Abstract
The rs17070145 polymorphism (C → T substitution, intron 9) of the KIBRA gene has recently been associated with episodic memory and cognitive flexibility. These findings were inconsistent across reports though, and largely lacked gene–gene or gene–environment interactions. The aim of the present study was to determine the impact of the rs17070145 polymorphism on clinically relevant cognitive domains and its interaction with the modifiers ‘lifestyle' and ‘cardiovascular risk factors'. Five-hundred forty-five elderly volunteers (mean age 64 years, ±7 years, 56% women) accomplished a comprehensive cognitive testing. Principal component analysis was used to reveal the internal structure of the data, rendering four composite scores: verbal memory, word fluency, executive function/psychomotor speed, and working memory. Lifestyle was assessed with a detailed questionnaire, age-associated risk factors by clinical interview and examination. There was no main effect of the rs17070145 genotype on any cognitive composite scores. However, we found worse performance in executive functions for T-allele carriers in the presence of arterial hypertension (β=−0.365, p=0.0077 and 0.031 after Bonferroni correction). This association was further modified by gender, showing the strongest association in hypertensive females (β=−0.500, p=0.0072 and 0.029 after Bonferroni correction). The effect of KIBRA on cognitive function seems to be complex and modified by gender and arterial hypertension.
Keywords: aging, memory, executive functions, vascular risk factors, gender, ecogenetic context
INTRODUCTION
Cognitive impairment is of substantial socioeconomic concern in the aging populations (Ferri et al, 2005; Wegman, 2006). Several risk factors for the development of dementia and cognitive impairment have been described, most importantly common vascular and metabolic diseases such as hypertension, diabetes, and atherosclerosis (Arvanitakis et al, 2004; Biessels et al, 2006; Elias et al, 2005; Knecht et al, 2008). There is also evidence that lifestyle factors, such as physical activity and dietary habits, exert influence on cognitive functions (Laurin et al, 2001; Ruscheweyh et al, 2009; Witte et al, 2009).
Besides these potentially ‘modifiable' conditions, genetic factors have been linked to cognitive performance (McClearn et al, 1997; Savitz et al, 2006). Common polymorphisms in several genes have been identified to influence cognitive function, including catechol-O-methyltransferase (Harris et al, 2005; Savitz et al, 2006), dopamine receptor D2 (Duan et al, 2003), brain-derived neurotrophic factor (BDNF) (Chen et al, 2006; Sklar et al, 2002), and apolipoprotein E (Bertram et al, 2007).
Rather than directly accounting for cognitive abilities, genetic factors might interact with external modifiers (eg, cardiovascular risk factors) in determining cognitive functions. Such interactions have already been demonstrated, for example, for APOE-carrier status and cholesterol in the development of dementia of the Alzheimer type (AD) (Chandra and Pandav, 1998).
A single-nucleotide polymorphism (SNP) in intron 9 of the KIBRA gene (common C → T substitution, rs17070145, GenBank accession number NM_015238) has recently been associated with episodic memory function and cognitive flexibility (Almeida et al, 2008; Bates et al, 2009; Nacmias et al, 2008; Papassotiropoulos et al, 2006; Schaper et al, 2008; Zhang et al, 2009; Schneider et al, 2010), and even with the risk of developing AD (Burgess et al, 2010; Corneveaux et al, 2008). The KIBRA protein is discussed to be involved in brain development and memory formation as a postsynaptic scaffold protein connecting cytoskeletal and signaling molecules (Johannsen et al, 2008; Yoshihama et al, 2009). However, results for episodic memory have been inconsistent across studies, ranging from the originally reported beneficial effects of the T-allele on memory functions (Almeida et al, 2008; Papassotiropoulos et al, 2006; Schaper et al, 2008; Yasuda et al, 2010; Vassos et al, 2010) to null findings (Need et al, 2008) and to even poorer memory in T-allele carriers (Nacmias et al, 2008). Considering other cognitive functions, two reports looked at an association of executive functions with the rs17070145 polymorphism: Zhang et al (2009) found an inverse association with T-allele-carrier status, revealing worse performance in the Wisconsin Card Sorting Test in T-allele carriers. Schaper et al (2008), however, found no association of KIBRA with tasks of executive function. With exception of the study by Vassos et al (2010) who used a memory composite score, previous findings were based on single neuropsychological tests (Almeida et al, 2008; Papassotiropoulos et al, 2006; Schaper et al, 2008), rather than composite scores of related cognitive tests. Moreover, only few studies so far have assessed gene–gene or gene–environment interactions, which are of particular relevance in a clinical context. One study by Zhang et al (2009) reported an interaction between KIBRA rs17070145 genotype and smoking habits and Preuschhof et al (2010) showed an interaction between KIBRA and CLSTN2 in the modulation of episodic memory performance. Taken together, the differential impact of the KIBRA rs17070145 polymorphism on cognitive function as well as its clinical significance and interaction with environmental and other genetic factors is not completely understood.
