Abstract
Identifying autoimmune hepatitis as the etiology of acute liver failure (ALF) is potentially important, since administering corticosteroids might avoid the need for liver transplantation. However, clinical and histological criteria of autoimmune (AI)-ALF have not been defined. Liver sections (biopsies and explants) from a 72-patient subset of the ALF Study Group Registry with indeterminate ALF were reviewed by a pathologist blinded to all clinical data, and were diagnosed with probable AI-ALF based upon 4 features suggestive of an autoimmune pathogenesis: distinctive patterns of massive hepatic necrosis (present in 42% of sections), presence of lymphoid follicles (32%), a plasma cell-enriched inflammatory infiltrate (63%), and central perivenulitis (65%). Forty-two sections (58%) were considered probable AI-ALF; this group demonstrated higher serum globulins [3.7 ± 0.2 vs. 3.0 ± 0.2 g/dL; P = 0.037] and a higher prevalence of antinuclear and/or anti-smooth muscle antibodies (73 vs. 48%; P = 0.034), compared to those without histology suggestive of probable AI-ALF. Thirty patients concordant for autoantibodies and probable AI-ALF on histology were more likely to have the classical AIH phenotype (female predominance [72 vs. 48%; P < 0.05], higher globulins [3.9 ± 0.2 vs. 3.0 ± 0.2 g/dL, P < 0.005], and higher incidence of chronic hepatitis in long-term follow-up (67 vs. 17%, P = 0.019), compared to the population without concordant AI-ALF histology and autoantibodies.
Conclusions
Patients with indeterminate ALF often have features of autoimmune disease by histology, serological testing, and clinical recurrence during follow-up. In contrast to classical autoimmune hepatitis, histological features of AI-ALF predominate in the centrilobular zone.
Keywords: autoimmune hepatitis, liver transplantation, acute cellular rejection, corticosteroids, autoantibody
Although autoimmune hepatitis (AIH) typically presents as a chronic necroinflammatory liver disease, an acute presentation occurs in up to 25%, a small minority of whom progress to autoimmune acute liver failure (AI-ALF) (1). The diagnosis of AIH has historically presented a challenge for clinicians but is of critical importance, since many patients will have dramatic responses to the administration of corticosteroids (2). Diagnostic criteria for classical AIH have been standardized by consensus, and have included histological, demographic, and laboratory components, as well as the absence of data suggestive of another diagnosis (3, 4). However, these criteria were designed to differentiate AIH from other causes of chronic liver disease, rather than to address diagnostic considerations of ALF.
The etiology of ALF, the syndrome of abrupt liver injury with ensuing coagulopathy and hepatic encephalopathy, can be determined in the majority of patients on the basis of a history of drug exposure, positive acute viral serology, or other historical feature (for example, pregnancy, mushroom ingestion, malignancy, or cardiovascular instability). However, the second most common etiology of ALF after acetaminophen (APAP) overdose is unknown or indeterminate, representing approximately 20% in the U.S. ALF Study Group Registry (5). A small minority of patients with ALF of indeterminate etiology have detectable protein-APAP adducts suggesting a remote ingestion, but more than 80% remain undiagnosed even after retrospective analysis of stored specimens for occult viral infections. Some patients with indeterminate ALF may be suspected of having an autoimmune pathogenesis on the basis of positive autoantibodies and other clinical clues (for example, female gender and hyperglobulinemia). However, even in patients with demographic and laboratory evidence of AIH, the diagnosis of AI-ALF usually remains tentative since autoantibodies are non-specific, histology is usually not available, and the entity is so rare that diagnostic criteria have not been codified by consensus. The histological assessment of ALF is complicated further by the absence of a formal microscopic classification system and the often-presumed non-specificity of the changes comprising massive hepatic necrosis (MHN).
We hypothesized that many patients with ALF of indeterminate etiology have an autoimmune pathogenesis resembling AIH. Small series have suggested that the histological features of AIH presenting as an acute hepatitis include centrilobular lesions (6–12) similar to those in liver allograft specimens from patients with severe, “atypical,” acute cellular rejection (13, 14). Accordingly, we developed histological criteria based upon these observations to identify patients with a likely autoimmune pathogenesis, and compared their presenting clinical and serological characteristics to those without histological features of autoimmunity. Furthermore, since AIH can recur after liver transplantation, we hypothesized that those patients with histological features of autoimmunity would be more likely to develop chronic hepatitis in their native livers if they recovered from ALF, or to develop recurrent allograft hepatitis after liver transplantation.
Patients and Methods
Patients and follow-up
The study population was enrolled in the ALF Study Group Registry between 1998 and 2008. Entry criteria included ALF (coagulopathy [international normalized ratio (INR) ≥1.5] and hepatic encephalopathy within 26 weeks of the onset of illness, in a patient without previously recognized liver disease) (15). Study subjects were chosen from a total registry of 1100 patients on the basis of having an indeterminate evaluation for the etiology of ALF, defined as: (1) no serologic evidence of acute viral hepatitis, (2) no evidence of ischemic liver injury (acute Budd-Chiari syndrome or “shock liver,”), and (3) no evidence of drug-induced hepatotoxicity (history of APAP overdose or recent prescription drug or over-the-counter herbal exposure). Patients with suspected acute Wilson disease or liver failure related to malignancy or pregnancy were also excluded. These criteria identified a sub-population of 204 patients with ALF of indeterminate etiology, some of whom had detectable autoantibodies on admission and were thereby suspected of having AIH . However, since autoantibodies are often non-specific, they were considered non-diagnostic for the purposes of this study.
Attempts were made to recover liver samples from each of 204 patients meeting the inclusion criteria above. Records indicated that 61 subjects had neither liver biopsies nor explant pathology available. Of the 143 potential samples, tissue was recovered from 79 (55%) for histological evaluation. Adequate liver tissue for analysis was recovered in 72 cases (50% of total); specimens from 7 subjects were inadequate for diagnosis due to small size. Forty-six samples were obtained from liver explants and 26 from transjugular liver biopsies. Clinical and serological characteristics of the 204 total patients and the subset of 72 with liver tissue available were similar, suggesting that the latter was a balanced subset of the former (data not shown). Nineteen of 72 patients received corticosteroids for clinically suspected AIH. However, due to small sample size and lack of randomization, response to steroids could not be analyzed as a diagnostic marker for AI-ALF.
