.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 126.Kaya C, Pabuçcu R, Koca C, Oguz AK, Erkan AF, Korkmaz A, et al. Relationship between interleukin-6 levels and ambulatory blood pressure in women with polycystic ovary syndrome. Fertil Steril. 2010;94:1437–4. doi: 10.1016/j.fertnstert.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 127.Livadas S, Dracopoulou M, Vasileiadi K, Lazaropoulou C, Magiakou MA, Xekouki P, et al. Elevated coagulation and inflammatory markers in adolescents with a history of premature adrenarche. Metabolism. 2009;58:576–81. doi: 10.1016/j.metabol.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 128.Mak W, Dokras A. Polycystic ovarian syndrome and the risk of cardiovascular disease and thrombosis. Semin Thromb Hemost. 2009;35:613–20. doi: 10.1055/s-0029-1242715. [DOI] [PubMed] [Google Scholar]
- 129.Martínez-García MA, Luque-Ramírez M, San Millán JL, Escobar-Morreale HF. Body iron stores and glucose intolerance in premenopausal women: role of hyperandrogenism, insulin resistance, and genomic variants related to inflammation, oxidative stress, and iron metabolism. Diabetes Care. 2009;32:1525–30. doi: 10.2337/dc09-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moran LJ, Hutchison SK, Meyer C, Zoungas S, Teede HJ. A comprehensive assessment of endothelial function in overweight women with and without polycystic ovary syndrome. Clin Sci (Lond) 2009;116:761–70. doi: 10.1042/CS20080218. [DOI] [PubMed] [Google Scholar]
- 131.Moran LJ, Meyer C, Hutchison SK, Zoungas S, Teede HJ. Novel inflammatory markers in overweight women with and without polycystic ovary syndrome and following pharmacological intervention. J Endocrinol Invest. 2010;33:258–65. doi: 10.1007/BF03345790. [DOI] [PubMed] [Google Scholar]
- 132.Morin-Papunen LC, Duleba AJ, Bloigu A, Järvelin MR, Saikku P, Pouta A. Chlamydia antibodies and self-reported symptoms of oligoamenorrhea and hirsutism: A new etiologic factor in polycystic ovary syndrome? Fertil Steril. 2010;94:1799–804. doi: 10.1016/j.fertnstert.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 133.Oh JY, Lee JA, Lee H, Oh JY, Sung YA, Chung H. Serum C-reactive protein levels in normal- weight polycystic ovary syndrome. Korean J Intern Med. 2009;24:350–5. doi: 10.3904/kjim.2009.24.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oktem M, Ozcimen EE, Uckuyu A, Esinler I, Pamuk B, Bayraktar N, et al. Polycystic ovary syndrome is associated with elevated plasma soluble CD40 ligand, a marker of coronary artery disease. Fertil Steril. 2009;91:2545–50. doi: 10.1016/j.fertnstert.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 135.Rajedran S, Willoughby SR, Chan WP, Liberts EA, Heresztyn T, Saha M, et al. Polycystic ovary syndrome is associated with severe platelet and endothelial dysfunction in both obese and lean subjects. Atherosclerosis. 2009;204:509–14. doi: 10.1016/j.atherosclerosis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 136.Rizzo M, Berneis K, Spinas G, Rini GB, Carmina E. Long-term consequences of polycystic ovary syndrome on cardiovascular risk. Fertil Steril. 2009;91:1563–7. doi: 10.1016/j.fertnstert.2008.09.070. [DOI] [PubMed] [Google Scholar]
- 137.Ruan X, Dai Y. Study on chronic low-grade inflammation and influential factors of polycystic ovary syndrome. Med Princ Pract. 2009;18:118–22. doi: 10.1159/000189809. [DOI] [PubMed] [Google Scholar]
- 138.Samy N, Hashim M, Sayed M, Said M. Clinical significance of inflammatory markers in polycystic ovary syndrome: their relationship to insulin resistance and body mass index. Dis Markers. 2009;26:163–70. doi: 10.3233/DMA-2009-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effects of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. J Clin Endocrinol Metab. 2009;94:103–8. doi: 10.1210/jc.2008-1750. [DOI] [PubMed] [Google Scholar]