The aim of the present study was to comprehensively test for potential associations of the common KIBRA rs17070450 genotype with cognitive functions in a medically well-characterized cohort of community-dwelling elderly individuals. We focused on cognitive composite scores, reflecting clinically meaningful cognitive domains, and on interactions of rs17070450 with established risk factors for cognitive decline (arterial hypertension, depression, diabetes, and lifestyle habits (Daffner, 2009)). We also analyzed gender-specific effects, as gender has been shown to modify the relationship between cognitive decline and environmental as well as genetic traits (Kuznetsova et al, 2004; Raz et al, 2009b).
MATERIALS AND METHODS
Subjects and Design
Participants were recruited from the population-based SEARCH-Health study (Systematic evaluation and alteration of risk factors for cognitive health), which aims to examine the impact of modifiable risk factors on cognitive aging (Knecht et al, 2008). The study was approved by the local ethics committee of the Medical Association Westfalen-Lippe. Community-dwelling individuals from 40 to 85 years of age were randomly selected based only on dates of birth from the register of residents from the city of Münster, Germany. They were invited to participate in the study by letter and recruited after giving informed consent. All participants received a structured clinical face-to-face interview, a physical examination by a trained study physician, a non-fasting blood sampling, a comprehensive neuropsychological assessment and cerebral magnetic resonance imaging at 3.0 T. The time period of baseline recruitment was 53 months (January 2004 to June 2008). The entire SEARCH baseline cohort comprised 729 individuals.
For the present study 545 individuals were included. Main criteria were age above 50 years and completion of the neuropsychological and laboratory assessments as well as the lifestyle questionnaire. Individuals with a history or imaging evidence of stroke, or other severe neurological conditions were excluded. We further excluded one participant with complex psychiatric comorbidities. Individuals included in the present study did not differ significantly in terms of age, gender, and education from subjects who were excluded from this analysis.
Risk Factor Assessment
Assessment of cardiovascular risk factors was based on self-reported physician diagnoses of existing or past illnesses, including history of hypertension, history of diabetes, and history of hypercholesterolemia. Depression was defined by history, intake of antidepressants, or scores higher than 17 on the Beck Depression Inventory. Education was assessed as categorical variable (5 vs 7 vs 9 years of secondary school vs tertiary education according to the German educational system (Wersching et al, 2010).
Lifestyle Index
To create a lifestyle score as described in previous studies (Floel et al, 2008; Kurth et al, 2006), we considered self-reported lifestyle information from a detailed questionnaire incorporating information about body mass index (BMI), dietary habits, exercise, smoking, and alcohol consumption. The overall ‘lifestyle score' was calculated by assigning scores of 1–5 to each individual variable category, for which a higher point value indicates a healthier behavior. The healthiest behavior was defined as a BMI of 18.5–22 kg/m2, never smoking, intense physical activity (>13 000 kcal per week), moderate alcohol consumption (4–10 drinks per week), and a dietary pattern rich in fruits, vegetables, wholegrain products, and unsaturated fatty acids. For details on this score, please see Floel et al (2008).
Neuropsychological Assessment
The neuropsychological assessment was conducted by trained clinical psychologists supervised by two senior neuropsychologists. A comprehensive neuropsychological test battery was administered to assess the full range of cognitive functions (Table 1). A detailed description of the tests is found in Lezak (2004). A principal component analysis (PCA) was carried out to reduce the single tests to superordinate composite scores using varimax rotation and including coefficients with absolute values above 0.4. We used composite scores to decrease error associated with the analysis of multiple related cognitive outcomes and to extract theoretically relevant cognitive domains. We validated the resulting composite scores with regard to known cognitive domains (Lezak, 2004) and labeled them as ‘verbal memory', ‘word fluency', ‘working memory', and ‘executive functions/psychomotor speed' (Table 1). Using PCA, the data were z-transformed resulting in a mean of zero and an SD of 1.00 for each cognitive domain. This allows for a direct comparison of the cognitive domains in terms of SD units.
Table 1. Neuropsychometric Test Battery.
| Domain after PCA | Test | Subtests |
|---|---|---|
| Verbal memory | Auditory verbal learning test (AVLT; German version) | AVLT 1—immediate verbal span |
| AVLT 5—verbal learning | ||
| AVLT 1–5—slope of learning | ||
| AVLT 6—short-term retrieval | ||
| AVLT 7—long-term retrieval | ||
| AVLT 8—recognition | ||
| Word fluency | Category and letter fluency | Letter S |
| Category animals | ||
| Shifting categories: fruit—sport | ||
| Executive functions/psychomotor speed | Color-word-interference test (CWIT; stroop test) | CWIT part 3—interference condition |
| Digit symbol substitution test | — | |
| Trail-making-test (TMT) | TMT—part A | |
| TMT—part B | ||
| Working memory | Digit span (Wechsler Memory Scale—revised, | WMS—digit span forward |
| WMS-R; German version) | WMS—digit span backward | |
| Rey complex figure test | Recall trial | |
| Copy trial |
Seventeen neuropsychological subtests were grouped into clinically significant cognitive domains using principal component analysis.
PCA, principal component analysis.
We performed a power analysis, which showed that the power to detect a 0.1 mean difference in the z-scores across genotypes is 21%, to detect a mean difference of 0.2 the power is 64%, and for a mean difference of ⩾0.3 the power is above 90%.