Patients surviving ALF, either after spontaneous recovery or liver transplantation, were requested to return twice, at 12 and ≥ 24 months after the admission for ALF. Detailed medical history including laboratories and liver biopsy results were reviewed at each visit. Follow-up liver biopsies were read locally for histological evidence of hepatitis and rejection (in transplant recipients) by study site pathologists and were not retrieved for central re-analysis.
Methods and Statistical Analysis
Histological analysis
Samples of liver were evaluated in a blinded fashion on two occasions by an experienced hepatopathologist (J.H.L.). The first review was a survey to identify features of an acute autoimmune pathogenesis, as previously described (6–12). Particular attention was paid to the centrilobular region of the lobule (8, 9, 11, 12). The second review, performed blinded to the first review, was undertaken to ensure reproducibility of the findings, and to further sub-classify the types of MHN. Concordance for finding in the first and second reviews was 100% (data not shown).
During the first review, several variants of MHN were observed, and classified MHN 1–5 in the second review (Figures 1 and 2). Three patterns (MHN 1, 2, and 3) were considered relatively non-specific. MHN1 was characterized by classical massive necrosis with near-complete loss of hepatocytes throughout the lobules, residual intrasinusoidal inflammation, periportal neocholangiolar proliferation (ductular reaction) and portal/periportal inflammation. MHN2 was characterized by submassive necrosis, representing regions of MHN1 as well as regenerative nodules and areas of early fibrosis, and was considered to represent a more subacute clinical course than MHN1. MHN 3 demonstrated necroinflammatory changes of acute hepatitis in portions of the specimen (spotty necrosis) as well as other regions with more substantial confluent necrosis, including areas of bridging hepatic necrosis or multilobular necrosis with neocholangiolar proliferation. Two patterns of MHN (types 4 and 5) were considered more characteristic of an autoimmune pathogenesis. MHN4 showed the typical features of pan-lobular necrosis, but with prominence of centrilobular necroinflammation and hemorrhage, resembling the severe form of the centrilobular variant of autoimmune hepatitis (10–12) and the centrilobular variant of acute cellular rejection observed in transplant allografts (14, 16). MHN5 showed features of classical peri-portal AIH in conjunction with superimposed changes of massive necrosis and sometimes centrilobular necroinflammation. In addition to patterns of MHN, 3 additional features were considered supportive of an autoimmune pathogenesis of liver injury: the presence of central perivenulitis (chronic inflammation, including lymphocytes and/or plasma cells, in and around central veins, often accompanied by hepatocyte drop-out/necrosis (14); Figure 3), the presence of plasma cells in the inflammatory infiltrate, and the presence of portal tract lymphoid aggregates (evidence of a chronic inflammatory process).
Figure 1. Diagrammatic depiction of five patterns of massive hepatic necrosis (MHN).
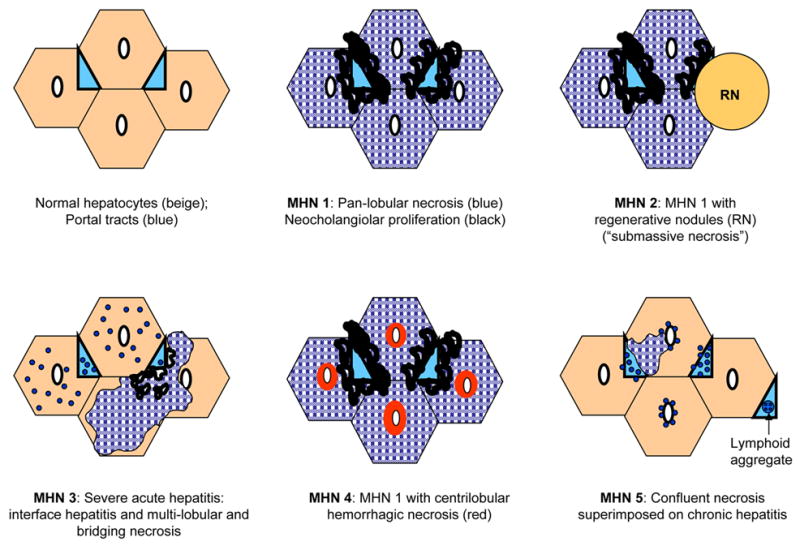
Normal hepatic architecture is depicted in the upper left diagram for comparison. Areas of hatched blue represent necrotic hepatocytes and areas in light blue represent portal tracts.
Figure 2. Histological variants of massive hepatic necrosis (MHN).