- 140.Soares GM, Vieira CS, Martins WP, Franceschini SA, dos Reis RM, Silva de Sá MF, et al. Increased arterial stiffness in nonobese women with polycystic ovary syndrome (PCOS) without comorbidities: one more characteristic inherent to the syndrome? Clin Endocrinol (Oxf) 2009;71:406–11. doi: 10.1111/j.1365-2265.2008.03506.x. [DOI] [PubMed] [Google Scholar]
- 141.Thomson RL, Buckley JD, Moran LJ, Noakes M, Clifton PM, Norman RJ, et al. Comparison of aerobic exercise capacity and muscle strength in overweight women with and without polycystic ovary syndrome. BJOG. 2009;116:1242–50. doi: 10.1111/j.1471-0528.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 142.Tosi F, Dorizzi R, Castello R, Maffeis C, Spiazzi G, Zoppini G, et al. Body fat and insulin resistance independently predict increased serum C-reactive protein in hyperandrogenic women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161:737–45. doi: 10.1530/EJE-09-0379. [DOI] [PubMed] [Google Scholar]
- 143.Tsilchorozidou T, Mohamed-Ali V, Conway GS. Determinants of interleukin-6 and C-reactive protein vary in polycystic ovary syndrome, as do effects of short- and long-term metformin therapy. Horm Res. 2009;71:148–54. doi: 10.1159/000197871. [DOI] [PubMed] [Google Scholar]
- 144.Verit FF. High sensitive serum C-reactive protein and its relationship with other risk factors in normoinsulinemic polycystic ovary patients without metabolic syndrome. Arch Gynecol Obstet. 2010;281:1009–14. doi: 10.1007/s00404-009-1226-6. [DOI] [PubMed] [Google Scholar]
- 145.Vrbikova J, Bendlova B, Vankova M, Dvorakova K, Grimmichova T, Vondra K, et al. Beta cell function and insulin sensitivity in women with polycystic ovary syndrome: influence of the family history of type 2 diabetes mellitus. Gynecol Endocrinol. 2009;9:597–602. doi: 10.1080/09513590902972133. [DOI] [PubMed] [Google Scholar]
- 146.Wu Y, Zhang J, Wen Y, Wang H, Zhang M, Cianflone K. Increased acylation-stimulating protein, C-reactive protein, and lipid levels in young women with polycystic ovary syndrome. Fertil Steril. 2009;91:213–9. doi: 10.1016/j.fertnstert.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 147.Yang HP, Kang JH, Su HY, Tzeng CR, Liu WM, Huang SY. Apnea-hypopnea index in nonobese women with polycystic ovary syndrome. Int J Gynaecol Onstet. 2009;105:226–9. doi: 10.1016/j.ijgo.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 148.Agarwal N, Rice SP, Bolusani H, Luzio SD, Dunseath G, Ludgate M, et al. Meformin reduces arterial stifness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95:722–30. doi: 10.1210/jc.2009-1985. [DOI] [PubMed] [Google Scholar]
- 149.Arikan S, Bahceci M, Tuzcu A, Kale E, Gökalp D. Serum resistin and adiponectin levels in young non-obese women with polycystic ovary syndrome. Gynecol Endocrinol. 2010;26:161–6. doi: 10.3109/09513590903247816. [DOI] [PubMed] [Google Scholar]
- 150.Kaya C, Pabuccu R, Berker B, Satiroglu H. Plasma interleukin-18 levels are increased in the polycystic ovary syndrome: relationship of carotid intima-media wall thickness and cardiovascular risk factors. Fertil Steril. 2010;93:1200–7. doi: 10.1016/j.fertnstert.2008.10.070. [DOI] [PubMed] [Google Scholar]
- 151.Pamuk BO, Torun AN, Kulaksizoglu M, Ertugrul D, Ciftci O, Kulaksizoglu S, et al. Asymmetric dimethylarginine levels and carotid intima-media thickness in obese patients with polycystic ovary syndrome and their relationship with metabolic parameters. Fertil Steril. 2010;93:1227–33. doi: 10.1016/j.fertnstert.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 152.Teede HJ, Meyer C, Hutchison SK, Zoungas S, McGrath BP, Moran LJ. Endothelial function and insulin resistance in polycystic ovary syndrome: the effects of medical therapy. Fertil Steril. 2010;93:184–91. doi: 10.1016/j.fertnstert.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 153.Zolti M, Bider D, Seidman DS, Mashiach S, Ben-Rafael Z. Cytokine levels in follicular fluid of polycystic ovaries in patients treated with dexamethasone. Fertil Steril. 1992;57:501–4. doi: 10.1016/s0015-0282(16)54891-2. [DOI] [PubMed] [Google Scholar]