Genotyping of KIBRA rs17070145 Polymorphism
Genomic DNA was extracted from white blood cells using QIAamp DNA Blood Kit (Qiagen, Hilden, Germany). From the published sequence of the KIBRA gene (Kremerskothen et al, 2003), a 198-bp fragment was chosen, which contained the polymorphic site (rs17070145, GenBank accession number NM_015238) using two primers (forward: atcctcttgaggcttcactgg, reverse: actttcaacacaatgaacaagg). The amplification was performed using the following PCR program: 95 °C for 15 min to denature, followed by 94 °C for 1 min, 68 °C for 1 min, and 72 °C for 1 min for 12 cycles, followed by 94 °C for 1 min, 56 °C for 1 min, 72 °C for 1 min for 25 cycles, and 72 °C for 10 min. PCR products were digested by addition of 1 U of MseI restriction enzyme for 12 h at 37 °C. The C allele lacks an MseI site present in the T-allele, so that only in the presence of the T-allele, the PCR product (198 bp) was cut into two fragments of 128 and 70 bp in length, visualized on ethidium bromide-stained 1.5% agarose gels.
Data Analysis
In line with previous studies, KIBRA rs17070145 CT and TT genotypes were combined and tested against KIBRA rs17070145 CC genotype (=reference) (Papassotiropoulos et al, 2006; Schaper et al, 2008). Student's t-tests, Fisher's exact tests, and χ2 tests were used to univariately test for differences in age, gender, education, and risk factors between CT/TT and CC genotypes.
After calculation of cognitive composite scores via PCA, effects of the KIBRA rs17070145 polymorphism on the cognitive domains were tested with multivariate analyses of variance using the general linear model procedure in SAS 9.2. The basic model addressing main effects included the following covariates: age, gender, education, history of arterial hypertension, intake of antihypertensive medication, history of diabetes, lifestyle index, history of depression, intake of antidepressive medication, and neuropsychologists involved. A second model addressing interactions between covariates further included second-order interaction terms among the genotype and the single risk factors including gender (arterial hypertension × rs17070145 genotype, diabetes × rs17070145 genotype, lifestyle index × rs17070145 genotype, depression × rs17070145 genotype, gender × rs17070145 genotype). Based on these results, further analyses were performed stratified by gender and hypertension. Statistical analyses were performed using SAS 9.2. All probability values are two-tailed, and we considered p<0.05 as statistically significant. We used Bonferroni correction to adjust for multiple testing.
RESULTS
Study Population
Of the 545 participants included in this study, 235 (43.12%) were carriers of the CC polymorphism of KIBRA rs17070145 in intron 9, 234 (42.94%) subjects were CT carriers, and 76 (13.94%) were homozygous TT carriers. The allelic distribution (35% T-allele frequency) was similar to previous studies (Almeida et al, 2008; Papassotiropoulos et al, 2006; Schaper et al, 2008) and proved to be in Hardy–Weinberg equilibrium (χ2 2.05, 1 df, p=0.15). The KIBRA rs17070145 polymorphism was not associated with age, gender, education, or any of the specified cerebrovascular risk factors or the lifestyle index (Table 2).
Table 2. Sample Characteristics.
| Characteristic | CT/TT genotypes N=310 | CC genotype N=235 | p-valuea |
|---|---|---|---|
| Age, years, mean±SD | 63.2±7.2 | 64.0±6.7 | 0.235 |
| Female, % | 56.5 | 54.9 | 0.728 |
| Education | |||
| Five years of secondary school, % | 30.0 | 35.7 | 0.431 |
| Seven years of secondary school, % | 22.9 | 20.9 | |
| Nine years of secondary school, % | 11.0 | 8.1 | |
| Tertiary education, % | 36.1 | 35.3 | |
| Arterial hypertension, % | 35.2 | 34.9 | 1.000 |
| Diabetes, % | 5.2 | 3.4 | 0.401 |
| Lifestyle index, mean±SD | 13.75±3.00 | 14.10±2.96 | 0.182 |
p-value denoting significance between groups.
PCA of the neuropsychological test scores rendered four meaningful cognitive domains: verbal memory, word fluency, executive functions/psychomotor speed, and working memory (see Table 1 for details).
KIBRA-Carrier Status and Cognition
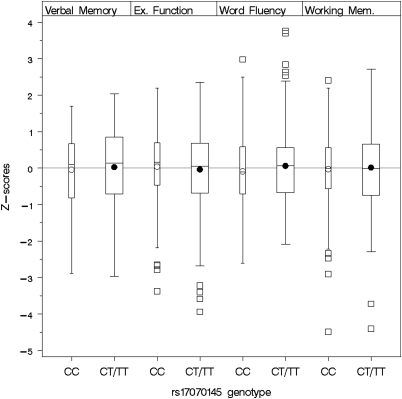
Unadjusted main effects of the KIBRA rs17070145 polymorphism on the cognitive domains are shown in Figure 1. The regression analyses adjusting for the full set of covariates revealed no significant associations of any of the cognitive composite scores with KIBRA rs17070145 genotype showing the following regression estimates: β=−0.118 for executive functions/psychomotor speed, β=0.037 for verbal memory, β=0.011 for working memory, and β=0.114 for verbal fluency; all p-values>0.1). Separate regression models for each single memory test of the composite score also revealed no significant association between KIBRA rs17070145 and any of the memory measures with or without adjustment for executive function (Supplementary Tables 1 and 2).