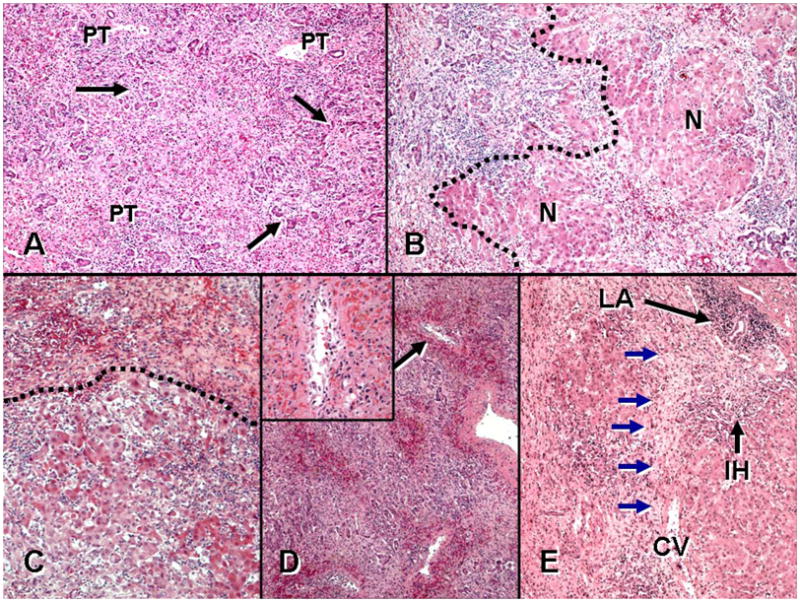
A. MHN type 1: “typical” MHN with panlobular necrosis and neocholangiolar proliferation (ductular reaction) (arrows) emerging from parenchyma near portal tracts (PT). B. MHN type 2: there is confluent necrosis in the field left of the hatched line, with early regenerative nodules (N), a pattern also referred to as ‘submassive necrosis’. C. MHN type 3: acute hepatitis with lobular disarray, inflammation and liver-cell damage is seen at bottom and is separated by the hatched line from more severe confluent necrosis above. D. MHN type 4: centrilobular hemorrhagic necrosis superimposed on the general features of massive necrosis is prominent at low power. Inset: one centrilobular region (arrow) shows lymphocytes and plasma cells around, infiltrating, and within the lumen of a central vein (‘central perivenulitis’), in association with hemorrhage and hepatocyte loss. E. MHN type 5: confluent necrosis is present superimposed on features of an underlying chronic hepatitis, including portal lymphoid aggregates (LA) and interface hepatitis (IH). Note confluent bridging hepatic necrosis (blue arrows) extending between central vein (CV) and portal tract at upper right. Multilobular necrosis is also present at the left of the field. (A-E: Hematoxylin and eosin stain, × 40. Inset to D: Hematoxylin and eosin stain, × 200.)
Figure 3. Central perivenulitits.
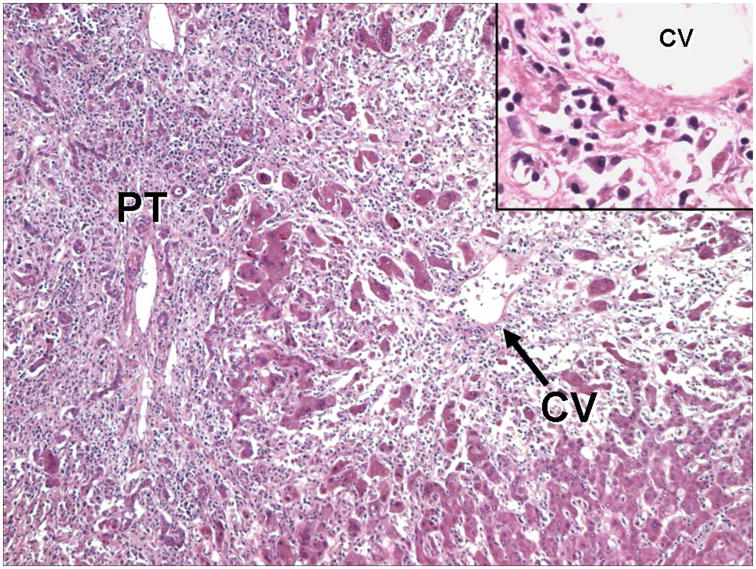
This section from an explanted liver shows severe hepatitis with extensive inflammation extending from the portal tract (PT) into the lobule, with marked loss of hepatocytes. Note the prominent hepatocyte loss and inflammation around the central vein (CV), “central perivenulitis.” Inset: Higher magnification of central perivenulitis highlighted by plasma cells surrounding and infiltrating the vein wall, associated with marked hepatocyte destruction. (Hematoxylin and eosin stain).
Simplified International Autoimmune Hepatitis Group (IAIHG) Criteria for AI-ALF
The diagnosis of AIH has traditionally relied upon a clinicopathological scoring system developed by the IAIHG (3), which was designed to differentiate AIH from other chronic liver diseases. More recently, Simplified Diagnostic Criteria (SDC) for AIH have been proposed consisting of points awarded for histology, serum globulin concentration, the absence of hepatitis viral markers, and the presence of autoantibodies (4). In the SDC for AIH, histological features are categorized as typical, compatible, and incompatible with AIH and awarded 2, 1, or 0 points, respectively. For purposes of validating our proposed histological features of AI-ALF using the SDC index, we assigned a similar point system, using the overall histological impression after the 2 reviews: 2 points were awarded for probable AI-ALF, 1 point for compatible with AI-ALF but with fewer autoimmune features, and 0 points for incompatible with AI-ALF. Patients were also given scores of up to 2 points each for autoantibodies and globulin concentration (upper limit of normal taken to be 3.5 g/dl); all patients were automatically awarded 2 points since, by inclusion criteria of the study, all had negative viral serologies. Therefore, the simplified IAIHG criteria modified for AI-ALF ranged from 2–8 points. By the SDC for AIH, ≥6 points is considered suggestive of, and ≥ 7 points considered diagnostic of, AIH.
Autoimmune serologies
Anti-nuclear (ANA) and anti-smooth muscle antibodies (ASMA) were obtained at the time of study admission from the laboratories of ALF Study Group sites in 168 (82%) and 144 (71%) of the 204 patients, respectively. A positive ANA and/or ASMA from local laboratories was defined as detectable at a serial dilution of ≥ 1:40 titer; titers < 1:40 were defined as negative. Stored sera from those patients without ANA/ASMA available from local laboratories were analyzed at a later date in a single laboratory, such that overall, ANA and ASMA results were available from 94 and 93% of subjects, respectively. A battery of additional autoimmune serologies were also determined for 109 (53%) patients by ELISA (QUANTA liteTM, INOVA Diagnostics, Inc.) and bead-based (QUANTA PlexTM , INOVA Diagnostics, Inc.) assays. For the 72 patients with liver tissue available for review, 61 (85%) had sera retrospectively re-evaluated for autoimmune serologies. Assays were performed using recombinant and native autoantigens as described (17).
Statistical analysis
Data are expressed as mean ± SEM. Dichotomous variables were analyzed for significance by Chi square test or two-tailed Fisher’s Exact Test, as appropriate, and continuous variables by ANOVA. Statistical analysis was performed with JMP Version 8.0. A P value of ≤0.05 was considered significant.