- 154.Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohí J, Simón C. The pathogenesis of ovarian hyperstimulation syndrome: in vivo studies investigating the role of interleukin-1beta, interleukin-6, and vascular endothelial growth factor. Fertil Steril. 1999;71:482–9. doi: 10.1016/s0015-0282(98)00484-1. [DOI] [PubMed] [Google Scholar]
- 155.Deshpande RR, Chang MY, Chapman JC, Michael SD. Alteration of cytokine production in follicular cystic ovaries induced in mice by neonatal estradiol injection. Am J Reprod Immunol. 2000;44:80–8. doi: 10.1111/j.8755-8920.2000.440203.x. [DOI] [PubMed] [Google Scholar]
- 156.Lédée-Bataille N, Laprée-Delage G, Taupin JL, Dubanchet S, Taieb J, Moreau JF, et al. Follicular fluid concentration of leukaemia inhibitory factor is decreased among women with polycystic ovarian syndrome during assisted reproduction cycles. Hum Reprod. 2001;16:2073–8. doi: 10.1093/humrep/16.10.2073. [DOI] [PubMed] [Google Scholar]
- 157.Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101:1177–82. doi: 10.1016/s0029-7844(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 158.Omu AE, Al-Azemi MK, Makhseed M, Al-Oattan F, Ismail AA, Al-Tahir S, et al. Differential expression of T-helper cytokines in the peritoneal fluid of women with normal ovarian cycle compared with women with chronic anovulation. Acta Obstet Gynecol Scand. 2003;82:603–9. doi: 10.1034/j.1600-0412.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 159.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 160.Ibañez L, de Zegher F. Ethinylestradiol-drospirenone, flutamide-metformin, or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89:1592–7. doi: 10.1210/jc.2003-031281. [DOI] [PubMed] [Google Scholar]
- 161.Ibáñez L, Valls C, Cabré S, De Zegher F. Flutamide-metformin plus ethinylestradiol-drospirenone for lipolysis and antiatherogenesis in young women with ovarian hyperandrogenism: the key role of early, low-dose flutamide. J Clin Endocrinol Metab. 2004;89:4716–20. doi: 10.1210/jc.2004-0047. [DOI] [PubMed] [Google Scholar]
- 162.Ibañez L, Valls C, Marcos MV, Ong K, Dunger DB, De Zegher F. Insulin sensitization for girls with precocious pubarche and with risk for polycystic ovary syndrome: effects of prepubertal initiation and postpuberal discontinuation of metformin treatment. J Clin Endocrinol Metab. 2004;89:4331–7. doi: 10.1210/jc.2004-0463. [DOI] [PubMed] [Google Scholar]
- 163.Walch K, Grimm C, Zeillinger R, Huber JC, Nagele F, Hefler LA. A common interleukin-6 gene promoter polymorphism influences the clinical characteristics of women with polycystic ovary syndrome. Fertil Steril. 2004;81:1638–41. doi: 10.1016/j.fertnstert.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 164.Ibáñez L, de Zegher F. Flutamide-metformin plus ethinylestradiol-drospirenone for lipolysys and antiatherogenesis in young women with ovarian hyperandrogenism: the key role of metformin at the start and after more than one year of therapy. J Clin Endocrinol Metab. 2005;90:39–43. doi: 10.1210/jc.2004-1405. [DOI] [PubMed] [Google Scholar]
- 165.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–24. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 166.Elkind-Hirsch KE. Thiazolidinediones for the therapeutic management of polycystic ovary syndrome. Treat Endocrinol. 2006;5:171–87. doi: 10.2165/00024677-200605030-00005. [DOI] [PubMed] [Google Scholar]