Figure 1.
Cognitive performance by cognitive domain and KIBRA rs17070145 genotype. Boxplots show medians, interquartile range, and outliers as well as arithmetic means (blank circle: CC, filled circle: CT/TT). CC: n=235 subjects and CT/TT: n=310 subjects: Ex. Function, executive function/psychomotor speed; Working Mem, working memory.
Interactions of KIBRA-Carrier Status and Risk Factors/Lifestyle
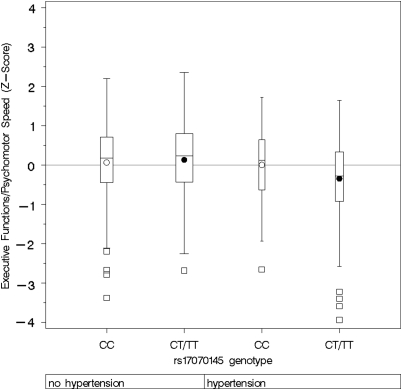
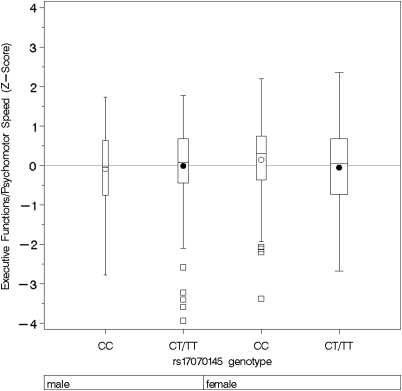
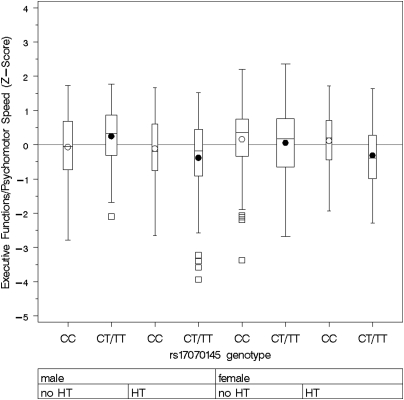
For executive functions/psychomotor speed, there was an interaction of KIBRA genotypes with hypertension status (p=0.049) and gender (p=0.022). In subjects with arterial hypertension, T-allele carriers performed significantly worse in executive functions than non-T-allele carriers (β=−0.365, p=0.0077 and 0.031 after Bonferroni correction), while this was not the case in subjects without arterial hypertension (β=0.031, p=0.756; Figure 2). Stratification by gender showed that the association of SNP rs17070145 and executive functions was statistically significant in women only (β=−0.273, p=0.012 and 0.048 after Bonferroni correction, men: β=−0.041, p=0.568; Figure 3). Again female T-allele carriers showed worse performance in executive tasks than female non-T-allele carriers. Stratification in four groups for both gender and hypertension status showed a strong association of SNP rs17070145 with executive function in hypertensive women only (β=−0.500, p=0.0072 and 0.029 after Bonferroni correction, Figure 4). There was also a significant interaction of KIBRA genotypes with gender for working memory (p=0.0075), and a trend for interaction of KIBRA genotypes with gender for verbal memory (p=0.107, not shown). The analyses revealed no significant gene–risk factor interaction for the cognitive domain of word fluency.
Figure 2.
Composite score of executive functions/psychomotor speed by rs17070145 genotype, stratified for arterial hypertension. Boxplots show medians, interquartile range, and outliers as well as arithmetic means (blank circle: CC, filled circle: CT/TT). HT=hypertension. CC, no HT: n=153; CT/TT, no HT: n=201; CC, HT: n=82; CT/TT, HT: n=109.
Figure 3.
Composite score of executive functions/psychomotor speed by rs17070145 genotype, stratified for gender. Boxplots show medians, interquartile range, and outliers as well as arithmetic means (blank circle: CC, filled circle: CT/TT). CC, male: n=106; CT/TT, male: n=135; CC, female: n=129; CT/TT, female: n=175.
Figure 4.
Composite score of executive functions/psychomotor speed by rs17070145 genotype, gender, and hypertension status. Boxplots show medians, interquartile range, and outliers as well as arithmetic means (blank circle: CC, filled circle: CT/TT). HT=hypertension. CC, male, no HT: n=65; CT/TT male, no HT: n=79; CC, male, HT: n=41; CT/TT, male, HT: n=56; CC, female, no HT: n=88; CT/TT female, no HT: n=122; CC, female, HT: n=41; CT/TT female, HT: n=53. Note that except for male non-hypertensives, T-allele carriers performed worse in tests of executive function. This difference is particularly evident in women with arterial hypertension.