Results
Clinical description of study population and prevalence of histological features of autoimmunity
Demographic and clinical characteristics of the 72-patient study population are shown in Table 1. Patients were predominantly young (mean age 41 years), female (58%), Caucasian (67%), and overweight (mean BMI 30 kg/m2). Admission laboratory data reflected severe hepatic dysfunction and frequent renal dysfunction, with mean INR 3.4 ± 0.2, bilirubin 24.7 ± 1.3 mg/dL, and creatinine 1.8 ± 0.3 mg/dL. Renal insufficiency often became more severe after admission, with a mean peak creatinine of 2.5 ± 0.2 mg/dL. Sixty-three percent of patients had anti-nuclear (ANA) and/or anti-smooth muscle antibodies (ASMA), 8% anti-tissue transglutaminase (tTG), 3% anti-liver/kidney microsome (LKM) or anti-soluble liver antigen (SLA) antibodies, and 15% anti-mitochondrial antibodies (AMA). The overall survival of the population was 71%, but 60% required liver transplantation; only 15% survived without transplantation.
Table 1. Demographic and clinical characteristics, and outcomes, of study population (N = 72).
Unless specified, laboratory data refer to admission values. Spontaneous survival refers to transplant-free survival. Outcomes refer to cumulative rates 21 days from admission.
| Clinical Parameter | N (%) | |
|---|---|---|
| Age (years) | 41.0 ± 1.7 | |
| Female Gender | 42 (58) | |
| Caucasian Race | 48 (67) | |
| Body Mass Index (kg/m2) | 30.0 ± 1.1 | |
| Jaundice-encephalopathy (days) | 17 ± 2 | |
| Hepatic encephalopathy grade 3/4 | 26 (39) | |
| Creatinine (mg/dl) | Admission | 1.8 ± 0.2 |
| Peak | 2.5 ± 0.2 | |
| INR | 3.2 ± 0.2 | |
| ALT (IU/L) | 1134 ± 137 | |
| AST (IU/L) | 1231 ± 197 | |
| Alkaline phosphatase (IU/L) | 205 ± 15 | |
| Total bilirubin (mg/dl) | 24.7 ± 1.3 | |
| Globulins (g/dl) | 3.4 ± 0.2 | |
| ANA | 35 (50) | |
| ASMA | 24 (34) | |
| ANA ± ASMA | 44 (63) | |
| Anti-LKM or anti-SLA | 2 (3) | |
| Anti-tTG | 5 (8) | |
| AMA | 10 (15) | |
| Spontaneous survival | 11 (15) | |
| Liver transplantation | 43 (60) | |
| Overall survival | 51 (71) | |
The prevalence of the four proposed histological features of autoimmunity, and the concurrence of these features in the same liver specimen, is depicted in Table 2. The most common feature of autoimmunity was central perivenulitis (65%), followed by plasma cell enrichment (63%), an autoimmune-type of MHN (type 4 or 5; 42%), and lymphoid aggregates (32%). Concurrence of autoimmune features was frequent, with 2 features noted in 15 (21%), 3 features in 19 (26%), and all 4 features in 14 (19%) sections. No features of autoimmunity were observed in 21 (29%) sections. The presence of an autoimmune type of MHN (4 or 5), lymphoid aggregates, and plasma cell enrichment of inflammation was highly predictive of the concurrence of central perivenulitis (in 93%, 87%, and 100%, respectively).
Table 2.
Prevalence and concurrence of proposed histological features of autoimmunity in 72 liver specimens from patients with ALF.
| Histological Feature of AI-ALF | Type 4 or 5 Massive Hepatic Necrosis | Lymphoid Aggregates | Central Perivenulitis | Plasma Cell Enrichment | ||||
|---|---|---|---|---|---|---|---|---|
| Present N = 30 | Absent N = 42 | Present N = 23 | Absent N = 49 | Present N = 47 | Absent N = 25 | Present N = 45 | Absent N = 27 | |
| Type 4 or 5 MHN (%) | -- | -- | 65** | 31 | 60† | 8 | 60† | 11 |
| Lymphoid Aggregates (%) | 50** | 19 | -- | -- | 43** | 12 | 42* | 15 |
| Central Perivenulitis (%) | 93† | 45 | 87** | 55 | -- | -- | 100† | 7 |
| Plasma Cell Enrichment (%) | 90† | 43 | 83* | 53 | 96† | 0 | -- | -- |
| Histological Diagnosis AI-ALF (%) | 93† | 33 | 83** | 47 | 87† | 4 | 87† | 11 |
P≤0.05,
P < 0.01,
P < 0.001,
P < 0.0001 vs. “absent” group
Correlation of proposed histological features of AI-ALF with clinical features of AIH and ALF
As evidence that the 4 proposed histological features of AI-ALF represented an autoimmune etiology, we compared the individual features of autoimmunity with well-recognized clinical and laboratory features of AIH and with specific features of ALF known to vary by etiology (Table 3). Individually, histological features of AI-ALF except for the type of MHN were more frequently observed with certain clinical markers of AIH. The presence of lymphoid aggregates was associated with lower alkaline phosphatase (156 ± 25 vs. 229 ± 18 IU/L, respectively; P = 0.02) and admission bilirubin (20.2 ± 2.3 vs. 26.9 ± 1.6 mg/dl, respectively; P = 0.02), compared to biopsies without lymphoid aggregates. Lower alkaline phosphatase is a criterion favoring AIH according to the IAIHG (3). The presence of central perivenulitis or plasma cell enrichment of inflammation was noted in patients with a more chronic clinical course (longer jaundice-to-encephalopathy interval [JEI]) than in patients without these features (20 ± 3 vs. 11 ± 4 days, respectively; P = 0.032 and 21 ± 3 vs. 10 ± 3 days, respectively; P = 0.015), also a feature of AIH. Perhaps as a result of this more prolonged course, overall survival was significantly higher in patients with central perivenulitis and plasma cell enrichment than in those without these features (83 vs. 48%, respectively; P = 0.003 and 82 vs. 52%, respectively; P = 0.008) due to an increased rate of liver transplantation (72 vs. 36%, respectively; P = 0.005 and 72 vs. 37%, respectively; P = 0.003). There was no difference in transplant-free survival in the presence or absence of any histological feature, although the number of spontaneous survivors was small (N = 11; data not shown). Although all 4 proposed histological features of AI-ALF were more frequently observed in patients with classical features of AIH (female gender, presence of ANA ± ASMA, and higher serum globulins), none reached statistical significance.
