Abstract
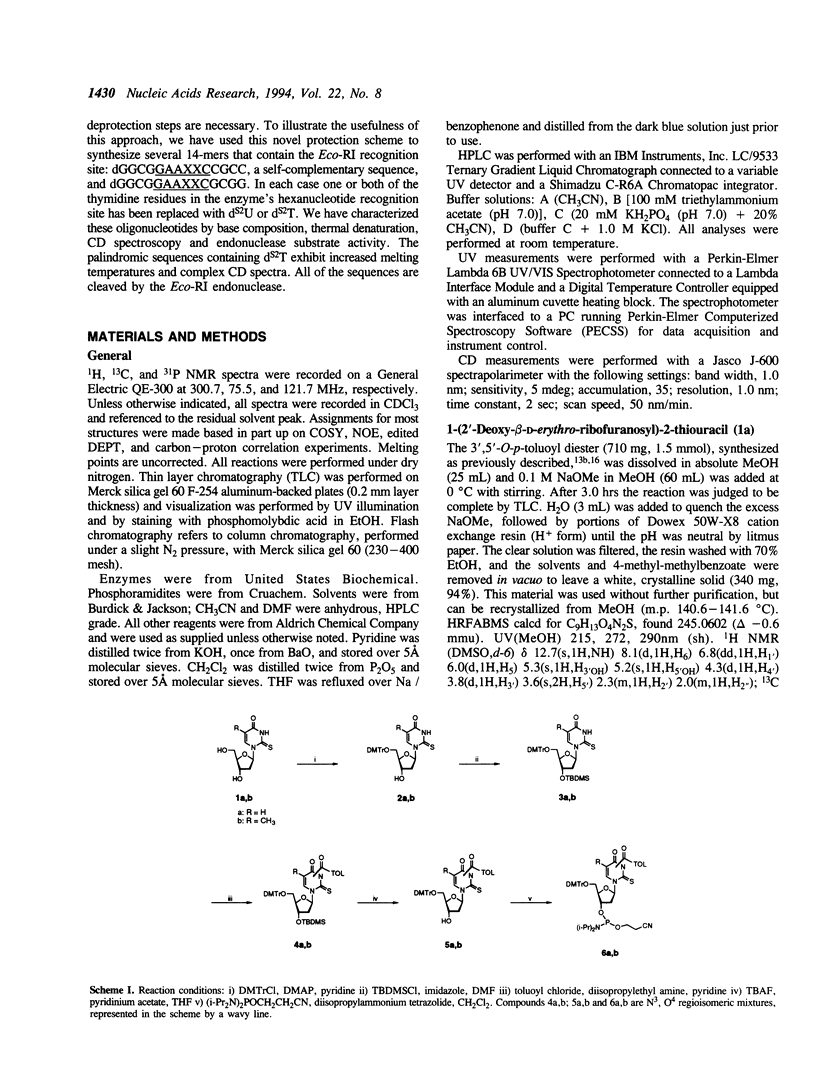
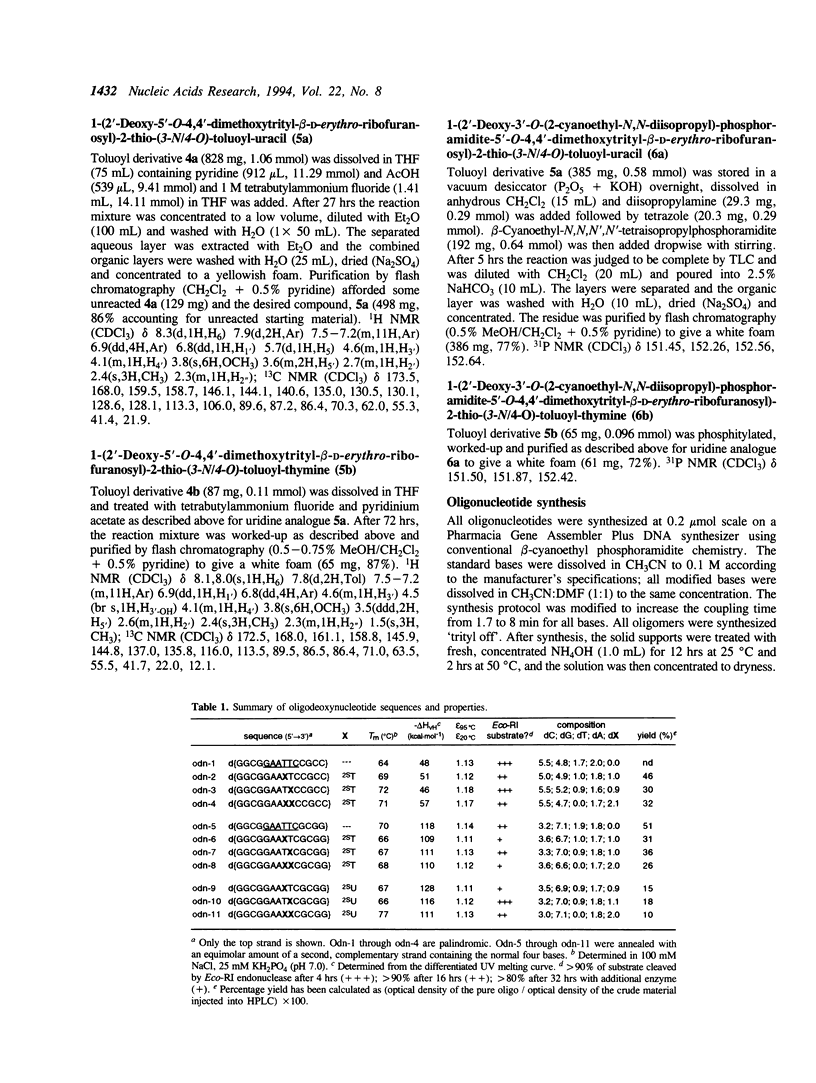
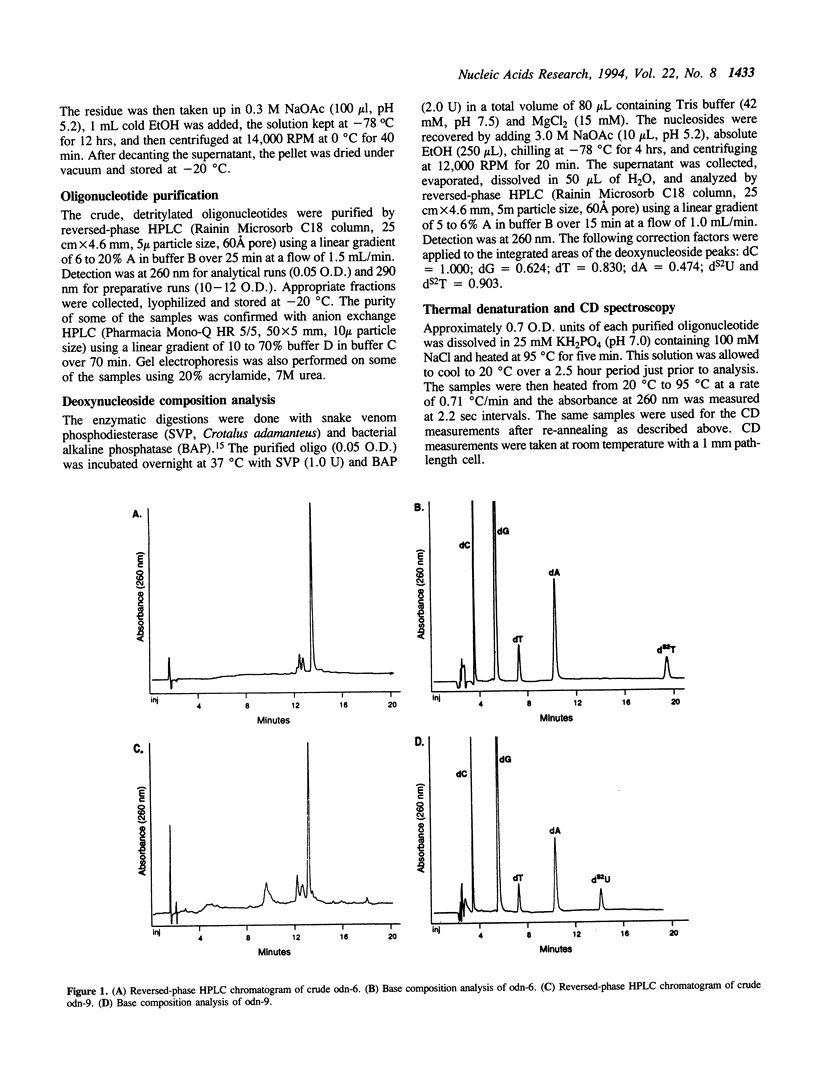
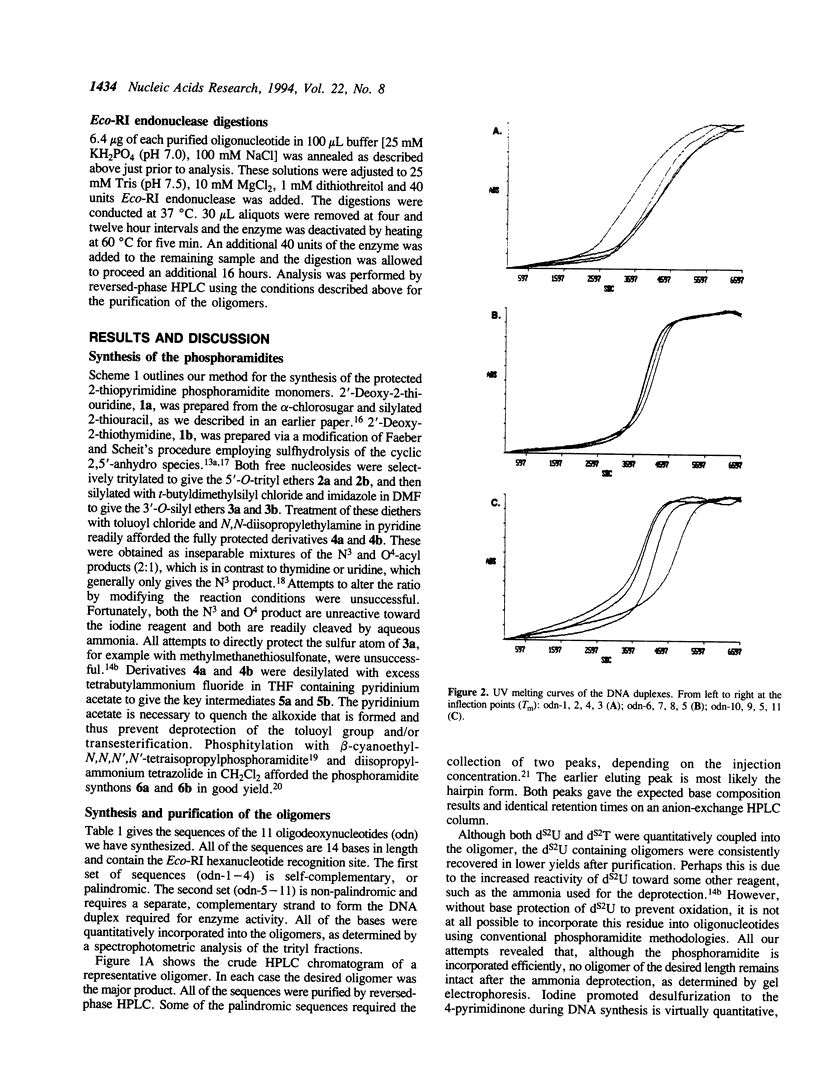
A method is described for the incorporation of 2'-deoxy-2-thiouridine (dS2U) and 2'-deoxy-2-thiothymidine (dS2T) into oligodeoxynucleotides at predetermined positions. This requires N3 or O4-acylation of dS2U and dS2T with toluoyl chloride. These base-protected thiopyrimidines are completely stable toward the aqueous iodine oxidation reagent used in the phosphoramidite DNA synthesis method. The toluoyl protecting group is removed during the standard post-synthetic ammonia treatment. This novel protection strategy allows dS2U and dS2T to be efficiently incorporated into oligodeoxynucleotides at predetermined sites without the usual problem of desulfurization and decomposition. Several 14-mers containing the Eco-RI recognition site (dGGCGGAAXXCCGCC and dGGCGGAAXXCGCGG, where X represents dT, dS2U or dS2T) have been synthesized and characterized by base composition, thermal denaturation, CD spectroscopy and endonuclease substrate activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan C. A., Van Cleve M. D., Gumport R. I. The effects of base analogue substitutions on the cleavage by the EcoRI restriction endonuclease of octadeoxyribonucleotides containing modified EcoRI recognition sequences. J Biol Chem. 1986 Jun 5;261(16):7270–7278. [PubMed] [Google Scholar]