- 167.Tarkun I, Cetinarslan B, Türemen E, Cantürk Z, Biyikli M. Association between circulating tumor necrosis factor-alpha, interleukin-6, and insulin resistance in normal-weight women with polycystic ovary syndrome. Metab Syndr Relat Disord. 2006;4:122–8. doi: 10.1089/met.2006.4.122. [DOI] [PubMed] [Google Scholar]
- 168.Vgontzas AN, Trakada G, Bixler EO, Lin HM, Pejovic S, Zoumakis E, et al. Plasma interleukin 6 levels are elevated in polycystic ovary syndrome independently of obesity or sleep apnea. Metabolism. 2006;55:1076–82. doi: 10.1016/j.metabol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 169.Corbould A, Dunaif A. The adipose cell lineage is not intrinsically insulin resistant in polycystic ovary syndrome. Metabolism. 2007;56:716–22. doi: 10.1016/j.metabol.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Engler D. Hypothesis: Musculin is a hormone secreted by skeletal muscle, the body’s largest endocrine organ. Evidence for actions on the endocrine pancreas to restrain the beta-cell mass and to inhibit insulin secretion and on the hypothalamus to co-ordinate the neuroendocrine and appetite responses to exercise. Acta Biomed. 2007;78:156–206. [PubMed] [Google Scholar]
- 171.Mlinar B, Marc J, Janez A, Pfeifer M. Molecular mechanism of insulin resistance and associated diseases. Clin Chim Acta. 2007;375:20–35. doi: 10.1016/j.cca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 172.Olszanecka-Glinianowicz M, Banás M, Zahorska-Markiewicz B, Janowska J, Kocelak P, Madej P, et al. Is the polycystic ovary syndrome associated with chronic inflammation per se? Eur J Obstet Gynecol Reprod Biol. 2007;133:197–202. doi: 10.1016/j.ejogrb.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 173.Wu R, Fujii S, Ryan NK, Van der Hoek KH, Jasper MJ, Sini I, et al. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum Reprod. 2007;22:527–35. doi: 10.1093/humrep/del371. [DOI] [PubMed] [Google Scholar]
- 174.Knebel B, Janssen OE, Hahn S, Jacob S, Gleich J, Kotzka J, et al. Increased low grade inflammatory serum markers in patients with polycystic ovary syndrome (PCOS) and their relationship to PPARgamma gene variants. Exp Clin Endocrinol Diabetes. 2008;116:481–6. doi: 10.1055/s-2008-1058085. [DOI] [PubMed] [Google Scholar]
- 175.Manneras L, Jonsdottir IH, Holmäng A, Lönn M, Stener-Victorin E. Low-frequency of electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology. 2008;149:3559–68. doi: 10.1210/en.2008-0053. [DOI] [PubMed] [Google Scholar]
- 176.Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Kocelak P, Janowska J, Semik-Grabarczyk E. The effect of weight loss on inflammation in obese women with polycystic ovary syndrome. Endokrynol Pol. 2008;59:13–7. [PubMed] [Google Scholar]
- 177.Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem. 2008;114:211–23. doi: 10.1080/13813450802364627. [DOI] [PubMed] [Google Scholar]
- 178.Benson S, Arck PC, Tan S, Hahn S, Mann K, Rifaie N, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology. 2009;34:727–35. doi: 10.1016/j.psyneuen.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 179.Chen Q, Sun X, Chen J, Cheng L, Wang J, Wang Y, et al. Direct rosiglitazone action on steroidogenesis and proinflammatory factor production in human granulosa-lutein cells. Reprod Biol Endocrinol. 2009;7:147. doi: 10.1186/1477-7827-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liang FJ, Wu XK, Qu JW, Ke L, Sun J, Wang Y. Response of IGF and IL-6 to ovarian stimulation in PCOS and normal women. Syst Biol Reprod Med. 2009;55:227–35. doi: 10.3109/19396360903247922. [DOI] [PubMed] [Google Scholar]