Table 3.
Proposed histological features of AI-ALF vs. clinical and laboratory characteristics of autoimmune hepatitis and acute liver failure. Overall survival and liver transplantation refers to cumulative rates at 21 days from admission.
| Clinical Characteristic | Histological Feature of Autoimmune Acute Liver Failure | |||||||
|---|---|---|---|---|---|---|---|---|
| Type 4 or 5 Massive Hepatic Necrosis | Lymphoid Aggregates | Central Perivenulitis | Plasma Cell Enrichment | |||||
| Present N = 30 | Absent N = 42 | Present N = 23 | Absent N = 49 | Present N = 47 | Absent N = 25 | Present N = 45 | Absent N = 27 | |
| Female Gender (%) | 60 | 57 | 65 | 55 | 60 | 56 | 60 | 56 |
| Jaundice-to-coma (days) | 20 ± 3 | 14 ± 3 | 18 ± 4 | 16 ± 3 | 20 ± 3* | 11 ± 4 | 21 ± 3* | 10 ± 3 |
| Admission INR | 3.1 ± 0.4 | 3.3 ± 0.3 | 3.3 ± 0.4 | 3.2 ± 0.3 | 3.4 ± 0.3 | 2.9 ± 0.4 | 3.4 ± 0.3 | 2.9 ± 0.4 |
| Peak Creatinine (mg/dl) | 2.3 ± 0.4 | 2.7 ± 0.3 | 2.3 ± 0.4 | 2.7 ± 0.3 | 2.2 ± 0.3 | 3.1 ± 0.4 | 2.2 ± 0.3 | 3.1 ± 0.4 |
| Admission Bilirubin (mg/dl) | 23.9 ± 2.1 | 25.3 ± 1.8 | 20.2 ± 2.3* | 26.9 ± 1.6 | 24.8 ± 1.7 | 24.7 ± 2.3 | 24.3 ± 1.7 | 25.4 ± 2.2 |
| Admission ALT (IU/L) | 1075 ± 213 | 1193 ± 180 | 915 ± 241 | 1252 ± 165 | 991 ± 167 | 1432 ± 229 | 984 ± 171 | 1411 ± 221 |
| Admission Alkaline Phosphatase (IU/L) | 215 ± 23 | 198 ± 20 | 156 ± 25* | 229 ± 18 | 200 ± 18 | 215 ± 26 | 193 ± 19 | 225 ± 25 |
| Admission Globulins (g/dL) | 3.7 ± 0.2 | 3.2 ± 0.2 | 3.7 ± 0.3 | 3.2 ± 0.2 | 3.5 ± 0.2 | 3.2 ± 0.3 | 3.6 ± 0.2 | 3.1 ± 0.3 |
| ANA ± ASMA (%) | 72 | 56 | 77 | 56 | 70 | 50 | 68 | 54 |
| Overall Survival (%) | 80 | 64 | 74 | 69 | 83** | 48 | 82** | 52 |
| Liver Transplantation (%) | 60 | 59 | 57 | 61 | 72** | 36 | 72** | 37 |
| Hepatitis in Follow- Up (%) | 47 | 42 | 42 | 47 | 48 | 25 | 50 | 20 |
P ≤ 0.05,
P < 0.01,
P < 0.001,
P < 0.0001 vs. “absent” group.
Correlation of clinico-pathological schemes of diagnosing AI-ALF with classical features of AIH and clinical features of ALF
Although individual histological features of AI-ALF were weakly associated with clinical features of autoimmunity, an overall histological diagnosis of probable AI-ALF conferred a discriminating clinical phenotype of autoimmunity as compared to those without probable AI-ALF (Table 4). Patients with a histological diagnosis of probable AI-ALF had a more subclinical course (JEI of 21 ± 3 vs. 11 ± 3 days; P = 0.024), milder degree of renal failure (peak creatinine 2.1 ± 0.3 vs. 3.2 ± 0.4 mg/dl; P = 0.025), lower admission ALT (921 ± 125 vs. 1456 ± 207 IU/L; P = 0.053), higher serum globulins (3.7 ± 0.2 vs. 3.0 ± 0.2 g/dL; P = 0.037), higher prevalence of ANA ± ASMA (73 vs. 48%; P = 0.034), and higher 21-day survival (86 vs. 50%; P = 0.002). Since the diagnosis of AIH also relies on laboratory markers of autoimmunity, we also examined the ability of ANA ± ASMA and serum globulins to improve the identification of an autoimmune phenotype beyond histology alone (Table 4). The addition of ANA ± ASMA to the histological diagnosis of probable AI-ALF better identified a population with a classical AIH phenotype, in that patients were also predominantly female (72 vs. 48%; P < 0.05), had higher serum globulins (3.9 ± 0.2 vs. 3.0 ± 0.2 g/dL, respectively; P < 0.005), and a higher incidence of hepatitis in long-term follow-up (67 vs. 17%, respectively; P = 0.019) compared to those without concordant histology for probable AI-ALF and the presence of autoantibodies. Similarly, the subgroup of patients with higher SDC for AIH scores (≥6), which takes into account histology, globulins, and autoantibodies, also more closely resembled patients with classical AIH, although the incidence of hepatitis in follow-up was not statistically different from patients with low SDC scores (< 6).
Table 4. Diagnostic schemes for AI-ALF vs. clinical and laboratory characteristics of autoimmune hepatitis and acute liver failure.