- Brennan C. A., Van Cleve M. D., Gumport R. I. The effects of base analogue substitutions on the methylation by the EcoRI modification methylase of octadeoxyribonucleotides containing modified EcoRI recognition sequences. J Biol Chem. 1986 Jun 5;261(16):7279–7286. [PubMed] [Google Scholar]
- Dubendorff J. W., deHaseth P. L., Rosendahl M. S., Caruthers M. H. DNA functional groups required for formation of open complexes between Escherichia coli RNA polymerase and the lambda PR promoter. Identification via base analog substitutions. J Biol Chem. 1987 Jan 15;262(2):892–898. [PubMed] [Google Scholar]
- Eadie J. S., McBride L. J., Efcavitch J. W., Hoff L. B., Cathcart R. High-performance liquid chromatographic analysis of oligodeoxyribonucleotide base composition. Anal Biochem. 1987 Sep;165(2):442–447. doi: 10.1016/0003-2697(87)90294-6. [DOI] [PubMed] [Google Scholar]
- Faerber P., Scheit K. H. Die chemische Synthese von 2.4-Dithio-thymin-nucleosiden. Chem Ber. 1970;103(5):1307–1311. doi: 10.1002/cber.19701030502. [DOI] [PubMed] [Google Scholar]
- Fu D. J., McLaughlin L. W. Importance of specific adenosine N7-nitrogens for efficient cleavage by a hammerhead ribozyme. A model for magnesium binding. Biochemistry. 1992 Nov 17;31(45):10941–10949. doi: 10.1021/bi00160a001. [DOI] [PubMed] [Google Scholar]