- 181.Giudice LC, Chandrasekar B, Cataldo NA. The potential roles of intraovarian peptides in normal and abnormal mechanisms of reproductive physiology. Curr Opin Obstet Gynecol. 1993;5:350–9. [PubMed] [Google Scholar]
- 182.Jasper M, Norman RJ. Immunoactive interleukin-1 beta and tumour necrosis factor-alpha in thecal, stromal and granulosa cell cultures from normal and polycystic ovaries. Hum Reprod. 1995;10:1352–4. doi: 10.1093/humrep/10.6.1352. [DOI] [PubMed] [Google Scholar]
- 183.Naz RK, Thurston D, Santoro N. Circulating tumor necrosis factor (TNF)-alpha in normally cycling women and patients with premature ovarian failure and polycystic ovaries. Am J Reprod Immunol. 1995;34:170–5. doi: 10.1111/j.1600-0897.1995.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 184.Gonzalez F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48:437–41. doi: 10.1016/s0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- 185.Hotamisligil GS. The role of TNF-alpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–5. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 186.Milner CR, Craig JE, Hussey ND, Norman RJ. No association between the -308 polymorphism in the tumour necrosis factor alpha (TNFalpha) promoter region and polycystic ovaries. Mol Hum Reprod. 1999;5:5–9. doi: 10.1093/molehr/5.1.5. [DOI] [PubMed] [Google Scholar]
- 187.Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61:993–8. doi: 10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- 188.Solomon CG, Seely EW. Brief review: hypertension in pregnancy: a manifestation of the insulin resistance syndrome? Hypertension. 2001;37:232–9. doi: 10.1161/01.hyp.37.2.232. [DOI] [PubMed] [Google Scholar]
- 189.Araya AV, Aguirre A, Romero C, Miranda C, Molina MC, Ferreira A. Evaluation of tumor necrosis factor alpha production in ex vivo short term culture whole blood from women with polycystic ovary syndrome. Eur Cytokine Netw. 2002;13:419–24. [PubMed] [Google Scholar]
- 190.Korhonen S, Romppanen EL, Hiltunen M, Mannermaa A, Punnonen K, Hippelainen M, et al. Lack of association between C-850T polymorphism of the gene encoding tumor necrosis factor-alpha and polycystic ovary syndrome. Gynecol Endocrinol. 2002;16:271–4. doi: 10.1080/gye.16.4.271.274. [DOI] [PubMed] [Google Scholar]
- 191.Davis JS, Rueda BR, Spanel-Borowski K. Microvascular endothelial cells of the corpus luteum. Reprod Biol Endocrinol. 2003;1:89. doi: 10.1186/1477-7827-1-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sayin NC, Gucer F, Balkanli-Kaplan P, Yuce MA, Ciftci S, Kucuk M, et al. Elevated serum TNF- alpha levels in normal-weight women with polycystic ovaries or the polycystic ovary syndrome. J Reprod Med. 2003;48:165–70. [PubMed] [Google Scholar]
- 193.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–82. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 194.Proietto J. Mechanisms of insulin resistance caused by nutrient toxicity. Hepatol Res. 2005;33:87–91. doi: 10.1016/j.hepres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 195.Babayof R, Margalioth EJ, Huleihel M, Amash A, Zylber-Haran E, Gal M, et al. Serum inhibin A, VEGF and TNFalpha levels after triggering oocyte maduration with GnRH agonist compared with HCG in women with polycystic ovaries undergoing IVF treatment: a prospective randomized trial. Hum Reprod. 2006;21:1260–5. doi: 10.1093/humrep/dei475. [DOI] [PubMed] [Google Scholar]
- 196.Minge CE, Ryan NK, Van der Hoek KH, Robker RL, Norman RJ. Troglitazone regulates peroxisome proliferator-activated receptors and inducible nitric oxide synthase in murine ovarian macrophages. Biol Reprod. 2006;74:153–60. doi: 10.1095/biolreprod.105.043729. [DOI] [PubMed] [Google Scholar]