Overall survival refers to cumulative rates at 21 days from admission. Simplified Diagnostic Criteria for AIH pertain to a composite score based upon histology, presence of autoantibodies, serum globulin concentrations, and the absence of hepatitis viral markers, as described by Hennes, et al (4).
| Clinical Characteristic | Histological Diagnosis Probable AI-ALF | Histological Diagnosis Probable AI-ALF and ANA ± ASMA | Simplified Diagnostic Criteria for AIH ≥ 6 Points | |||
|---|---|---|---|---|---|---|
| Present N = 42 | Absent N = 30 | Present N = 30 | Absent N = 42 | Present N = 29 | Absent N = 43 | |
| Female Gender (%) | 62 | 53 | 73* | 48 | 69 | 51 |
| Jaundice-to-coma (days) | 22 ± 3* | 11 ± 3 | 21 ± 3 | 14 ± 3 | 22 ± 3* | 13 ± 3 |
| Admission INR | 3.1 ± 0.3 | 3.4 ± 0.4 | 2.9 ± 0.4 | 3.5 ± 0.3 | 3.1 ± 0.4 | 3.3 ± 0.3 |
| Peak Creatinine (mg/dl) | 2.1 ± 0.3* | 3.2 ± 0.4 | 2.2 ± 0.3 | 2.7 ± 0.3 | 2.2 ± 0.4 | 2.7 ± 0.3 |
| Admission Bilirubin (mg/dl) | 25.4 ± 1.8 | 27.8 ± 2.1 | 24.1 ± 2.1 | 24.7 ± 2.8 | 25.3 ± 2.1 | 24.3 ± 2.7 |
| ALT on Admission (IU/L) | 921 ± 175* | 1456 ± 207 | 851 ± 210* | 1393 ± 181 | 936 ± 214 | 1284 ± 176 |
| Admission Alkaline Phosphatase (IU/L) | 192 ± 19 | 224 ± 23 | 190 ± 23 | 221 ± 20 | 196 ± 24 | 211 ± 20 |
| Admission Globulins (g/dL) | 3.7 ± 0.2* | 3.0 ± 0.2 | 3.9 ± 0.2** | 3.0 ± 0.2 | 4.3 ± 0.2† | 2.7 ± 0.2 |
| ANA ± ASMA (%) | 73* | 48 | 100† | 35 | 93† | 42 |
| Overall Survival (%) | 86** | 50 | 87* | 60 | 86* | 61 |
| Hepatitis in Follow-Up (%) | 52 | 17 | 67** | 17 | 60 | 25 |
P ≤ 0.05,
P < 0.01,
P < 0.0001 vs. “absent” group.
Specificity of AI-ALF Histology Index in Patients with Defined Etiologies
Since liver biopsies are less likely to be performed in patients with ALF of defined etiology and OLT is infrequently performed for APAP-induced ALF, only a limited number of tissue samples were available to test the specificity of the 4 proposed features of AI-ALF against other etiologies. Specimens from 16 subjects, 9 with APAP-induced ALF, 5 with hepatitis B virus (HBV)-, and 2 with hepatitis A virus (HAV)-induced ALF were assessed in a blinded fashion as described above. All 5 of the sections from patients with HBV-ALF were characterized by central perivenulitis typically with lymphoid aggregates. In contrast, 7 of 9 of specimens with APAP-ALF and both of those from HAV-ALF were deemed not compatible with AI-ALF. Sections from 2 other patients with APAP-ALF showed plasma cell-predominant inflammation and central perivenulitis, 3 had MHN4, and 1 had lymphoid aggregates (data not shown).
Discussion
The identification of a potentially reversible etiology of ALF, such as AI-ALF, is a primary goal in management. However, the absence of a formal classification system based upon morphology remains a major obstacle. A broad range of terms has been used to describe the MHN of ALF, including “map-like” (18), “zonal,” or “panlobular” (19), changes interpreted as non-specific. Therefore, this study focused on characterizing specific patterns of MHN as well as other specific histological features which favor an autoimmune pathogenesis.
In contrast to classical AIH, there are no consensus guidelines to distinguish AI-ALF from other etiologies of ALF. Moreover, adequate numbers of patients with ALF and liver histology are not available to prospectively test our observations, even within a large research consortium devoted to the study of ALF. Consequently, we analyzed our observations in terms of their ability to identify a classical autoimmune phenotype assuming the phenotype for patients with AIH is similar to patients with AI-ALF. We found that the 4 histological features proposed to represent AI-ALF are common in patients with ALF of indeterminate etiology, and that the features usually occur concurrently in the same liver specimen (Table 2). Although certain histological features of autoimmunity are associated with clinical features suggestive of AIH (Table 3), an overall histological impression of AI-ALF is associated with a decidedly autoimmune phenotype (subacute clinical course, higher globulins, higher prevalence of autoantibodies; Table 4). Furthermore, the addition of ANA±ASMA to a histological diagnosis of probable AI-ALF appears to strengthen this autoimmune phenotype to include a predominantly female population with a higher incidence of hepatitis in long-term follow-up (Table 4). The SDC for AIH, which identified 24% of patients with non-acetaminophen ALF as having possible or probable AIH in a recent study (20), did not appear to improve the identification of patients with an autoimmune phenotype over concordance for final histological diagnosis of AI-ALF and the presence of ANA±ASMA.
Classical histological features of non-fulminant AIH include a portal tract-based necroinflammatory process with interface hepatitis, often with lobular (zone 2 and 3) involvement (1, 21, 22); centrilobular predominance is distinctly unusual. A centrilobular variant of AIH first proposed by Pratt, et al. (6) was followed by several other case reports and small series (7–12), suggesting that the centrilobular variant represents an early, severe or acute presentation of AIH, which may either evolve into the classical portal-based hepatitis or remain centrilobular (11, 12). In the largest series to date, 20 of 114 (18%) of liver biopsies from classical AIH patients (none with ALF) had predominantly centrilobular necroinflammation, 4 of whom had exclusive centrilobular disease (11). Patients with the centrilobular variant more often presented as an acute hepatitis, had higher hepatic activity indices, and had less fibrosis than did the classical portal-based variants; centrilobular hemorrhage resembling hepatic venous outflow obstruction (MHN4 in the present work) has also been noted (12). Although these reports described patients with acute AIH without ALF, a few subsequent cases of ALF considered likely autoimmune feature central perivenulitis as the histological hallmark of severe, immune-mediated liver injury (8, 9).
