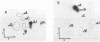Abstract
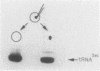
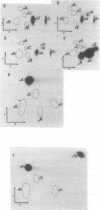
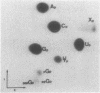
We examined the base modification pattern of Xenopus tRNA(Sec) using microinjection into Xenopus oocytes, with particular focus on the wobble base U34 at the first position of the anticodon. We found that U34 becomes modified to mcm5U34 (5-methylcarboxymethyluridine) in the oocyte cytoplasm in a rather complex manner. When the tRNA(Sec) gene is injected into Xenopus oocyte nuclei, psi 55 and m1A58 are readily obtained, but not mcm5U34. This will appear only upon cytoplasmic injection of the gene product arising from the first nuclear injection. In contrast, tRNA(Sec) produced by in vitro transcription with T7 RNA polymerase readily acquires i6A37, psi 55, m1A58, and mcm5U34. The latter is obtained after direct nuclear or cytoplasmic injections. It has been reported by others that mcm5Um, a 2'-O-methylated derivative of mcm5U34, also exists in rat and bovine tRNA(Sec). With both the gene product and the in vitro transcript, and using the sensitive RNase T2 assay, we were unable to detect under our conditions the presence of a dinucleotide carrying mcm5Um and that would be therefore refractory to hydrolysis. We showed that the unusual mcm5U acquisition pathway does not result from impairment of nucleocytoplasmic transport. Rather, these data can be interpreted to mean that the modification is performed by a tRNA(Sec) specific enzyme, limiting in the oocyte cytoplasm.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amberg R., Urban C., Reuner B., Scharff P., Pomerantz S. C., McCloskey J. A., Gross H. J. Editing does not exist for mammalian selenocysteine tRNAs. Nucleic Acids Res. 1993 Dec 11;21(24):5583–5588. doi: 10.1093/nar/21.24.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Böck A. The length of the aminoacyl-acceptor stem of the selenocysteine-specific tRNA(Sec) of Escherichia coli is the determinant for binding to elongation factors SELB or Tu. J Biol Chem. 1991 Oct 25;266(30):20375–20379. [PubMed] [Google Scholar]
- Baron C., Westhof E., Böck A., Giegé R. Solution structure of selenocysteine-inserting tRNA(Sec) from Escherichia coli. Comparison with canonical tRNA(Ser). J Mol Biol. 1993 May 20;231(2):274–292. doi: 10.1006/jmbi.1993.1282. [DOI] [PubMed] [Google Scholar]
- Böck A., Forchhammer K., Heider J., Baron C. Selenoprotein synthesis: an expansion of the genetic code. Trends Biochem Sci. 1991 Dec;16(12):463–467. doi: 10.1016/0968-0004(91)90180-4. [DOI] [PubMed] [Google Scholar]
- Carbon P., Krol A. Transcription of the Xenopus laevis selenocysteine tRNA(Ser)Sec gene: a system that combines an internal B box and upstream elements also found in U6 snRNA genes. EMBO J. 1991 Mar;10(3):599–606. doi: 10.1002/j.1460-2075.1991.tb07987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. M., Choi I. S., Crain P. F., Hashizume T., Pomerantz S. C., Cruz R., Steer C. J., Hill K. E., Burk R. F., McCloskey J. A. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA([Ser]Sec). J Biol Chem. 1993 Jul 5;268(19):14215–14223. [PubMed] [Google Scholar]
- Diamond A., Dudock B., Hatfield D. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell. 1981 Aug;25(2):497–506. doi: 10.1016/0092-8674(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Droogmans L., Haumont E., de Henau S., Grosjean H. Enzymatic 2'-O-methylation of the wobble nucleoside of eukaryotic tRNAPhe: specificity depends on structural elements outside the anticodon loop. EMBO J. 1986 May;5(5):1105–1109. doi: 10.1002/j.1460-2075.1986.tb04329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edqvist J., Grosjean H., Stråby K. B. Identity elements for N2-dimethylation of guanosine-26 in yeast tRNAs. Nucleic Acids Res. 1992 Dec 25;20(24):6575–6581. doi: 10.1093/nar/20.24.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Droogmans L., Giégé R., Uhlenbeck O. C. Guanosine modifications in runoff transcripts of synthetic transfer RNA-Phe genes microinjected into Xenopus oocytes. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):267–273. doi: 10.1016/0167-4781(90)90179-6. [DOI] [PubMed] [Google Scholar]
- Houssier C., Degée P., Nicoghosian K., Grosjean H. Effect of uridine dethiolation in the anticodon triplet of tRNA(Glu) on its association with tRNA(Phe). J Biomol Struct Dyn. 1988 Jun;5(6):1259–1266. doi: 10.1080/07391102.1988.10506468. [DOI] [PubMed] [Google Scholar]
- Lee B. J., Kang S. G., Hatfield D. Transcription of Xenopus selenocysteine tRNA Ser (formerly designated opal suppressor phosphoserine tRNA) gene is directed by multiple 5'-extragenic regulatory elements. J Biol Chem. 1989 Jun 5;264(16):9696–9702. [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Lee B. J., de la Peña P., Tobian J. A., Zasloff M., Hatfield D. Unique pathway of expression of an opal suppressor phosphoserine tRNA. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6384–6388. doi: 10.1073/pnas.84.18.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Differential accumulation of U1 and U4 small nuclear RNAs during Xenopus development. Genes Dev. 1987 Mar;1(1):39–46. doi: 10.1101/gad.1.1.39. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. In vitro synthesis of vertebrate U1 snRNA. EMBO J. 1989 Jan;8(1):287–292. doi: 10.1002/j.1460-2075.1989.tb03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslinski E., Schuster C., Huet J., Sentenac A., Krol A., Carbon P. Point mutations 5' to the tRNA selenocysteine TATA box alter RNA polymerase III transcription by affecting the binding of TBP. Nucleic Acids Res. 1993 Dec 25;21(25):5852–5858. doi: 10.1093/nar/21.25.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslinski E., Schuster C., Krol A., Carbon P. Promoter strength and structure dictate module composition in RNA polymerase III transcriptional activator elements. J Mol Biol. 1993 Nov 20;234(2):311–318. doi: 10.1006/jmbi.1993.1588. [DOI] [PubMed] [Google Scholar]
- Schön A., Böck A., Ott G., Sprinzl M., Söll D. The selenocysteine-inserting opal suppressor serine tRNA from E. coli is highly unusual in structure and modification. Nucleic Acids Res. 1989 Sep 25;17(18):7159–7165. doi: 10.1093/nar/17.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Steinberg S., Misch A., Sprinzl M. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1993 Jul 1;21(13):3011–3015. doi: 10.1093/nar/21.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler C., Westhof E., Carbon P., Krol A. Unique secondary and tertiary structural features of the eucaryotic selenocysteine tRNA(Sec). Nucleic Acids Res. 1993 Mar 11;21(5):1073–1079. doi: 10.1093/nar/21.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]