- Fu D. J., Rajur S. B., McLaughlin L. W. Importance of specific guanosine N7-nitrogens and purine amino groups for efficient cleavage by a hammerhead ribozyme. Biochemistry. 1993 Oct 12;32(40):10629–10637. doi: 10.1021/bi00091a013. [DOI] [PubMed] [Google Scholar]
- Grasby J. A., Jonathan P., Butler G., Gait M. J. The synthesis of oligoribonucleotides containing O6-methylguanosine: the role of conserved guanosine residues in hammerhead ribozyme cleavage. Nucleic Acids Res. 1993 Sep 25;21(19):4444–4450. doi: 10.1093/nar/21.19.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Circular dichroism and its empirical application to biopolymers. Methods Biochem Anal. 1985;31:61–163. doi: 10.1002/9780470110522.ch2. [DOI] [PubMed] [Google Scholar]
- Khamis M. I., Casas-Finet J. R., Maki A. H. Stacking interactions of tryptophan residues and nucleotide bases in complexes formed between Escherichia coli single-stranded DNA binding protein and heavy atom-modified poly(uridylic) acid. A study by optically detected magnetic resonance spectroscopy. J Biol Chem. 1987 Feb 5;262(4):1725–1733. [PubMed] [Google Scholar]
- Lesser D. R., Kurpiewski M. R., Waters T., Connolly B. A., Jen-Jacobson L. Facilitated distortion of the DNA site enhances EcoRI endonuclease-DNA recognition. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7548–7552. doi: 10.1073/pnas.90.16.7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 1987 Sep;26(9):1601–1620. doi: 10.1002/bip.360260911. [DOI] [PubMed] [Google Scholar]