The individual histological features of autoimmunity are not entirely specific to AI-ALF. Although the 16 liver specimens from patients with “defined” etiology exhibited fewer features of autoimmunity, those from all 5 patients with HBV-ALF and 2/9 from APAP-ALF were characterized by at least some autoimmune features. Several explanations are plausible, including more than one etiology, misdiagnosis, similar immunopathogenesis, or evolution from an early metabolite-mediated necrosis to a lymphocyte-plasma cell mediated injury following exposure of autoantigens. A similar immunopathogenesis between AI-ALF and HBV-induced ALF seems likely, as overwhelming viral infections are known to activate B-lymphocytes to differentiate into plasma cells secreting IgM and IgG against the hepatitis B core antigen (23, 24). Moreover, despite the classical description of APAP-induced hepatotoxicity as bland centrilobular necrosis, the innate immune system also participates in liver injury (25). It should be emphasized that the aim of the current study was to identify AI-ALF among subjects with indeterminate etiology, and no single test, including liver histology, is capable of cinching the diagnosis; AI-ALF remains a diagnosis based on exclusion of viral and drug etiologies first, but also requires histological and serological evaluation. Although liver biopsies are not performed routinely in ALF due to bleeding risk, our observations suggest that the information provided by histology may be worth the risk in indeterminate cases.
It remains unclear whether the centrilobular variant of AIH represents the same or a different disease as the classical portal-based variant. Perivenulitis and centrilobular necrosis are also features of atypical liver allograft rejection, which appears to be distinct from classical, portal-based rejection in that it resists immunosuppression and may presage chronic rejection (13). Liver allografts with centrilobular acute rejection can also evolve into a state of veno-occlusive congestion similar to the hemorrhagic MHN4 lesion noted in many patients herein with AI-ALF (13, 26, 27). These histological and clinical features are distinct from classical descriptions of acute allograft rejection (28). Hepatotoxicity due to certain drugs can also target the centrilobular region (29); halothane hepatitis, the best defined, results from metabolic idiosyncrasy and autoantibody formation toward antigens located within pericentral hepatocytes (30). Therefore, it seems plausible that specific antigens, whether environmental, drug-derived, or expressed on non-self hepatocytes, may trigger an immune-mediated injury to the centrilobular region by targeting antigens preferentially expressed by zone 3 hepatocytes.
The fact that no true gold standard exists for the diagnosis of AIH represents a limitation of our observations. Accordingly, we reasoned that patients with bona fide AI-ALF would more often develop chronic hepatitis in their native livers (in spontaneous survivors) or allografts (in transplant recipients) than those with indeterminate ALF. After 1-4 years follow-up, we found a high (44%) incidence of hepatitis in the study population, and those with histologically proven hepatitis were more frequently those with positive ANA ± ASMA who were given a final histological diagnosis of probable AI-ALF. These data seem further supported by the fact that none of these markers of autoimmunity before transplant were associated with allograft rejection (data not shown).
The clinical relevance of non-organ specific autoantibodies in ALF remains uncertain. A recent screen of ANA, ASMA, AMA, and LKM autoantibodies in patients with ALF revealed a prevalence of 25% in patients with non-acetaminophen drug reactions and hepatotrophic viral infections, but in none of patients with acetaminophen-induced ALF (31), suggesting that their presence may non-specifically accompany overwhelming immune activation. Our observations suggest that the presence of autoantibodies correlate with histological diagnosis of AI-ALF. Specifically, patients with AI-ALF more frequently had ANA and/or ASMA, and anti-LKM, anti-SLA, and anti-tTG were exclusively detected in patients with histological AI-ALF (data not shown). Moreover, the addition of ANA ± ASMA to a histological diagnosis of AI-ALF appeared to improve the detection of histology alone to identify an autoimmune phenotype.
In conclusion, we propose that 4 histological features of autoimmune liver disease can be interpreted as probable AI-ALF. Patients with probable AI-ALF on histology have a distinctly autoimmune clinical phenotype, and the presence of ANA and/or ASMA may improve the distinction of AI-ALF from other cases of indeterminate ALF. Similar to aggressive, refractory cases of acute cellular allograft rejection, centrilobular necroinflammatory features appear to be a hallmark of AI-ALF.
Acknowledgments
Financial Support: This work was supported by NIDDK grant # U-01 58369, William M. Lee, Principal Investigator, and #DK29588, M.E. Gershwin, P.I.