DISCUSSION
The present study revealed these three major findings: (1) We did not find any main effects of the KIBRA rs17070145 genotype on cognitive functions. Neither the composite scores nor the single memory tests were significantly associated with T-allele status. Hence, we could not replicate recent findings of the association of rs17070145 and immediate and delayed memory performance. (2) For the first time, we showed an interaction of the rs17070145 polymorphism (common C → T substitution within the ninth intron) of the KIBRA gene with arterial hypertension: in subjects with arterial hypertension, T-allele carriers performed worse in executive functions compared with CC carriers. In subjects without a diagnosis of hypertension, there was no effect of rs17070145 genotype on executive functions. (3) We found gender to modify the effects of rs17070145 on performance in executive functions/psychomotor speed showing a negative effect of the T-allele only in females, whereas in men, there was no difference in executive function across genotypes.
When dividing the cohort into four strata by gender and hypertension status, we observed an additive effect showing the largest genotype-related difference in executive function for women with arterial hypertension.
Moreover, we found a significant interaction of gender and rs17070145 genotype for the cognitive domain of working memory, resulting from a different effect of gender on working memory by rs17070145 genotype. This means, in the group of T-allele carriers women performed significantly worse than men. Hence, we see a modulation of gender-specific effects by KIBRA genotype rather than vice versa.
A comprehensive evaluation of cognitive function was conducted in this study. A subsequent PCA then reduced the single test scores to four main factors, that is the four clusters of tests that best explain the variance of the data, thereby reflecting physiologically meaningful phenotypes. Previous studies used only a restricted number of neuropsychological assessments, and found associations for only subtests of the respective domains tested (Almeida et al, 2008; Papassotiropoulos et al, 2006; Schaper et al, 2008; Zhang et al, 2009). However, even when analyzing the single components of the AVLT, we were not able to replicate the original findings (Papassotiropoulos et al, 2006; Schaper et al, 2008) on episodic memory functions. However, since these first reports, a number of studies have reported no association, or even an opposite association, of T-carrier status for memory performance (Nacmias et al, 2008; Need et al, 2008; Zhang et al, 2009), while others were able to corroborate the finding of a better memory performance in T-allele carriers (Almeida et al, 2008; Bates et al, 2009; Ehret, 2010). Similarly, there are conflicting reports on the impact of the rs17070145 polymorphism on executive functions: Zhang et al (2009) found an inverse association of T-allele-carrier status, revealing worse performance in the Wisconsin Card Sorting Test in T-allele carriers, whereas Schaper et al (2008) found no association of KIBRA with the Trail-Making Test and a lexical fluency task.
Conflicting results in previous studies may be due to the fact that several single test scores, instead of cognitive domains, were employed, thus increasing the chance of significant correlations in both the positive and the negative direction. On the other hand, inconsistencies might be due to differences in the underlying study population, particularly if sample sizes are very small (Schaper et al, 2008). Even more important, the impact of environmental factors and traditional vascular risk factors (such as arterial hypertension) has been largely neglected so far. Besides being an important cardiovascular risk factor, hypertension itself is being influenced by genetic (31–68%) and environmental factors (Ehret, 2010). Genome-wide association studies identified more than a dozen loci that are associated with blood pressure traits in large cohorts. However, the effect sizes of the variants are small and explain only about 1% of the phenotypic variability to date (Ehret, 2010).
While previous studies consistently corrected for age, gender, and education, they largely missed adjustment for vascular risk factors and lifestyle choices. Since the latter factors are known to influence development of atherosclerotic disease and dementia, they should be taken into account when examining gene–cognition associations, particularly in an elderly population in which a large proportion shows one or more of these risk factors (Wolf-Maier et al, 2003).
Gene–risk factor interactions regarding cognitive performance have been found for other genes and vascular risk factors: APOE-carrier status modified the negative effects of atherosclerosis and diabetes on cognition (Dore et al, 2009; Haan et al, 1999). BDNF, which is involved in brain development and memory formation (Savitz et al, 2006), modified the effects of vascular risk factors on cognitive skills (Raz et al, 2008). Importantly, in line with our findings, the BDNF Val66Met polymorphism seemed to influence psychomotor speed particularly in women and in subjects with arterial hypertension (Raz et al, 2009b).
Our finding of a gender difference in the relationship between KIBRA T-allele-carrier status and cognition further argues for a genetically determined modification of the association between hormonal status and cognitive performance. An interaction of estrogen and APOE-carrier status on cognitive decline was recently found by Yaffe et al (2000). KIBRA itself has been shown to have a role in the activation of the estrogen receptor-α in mammalian cells (Rayala et al, 2006). The impact of gender on the associations between KIBRA and cognition can also be seen in light of a differential impact of vascular risk factors on cognitive function. In our study, women on average had less vascular risk factors than men, possibly leaving more impact for genetic factors to influence cognitive functions (Raz et al, 2009a).
Mechanistically, KIBRA encodes a postsynaptic scaffold protein, which is predominately expressed in the kidney and the brain (‘KIdney and BRAin' (Kremerskothen et al, 2003)). KIBRA RNA is expressed in the whole brain with a peak in memory-related structures such as the hippocampus and the temporal lobe (Papassotiropoulos et al, 2006). As a component of the neuronal cytoskeleton, KIBRA might have a role in brain development connecting cytoskeletal and signaling molecules (Johannsen et al, 2008; Yoshihama et al, 2009). KIBRA is also a substrate for protein kinase Cζ, which is crucially involved in synaptic plasticity and memory formation (Buther et al, 2004).