- 197.Puder JJ, Varga S, Nusbaumer CP, Zulewski H, Bilz S, Muller B, et al. Women with polycystic ovary syndrome are sensitive to the TNF-alpha-lowering effect of glucose-induced hyperinsulinaemia. J Clin Invest. 2006;36:883–9. doi: 10.1111/j.1365-2362.2006.01734.x. [DOI] [PubMed] [Google Scholar]
- 198.Sander V, Luchetti CG, Solano ME, Elia E, Di Girolamo G, Gonzalez C, et al. Role of the N, N’-dimethylbiguanide metformin in the treatment of female prepuberal BALB/c mice hyperandrogenized with dehydroepiandrosterone. Reproduction. 2006;131:591–602. doi: 10.1530/rep.1.00941. [DOI] [PubMed] [Google Scholar]
- 199.Garruti G, Depalo R, Vita MG, Lorusso F, Giampetruzzi F, Damato AB, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biol Endocrinol. 2009;19:552–63. doi: 10.1016/j.rbmo.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 200.Makimura H, Wei J, Dolan-Looby SE, Ricchiuti V, Grinspoon S. Retinol-binding protein levels are increased in association with gonadotropin levels in healthly women. Metabolism. 2009;58:479–87. doi: 10.1016/j.metabol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Victor VM, Rocha M, Bañuls C, Sanchez-Serrano M, Sola E, Gomez M, et al. Mitochondrial complex I impairment in leukocytes from polycystic ovary syndrome patients with insulin resistance. J Clin Endocrinol Metab. 2009;94:3505–12. doi: 10.1210/jc.2009-0466. [DOI] [PubMed] [Google Scholar]
- 202.Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millan JL. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: Relationship to insulin resistance and to obesity. J Clin Endocrinol Metab. 2004;89:806–11. doi: 10.1210/jc.2003-031365. [DOI] [PubMed] [Google Scholar]
- 203.Zhang YF, Yang YS, Hong J, Gu WQ, Shen CF, Xu M, et al. Elevated serum levels of interleukin-18 are associated with insulin resistance in women with polycystic ovary syndrome. Endocrine. 2006;29:419–24. doi: 10.1385/ENDO:29:3:419. [DOI] [PubMed] [Google Scholar]
- 204.Nasiek M, Kos-Kudla B, Ostrowska Z, Marek B, Kajdaniuk D, Sieminska L, et al. Plasma concentration of soluble intercellular adhesion molecule-1 in women with polycystic ovary syndrome. Gynecological Endocrinology. 2004;19:208–15. doi: 10.1080/09513590400014313. [DOI] [PubMed] [Google Scholar]
- 205.Kebapcilar L, Taner CE, Kebapcilar AG, Sari I. High mean platelet volume, low-grade systemic coagulation and fibrinolytic activation are associated with androgen and insulin levels in polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280:187–93. doi: 10.1007/s00404-008-0884-0. [DOI] [PubMed] [Google Scholar]
- 206.Liu B, Cai LY, Lv HM, Xia L, Zhang YJ, Zhang HX, et al. Raised serum levels of matrix metalloproteinase-9 in women with polycystic ovary syndrome and its association with insulin-like growth factor binding protein-1. Gynecol Endocrinol. 2008;24:285–8. doi: 10.1080/09513590802056995. [DOI] [PubMed] [Google Scholar]
- 207.Glintborg D, Andersen M, Richelsen B, Bruun JM. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin Endocrinol (Oxf) 2009;71:652–8. doi: 10.1111/j.1365-2265.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- 208.González F, Rote NS, Minium J, Weaver AL, Kirwan JP. Elevated circulating levels of macrophage migration inhibitory factor in polycystic ovary syndrome. Cytokine. 2010;51:240–4. doi: 10.1016/j.cyto.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor- alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–10. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 210.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 211.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–42. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 212.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
QUORUM flowcharts of the systematic review and meta-analysis of serum C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) concentrations in women with PCOS and controls.