- Mazzarelli J. M., Rajur S. B., Iadarola P. L., McLaughlin L. W. Interactions between the trp repressor and its operator sequence as studied by base analogue substitution. Biochemistry. 1992 Jun 30;31(25):5925–5936. doi: 10.1021/bi00140a032. [DOI] [PubMed] [Google Scholar]
- McLaughlin L. W., Benseler F., Graeser E., Piel N., Scholtissek S. Effects of functional group changes in the EcoRI recognition site on the cleavage reaction catalyzed by the endonuclease. Biochemistry. 1987 Nov 17;26(23):7238–7245. doi: 10.1021/bi00397a007. [DOI] [PubMed] [Google Scholar]
- Nikiforov T. T., Connolly B. A. Oligodeoxynucleotides containing 4-thiothymidine and 6-thiodeoxyguanosine as affinity labels for the Eco RV restriction endonuclease and modification methylase. Nucleic Acids Res. 1992 Mar 25;20(6):1209–1214. doi: 10.1093/nar/20.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A., d'Ischia M., Misuraca G., Iannone A., Prota G. Selective uptake of 2-thiouracil into melanin-producing systems depends on chemical binding to enzymically generated dopaquinone. Biochim Biophys Acta. 1990 Dec 6;1036(3):221–227. doi: 10.1016/0304-4165(90)90038-x. [DOI] [PubMed] [Google Scholar]