Interestingly, executive functions were only significantly affected in individuals that suffered from hypertension. Prefrontal lobe functioning is dependent to a large degree on subcortical white matter integrity underlying prefrontal cortex. In both healthy individuals (Floel et al, 2009; Gold et al, 2007) and patient populations (Guerini et al, 2004; Delano-Wood et al, 2008), integrity of white matter fiber tracts connecting the prefrontal cortex to other cortical, as well as subcortical structures has been shown to correlate with success of learning and memory formation. Thus, prefrontal functions are particularly vulnerable to cerebral vascular damage (Prins et al, 2005). Our findings of worse effects for T-allele-carrier status with regard to executive functions, as well as effect modification by the presence of arterial hypertension (resulting in even worse performance for executive functions in hypertensive T-allele carriers) may thus be due to a differential modification of prefrontal functions by vascular risk factors.
An alternative explanation for the differential effects of T-allele-carrier status on different cognitive domains has been proposed by Zhang et al (2009). The authors postulated a differential effect of KIBRA-carrier status on long-lasting memory (hippocampus dependent) on the one hand, and cognitive flexibility, short-term memory, and working memory (prefrontal cortex dependent) on the other hand. However, note that the classification of cognitive tests varies across studies. In our study, using a PCA first to identify superordinate composite scores for cognitive domains, tests of cognitive flexibility fell within the category of executive functions/psychomotor speed. In the study by Zhang et al, the Wisconsin Card Sorting tests, another test of cognitive flexibility, was designated as a test of working memory. Moreover, 5 min delayed free recall in the study by Papassotiropoulos et al (2006) has been termed ‘short-term memory' as well. Further studies will have to pay close attention to the definition of clinically significant cognitive domains from multiple neuropsychological tests first before progressing to correlation analyses.
The use of composite scores per se needs to be handled with caution when trying to identify molecular pathways related to human cognition. Many SNP effects have shown to be highly specific (Egan et al, 2003) and, therefore, might be distorted when constructing artificial composite scores. We therefore used a PCA to reveal the internal structure of the present data (Tabachnik and Fedell, 2011). The composite scores extracted this way comprise tests that share common variance, that is, they reflect underlying neuropsychological functions. For the cognitive domain of verbal memory, the composite score is highly specific, reflecting hippocampal-dependent episodic memory performance. The domain ‘executive function' is of a more widespread character. Consisting of the subtests TMT-A, TMT-B, Stroop, and DSST, it measures multiple-related skills such as cognitive flexibility and speed, attention, and visuomotor coordination (Lezak, 2004). Hence, physiologically, several brain regions are involved. However, all of these tests crucially depend on an intact function of the prefrontal cortex (therefore, the term ‘frontal-executive functions' is often used).
Even though the present study included a large and well-characterized population in terms of vascular risk factors and cognitive profile, some limitations need to be discussed. First, given the complexity of gene–gene and gene–environment interactions, the statistical model including main effects and second-order interaction terms might not be complex enough to capture the full set of associations. However, to include yet more factors, an even larger cohort will be necessary. Next, a larger number of common genetic polymorphisms in possibly learning-relevant genes (eg, BDNF, COMT, dopamine receptor D2, or APOE) could have been included, but similarly, this would have resulted in an excessive number of factors to be included in the model. Third, we analyzed only one polymorphism of the KIBRA gene (SNP rs17070145). Although this approach is suitable for replication of and comparison with previous studies, which mainly analyzed the SNP rs17070145, there might be several other SNPs of the KIBRA gene that may impact cognitive function, so that a tagging SNP approach could be helpful for a future study design. Moreover, the KIBRA rs17070145 polymorphism is found in an intron, and is supposed to be linked to causal polymorphisms, which are yet unknown. Further investigations are needed to determine the causative polymorphism in the KIBRA gene.
Finally, the lack of main effects of SNP rs17070145 on cognitive measures in our study might be a problem of study power. We calculated a power of only ∼20% to detect a mean difference of 0.1 in the z-scores, which is about the average difference we observed in the four cognitive domains across genotypes. Hence, the power to detect main effects is very low in our cohort. However, some of the previous studies were based on a smaller study population and yet reported significant findings (Nacmias et al, 2008; Schaper et al, 2008). These studies differed from our cohort with respect to the study population in terms of age and premorbidity, respectively. The largest study to date reporting an association between KIBRA and memory function is published by Bates et al (2009). Based on >2000 participants, the power to detect a significant difference in memory measures across genotypes was very high. However, Bates et al did not apply tests of executive function, therefore, an even larger effect of rs17070145 on executive function cannot be excluded. Thus, the characteristics of the study participants, the neuropsychological tests applied and possible modifying effects should be carefully evaluated when analyzing and interpreting effects of the KIBRA gene on cognition.
In conclusion, the effect of KIBRA on cognitive functions is more complex than previously thought, depending on the cognitive domain that is being assessed. Furthermore, the effect is modulated not only by gender, but also seems to be modified by arterial hypertension, which itself is being influenced by genetic and environmental factors, and which importantly contributes to atherosclerosis and cognitive decline. Thus, a comprehensive assessment of these factors should be conducted in studies on association of common genetic polymorphisms on cognition, at least in elderly populations. A better understanding of the complex interactions between genetic profile and cardiovascular risk factors will help to develop preventive or therapeutic strategies for memory improvement, ideally tailored to each individual's genetic profile (Mayor, 2007).