List of Abbreviations
- AI-ALF
autoimmune acute liver failure
- AIH
autoimmune hepatitis
- ALT
alanine aminotransferase
- AMA
anti-mitochondrial antibodies
- ANA
anti-nuclear antibodies
- Anti-LKM
anti-liver/kidney microsome
- Anti-SLA
anti-soluble liver antigen
- Anti-tTG
anti-tissue transglutaminase
- APAP
acetaminophen
- ASMA
anti-smooth muscle antibodies
- AST
aspartate aminotransferase
- HBV
hepatitis B virus
- IAIHG
International Autoimmune Hepatitis Group
- MHN
massive hepatic necrosis
- OLT
orthotopic liver transplantation
- SDC
simplified diagnostic criteria for AIH
Reference List
- 1.Manns MP, Strassburg CP. Autoimmune hepatitis: clinical challenges. Gastroenterology. 2001;120:1502–1517. doi: 10.1053/gast.2001.24227. [DOI] [PubMed] [Google Scholar]
- 2.Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnick GL, Elveback IR, et al. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820–833. [PubMed] [Google Scholar]
- 3.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 4.Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 5.Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28:142–152. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- 6.Pratt DS, Fawaz KA, Rabson A, Dellelis R, Kaplan MM. A novel histological lesion in glucocorticoid-responsive chronic hepatitis. Gastroenterology. 1997;113:664–668. doi: 10.1053/gast.1997.v113.pm9247489. [DOI] [PubMed] [Google Scholar]
- 7.Te HS, Koukoulis G, Ganger DR. Autoimmune hepatitis: a histological variant associated with prominent centrilobular necrosis. Gut. 1997;41:269–271. doi: 10.1136/gut.41.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abe M, Onji M, Kawai-Ninomiya K, Michitaka K, Matsuura B, Hiasa Y, et al. Clinicopathologic features of the severe form of acute type 1 autoimmune hepatitis. Clin Gastroenterol Hepatol. 2007;5:255–258. doi: 10.1016/j.cgh.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Kessler WR, Cummings OW, Eckert G, Chalasani N, Lumeng L, Kwo PY. Fulminant hepatic failure as the initial presentation of acute autoimmune hepatitis. Clin Gastroenterol Hepatol. 2004;2:625–631. doi: 10.1016/s1542-3565(04)00246-0. [DOI] [PubMed] [Google Scholar]
- 10.Singh R, Nair S, Farr G, Mason A, Perrillo R. Acute autoimmune hepatitis presenting with centrizonal liver disease: case report and review of the literature. Am J Gastroenterol. 2002;97:2670–2673. doi: 10.1111/j.1572-0241.2002.06052.x. [DOI] [PubMed] [Google Scholar]
- 11.Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol. 2006;59:246–249. doi: 10.1136/jcp.2005.029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misdraji J, Thiim M, Graeme-Cook FM. Autoimmune hepatitis with centrilobular necrosis. Am J Surg Pathol. 2004;28:471–478. doi: 10.1097/00000478-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Turlin B, Slapak GI, Hayllar KM, Heaton N, Williams R, Portmann B. Centrilobular necrosis after orthotopic liver transplantation: a longitudinal clinicopathologic study in 71 patients. Liver Transpl Surg. 1995;1:285–289. doi: 10.1002/lt.500010503. [DOI] [PubMed] [Google Scholar]
- 14.Hubscher SG. Central perivenulitis: a common and potentially important finding in late posttransplant liver biopsies. Liver Transpl. 2008;14:596–600. doi: 10.1002/lt.21451. [DOI] [PubMed] [Google Scholar]
- 15.Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–298. [PubMed] [Google Scholar]
- 16.Abraham SC, Freese DK, Ishitani MB, Krasinskas AM, Wu TT. Significance of central perivenulitis in pediatric liver transplantation. Am J Surg Pathol. 2008;32:1479–1488. doi: 10.1097/PAS.0b013e31817a8e96. [DOI] [PubMed] [Google Scholar]
- 17.Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, Kikuchi K, et al. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology. 2007;46:1436–1442. doi: 10.1002/hep.21828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaglia A, Portmann BC, Knisely AS, Srinivasan P, Muiesan P, Wendon J, et al. Auxiliary transplantation for acute liver failure: Histopathological study of native liver regeneration. Liver Transpl. 2008;14:1437–1448. doi: 10.1002/lt.21568. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch R, Yap J, Roberts EA, Cutz E. Clinicopathologic spectrum of massive and submassive hepatic necrosis in infants and children. Hum Pathol. 2009;40:516–526. doi: 10.1016/j.humpath.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Yeoman AD, Westbrook RH, Al-Chalabi T, Carey I, Heaton ND, Portmann BC, et al. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology. 2009;50:538–545. doi: 10.1002/hep.23042. [DOI] [PubMed] [Google Scholar]
- 21.Burgart LJ, Batts KP, Ludwig J, Nikias GA, Czaja AJ. Recent-onset autoimmune hepatitis. Biopsy findings and clinical correlations. Am J Surg Pathol. 1995;19:699–708. doi: 10.1097/00000478-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Fink K, Manjarrez-Orduno N, Schildknecht A, Weber J, Senn BM, Zinkernagel RM, et al. B cell activation state-governed formation of germinal centers following viral infection. J Immunol. 2007;179:5877–5885. doi: 10.4049/jimmunol.179.9.5877. [DOI] [PubMed] [Google Scholar]
- 24.Farci P, Diaz G, Chen Z, Govindarajan S, Tice A, Agulto L, et al. B cell gene signature with massive intrahepatic production of antibodies to hepatitis B core antigen in hepatitis B virus –associated acute liver failure. Proc Natl Acad Sci. 2010;107:8766–8771. doi: 10.1073/pnas.1003854107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 26.Nakazawa Y, Jonsson JR, Walker NI, Kerlin P, Steadman C, Lynch SV, et al. Fibrous obliterative lesions of veins contribute to progressive fibrosis in chronic liver allograft rejection. Hepatology. 2000;32:1240–1247. doi: 10.1053/jhep.2000.20350. [DOI] [PubMed] [Google Scholar]
- 27.Sebagh M, Debette M, Samuel D, Emile JF, Falissard B, Cailliez V, et al. "Silent" presentation of veno-occlusive disease after liver transplantation as part of the process of cellular rejection with endothelial predilection. Hepatology. 1999;30:1144–1150. doi: 10.1002/hep.510300514. [DOI] [PubMed] [Google Scholar]
- 28.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 29.Demetris AJ. Central venulitis in liver allografts: considerations of differential diagnosis. Hepatology. 2001;33:1329–1330. doi: 10.1053/jhep.2001.24315. [DOI] [PubMed] [Google Scholar]
- 30.Smith GC, Kenna JG, Harrison DJ, Tew D, Wolf CR. Autoantibodies to hepatic microsomal carboxylesterase in halothane hepatitis. Lancet. 1993;342:963–964. doi: 10.1016/0140-6736(93)92005-e. [DOI] [PubMed] [Google Scholar]
- 31.Bernal W, Ma Y, Smith HM, Portmann B, Wendon J, Vergani D. The significance of autoantibodies and immunoglobulins in acute liver failure: a cohort study. J Hepatol. 2007;47:664–670. doi: 10.1016/j.jhep.2007.05.011. [DOI] [PubMed] [Google Scholar]