The project is supported by several public funding sources, which have no involvement in study design, in the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the paper for publication. HW, KG, SH, CH, MS, VW, JW, and HL report no disclosure. SJ is supported by ‘Interdisziplinäres Zentrum für klinische Forschung' (IZKF, Floe3/004/08). KD and JK were supported by DFG grant PA 483/14-2 and received support by IMF Münster 2009–2011. SK has received speaker's honoraria from Janssen-Cilag and Boehringer Ingelheim and travel support from Bayer Health Care. EB is supported by a Heisenberg professorship from the DFG (Br 1589/8-1 and BR 1589/8-2). AF is supported by a grant from the ‘Interdisziplinäres Zentrum für klinische Forschung' (IZKF, Floe3/004/08), the DFG (FL 379-4/2, FL 379-8/1; and DFG-Exc 257), and the BMBF (FKZ 0315673A and 01EO0801). SK received support from the BMBF-Competence Network Mednet Atrial Fibrillation, the European Commission (MRTN), and the Neuromedical Foundation Muenster.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Almeida OP, Schwab SG, Lautenschlager NT, Morar B, Greenop KR, Flicker L, et al. KIBRA genetic polymorphism influences episodic memory in later life, but does not increase the risk of mild cognitive impairment. J Cell Mol Med. 2008;12:1672–1676. doi: 10.1111/j.1582-4934.2008.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- Bates TC, Price JF, Harris SE, Marioni RE, Fowkes FG, Stewart MC, et al. Association of KIBRA and memory. Neurosci Lett. 2009;458:140–143. doi: 10.1016/j.neulet.2009.04.050. [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Burgess JD, Pedraza O, Graff-Radford NR, Hirpa M, Zou F, Miles R, et al. 2010Association of common KIBRA variants with episodic memory and AD risk Neurobiol Aging(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Buther K, Plaas C, Barnekow A, Kremerskothen J. KIBRA is a novel substrate for protein kinase Czeta. Biochem Biophys Res Commun. 2004;317:703–707. doi: 10.1016/j.bbrc.2004.03.107. [DOI] [PubMed] [Google Scholar]
- Chandra V, Pandav R. Gene-environment interaction in Alzheimer′s disease: a potential role for cholesterol. Neuroepidemiology. 1998;17:225–232. doi: 10.1159/000026175. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneveaux JJ, Liang WS, Reiman EM, Webster JA, Myers AJ, Zismann VL, et al. Evidence for an association between KIBRA and late-onset Alzheimer's disease. Neurobiol Aging. 2008;31:901–909. doi: 10.1016/j.neurobiolaging.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR. Promoting successful cognitive aging: a comprehensive review. J Alzheimers Dis. 2009;19:1101–1122. doi: 10.3233/JAD-2010-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano-Wood L, Abeles N, Sacco JM, Wierenga CE, Horne NR, Bozoki A. Regional white matter pathology in mild cognitive impairment: differential influence of lesion type on neuropsychological functioning. Stroke. 2008;39:794–799. doi: 10.1161/STROKEAHA.107.502534. [DOI] [PubMed] [Google Scholar]
- Dore GA, Elias MF, Robbins MA, Elias PK, Nagy Z. Presence of the APOE epsilon4 allele modifies the relationship between type 2 diabetes and cognitive performance: the Maine-Syracuse Study. Diabetologia. 2009;52:2551–2560. doi: 10.1007/s00125-009-1497-2. [DOI] [PubMed] [Google Scholar]
- Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ehret GB. Genome-wide association studies: contribution of genomics to understanding blood pressure and essential hypertension. Curr Hypertens Rep. 2010;12:17–25. doi: 10.1007/s11906-009-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham Heart Study. Neurobiol Aging. 2005;26 (Suppl 1:11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, de Vries MH, Scholz J, Breitenstein C, Johansen-Berg H. White matter integrity in the vicinity of Broca's area predicts grammar learning success. Neuroimage. 2009;47:1974–1981. doi: 10.1016/j.neuroimage.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Floel A, Witte AV, Lohmann H, Wersching H, Ringelstein EB, Berger K, et al. Lifestyle and memory in the elderly. Neuroepidemiology. 2008;31:39–47. doi: 10.1159/000137378. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45:2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerini F, Frisoni GB, Bellwald C, Rossi R, Bellelli G, Trabucchi M. Subcortical vascular lesions predict functional recovery after rehabilitation in patients with L-dopa refractory parkinsonism. J Am Geriatr Soc. 2004;52:252–256. doi: 10.1111/j.1532-5415.2004.52064.x. [DOI] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Harris SE, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The functional COMT polymorphism, Val 158 Met, is associated with logical memory and the personality trait intellect/imagination in a cohort of healthy 79 year olds. Neurosci Lett. 2005;385:1–6. doi: 10.1016/j.neulet.2005.04.104. [DOI] [PubMed] [Google Scholar]
- Johannsen S, Duning K, Pavenstadt H, Kremerskothen J, Boeckers TM. Temporal-spatial expression and novel biochemical properties of the memory-related protein KIBRA. Neuroscience. 2008;155:1165–1173. doi: 10.1016/j.neuroscience.2008.06.054. [DOI] [PubMed] [Google Scholar]
- Knecht S, Wersching H, Lohmann H, Bruchmann M, Duning T, Dziewas R, et al. High-normal blood pressure is associated with poor cognitive performance. Hypertension. 2008;51:663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577. [DOI] [PubMed] [Google Scholar]
- Kremerskothen J, Plaas C, Buther K, Finger I, Veltel S, Matanis T, et al. Characterization of KIBRA, a novel WW domain-containing protein. Biochem Biophys Res Commun. 2003;300:862–867. doi: 10.1016/s0006-291x(02)02945-5. [DOI] [PubMed] [Google Scholar]
- Kurth T, Moore SC, Gaziano JM, Kase CS, Stampfer MJ, Berger K, et al. Healthy lifestyle and the risk of stroke in women. Arch Intern Med. 2006;166:1403–1409. doi: 10.1001/archinte.166.13.1403. [DOI] [PubMed] [Google Scholar]
- Kuznetsova T, Staessen JA, Thijs L, Kunath C, Olszanecka A, Ryabikov A, et al. Left ventricular mass in relation to genetic variation in angiotensin II receptors, renin system genes, and sodium excretion. Circulation. 2004;110:2644–2650. doi: 10.1161/01.CIR.0000145541.63406.BA. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. Oxford University Press: New York, Oxford; 2004. [Google Scholar]
- Mayor S. Fitting the drug to the patient. BMJ. 2007;334:452–453. doi: 10.1136/bmj.39133.452315.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- Nacmias B, Bessi V, Bagnoli S, Tedde A, Cellini E, Piccini C, et al. KIBRA gene variants are associated with episodic memory performance in subjective memory complaints. Neurosci Lett. 2008;436:145–147. doi: 10.1016/j.neulet.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Need AC, Attix DK, McEvoy JM, Cirulli ET, Linney KN, Wagoner AP, et al. Failure to replicate effect of Kibra on human memory in two large cohorts of European origin. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:667–668. doi: 10.1002/ajmg.b.30658. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, et al. Common Kibra alleles are associated with human memory performance. Science. 2006;314:475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- Preuschhof C, Heekeren HR, Li SC, Sander T, Lindenberger U, Backman L. KIBRA and CLSTN2 polymorphisms exert interactive effects on human episodic memory. Neuropsychologia. 2010;48:402–408. doi: 10.1016/j.neuropsychologia.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den HT, Vermeer SE, Jolles J, Koudstaal PJ, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- Rayala SK, den HP, Manavathi B, Talukder AH, Song C, Peng S, et al. Essential role of KIBRA in co-activator function of dynein light chain 1 in mammalian cells. J Biol Chem. 2006;281:19092–19099. doi: 10.1074/jbc.M600021200. [DOI] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land S.2009aEffects of age, genes, and pulse pressure on executive functions in healthy adults Neurobiol Aging(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land SJ, Jacobs BS. Brain-derived neurotrophic factor Val66Met and blood glucose: a synergistic effect on memory. Front Hum Neurosci. 2008;2:12. doi: 10.3389/neuro.09.012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009b;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, et al. 2009Physical activity and memory functions: an interventional study Neurobiol Aging(e-pub ahead of print). [DOI] [PubMed]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Schaper K, Kolsch H, Popp J, Wagner M, Jessen F. KIBRA gene variants are associated with episodic memory in healthy elderly. Neurobiol Aging. 2008;29:1123–1125. doi: 10.1016/j.neurobiolaging.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schneider A, Huentelman MJ, Kremerskothen J, Duning K, Spoelgen R, Nikolich K. KIBRA: a new gateway to learning and memory. Front Aging Neurosci. 2010;2:4. doi: 10.3389/neuro.24.004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Tabachnik B, Fedell LS.2011Using Multivariate StatisticsPearson International Edition. 5th ed. 2007.
- Vassos E, Bramon E, Picchioni M, Walshe M, Filbey FM, Kravariti E, et al. Evidence of association of KIBRA genotype with episodic memory in families of psychotic patients and controls. J Psychiatr Res. 2010;44:795–798. doi: 10.1016/j.jpsychires.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Wegman DH. Aging and globalization. Med Lav. 2006;97:137–142. [PubMed] [Google Scholar]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Hashimoto R, Ohi K, Fukumoto M, Takamura H, Iike N, et al. Association study of KIBRA gene with memory performance in a Japanese population. World J Biol Psychiatry. 2010;11:852–857. doi: 10.3109/15622971003797258. [DOI] [PubMed] [Google Scholar]
- Yoshihama Y, Hirai T, Ohtsuka T, Chida K. KIBRA co-localizes with protein kinase Mzeta (PKMzeta) in the mouse hippocampus. Biosci Biotechnol Biochem. 2009;73:147–151. doi: 10.1271/bbb.80564. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kranzler HR, Poling J, Gruen JR, Gelernter J. Cognitive flexibility is associated with KIBRA variant and modulated by recent tobacco use. Neuropsychopharmacology. 2009;34:2508–2516. doi: 10.1038/npp.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.