Abstract
Objective
to evaluate evidence of placental hemorrhage (PH) obtained through maternal interviews, patient charts, and placental pathology examinations as potential indicators of a “bleeding pathway” to preterm delivery (PTD).
Design
Prospective cohort
Setting
Fifty-two clinics in five communities in Michigan, USA (1998–2004)
Population
A subset (N=996) of cohort participants with complete placental pathology data
Methods
First trimester bleeding and placental abruption were ascertained by mid-trimester interviews and chart review, respectively. Disc-impacting blood clot was defined as a gross placental examination finding of a blood clot impacting adjacent tissue. Microscopic hemorrhage was defined as “high” (top quintile) scores on an aggregate measure of placental pathology findings suggestive of atypical maternal vessel hemorrhage. These four PH indicators were compared with one another and with risk of PTD assessed by logistic regression analyses.
Main Outcome Measures
PTD and PTD subtypes (i.e., <35 weeks, 35–36 weeks; spontaneous, medically indicated) compared with term deliveries.
Results
Placental abruption cases had 2.3 to 5.5-fold increased odds of the other 3 PH indicators. Disc-impacting blood clots and microscopic hemorrhage were associated with one another (OR=4.6), but not with first trimester bleeding. In a multivariable model that included all four PH indicators and confounders, risk of PTD <35 weeks was elevated with first trimester bleeding (OR=1.9 (1.0, 3.4)), placental abruption (OR=5.2 (1.7, 16.2)), disc-impacting blood clots (OR=2.3 (1.0, 5.0)); and microscopic hemorrhage (OR=2.4 (1.4, 4.2)).
Conclusions
Multiple clinical and subclinical PH indicators are associated with PTD, particularly early PTD.
Keywords: Preterm delivery, placental abruption, bleeding, pregnancy outcomes, placental pathology
Introduction
Several etiologic pathways have been implicated in preterm delivery (PTD), including infection, stress, abnormal uterine distension, and uterine bleeding.1 Various types of direct or indirect evidence of placental hemorrhage (PH) may be manifestations of a uterine bleeding pathway. Vaginal bleeding in early to mid-pregnancy has been associated with PTD in many studies.2–4 Placental abruption, or premature separation of a normally placed placenta, may originate with bleeding into the decidua from ruptured decidual blood vessels5 and can result in major obstetric hemorrhage.6 Placental abruption is present in a larger proportion of preterm than term deliveries.7, 8 Subchorionic or retroplacental bleeding detected by prenatal ultrasonography9, 10 and hemosiderin (a breakdown product of old blood) in placental tissues detected through histologic examination11 have also been associated with PTD, although these have not been reported on extensively.
It is unclear whether these variable manifestations of bleeding belong to a single pathway with a common set of maternal risk factors. Vaginal bleeding early in pregnancy has been linked to placental abruption,2, 3, 12 suggesting that bleeding and abruption may share one or more common pathways. Many other maternal characteristics and clinical circumstances are shared risk factors for both PTD and placental abruption, including hypertension,13–15 African-American identity,16, 17 smoking,14, 18 cocaine abuse,19 and low body mass index (BMI).20, 21 Less has been published on maternal characteristics associated with other evidence of PH, such as early pregnancy bleeding22 or placental pathology findings.
While placental abruption is rare, complicating approximately 1% of pregnancies,6 placental pathology findings consistent with abruption are more common.23 We hypothesized that placental abruption may only represent a portion of the uterine bleeding that affects delivery timing – the “tip of the iceberg” – because subclinical gross or microscopic evidence of placental hemorrhage apparent only in placental examinations could also be related to PTD risk. The prospective Pregnancy Outcomes and Community Health (POUCH) Study collected multiple sources of evidence of PH, including mid-pregnancy maternal reports of prior vaginal bleeding episodes, clinical data from medical records and gross and microscopic placental pathology findings. The aim of this study was to evaluate four indicators of PH as potential components of a common “bleeding pathway” by (1) assessing their mutual associations, (2) describing their prevalence according to maternal characteristics, and (3) estimating their odds of PTD and its subtypes.
Methods
Study Protocol
The POUCH Study enrolled 3019 pregnant women from five Michigan communities at 15–27 weeks’ gestation (1998–2004). English-speaking women aged ≥15 years who had maternal serum alpha-fetoprotein (MSAFP) screening at 15–22 weeks, a singleton pregnancy with no known congenital anomalies or chromosomal abnormalities, and no pre-existing diabetes were eligible. MSAFP was of particular interest in the POUCH Study’s design because of this biomarker’s consistent association with PTD;24 thus, the POUCH cohort oversampled women who had unexplained high MSAFP (≥2 multiples of the median, 7% of cohort). The POUCH study protocol received institutional review board approval from Michigan State University, the Michigan Department of Community Health, and all nine delivery hospitals. All women provided informed written consent.
At enrollment, women participated in a structured interview with a study nurse and completed a self-administered questionnaire. The interview elicited information on demographics (including self-reported race/ethnicity), height and pre-pregnancy weight (used to calculate BMI), reproductive history, medical conditions, medication use and events during pregnancy. During the interview, women were asked, “Have you had any spotting or bleeding so far during this pregnancy?” and prompted to describe timing (gestational week) and heaviness (spotting, slight, about the same as usual period, or heavier than usual period) for up to seven episodes. The questionnaire was designed to collect data on potentially sensitive questions including substance use and physical abuse. Women were asked how often in the last six months they had been “shoved, hit, or physically abused by [their] parents or a partner or husband” (never, once or twice, several times, often, or very often).
Gestational age was estimated by last menstrual period, corroborated by an ultrasound scan conducted prior to 25 weeks (available for 97% of women). If the estimates differed by more than 2 weeks (17%) or if last menstrual period was unavailable (3.6%), the ultrasound date was used. PTD was defined as delivery prior to 37-0/7 weeks’ gestation.
Subcohort Sample
A subcohort (n=1371) was selected for detailed study of biologic samples and medical chart-level data. The subcohort included all PTDs, all women with unexplained high MSAFP and a stratified random sample of women with normal MSAFP and term deliveries, with oversampling of African-Americans. Subcohort analyses incorporate sampling weights to reconstitute the cohort distributions and further correct for overrepresentation of high MSAFP in the cohort, such that weighted proportions based on the subcohort can be interpreted as prevalence or risk.
Placenta protocol
After subcohort deliveries, placentas were formalin fixed at the delivery hospital prior to transport to the study’s pathology laboratory. For the gross examination, parallel slices were made through the placental disc 1 cm apart. The pathologist noted clots in the cut surface, along with indicators of adjacent tissue involvement, i.e. dissecting hemorrhage, and tissue infarction, compression, or red/brown discoloration. Disc-impacting blood clot was defined as a gross examination finding of a retro- or intraplacental blood clot impacting adjacent tissue.
The microscopic placental examination was conducted without the pathologist’s knowledge of gross examination findings and clinical outcome. Nine tissue samples per placenta were selected: two from the membrane roll, two from the cord, and five from the disc.25 The disc samples were chosen as follows: 1 at the cord insertion, 1 in grossly normal central tissue, 1 from marginal tissue, and 2 additional samples from central tissue, the latter representing grossly abnormal tissue if present.
Microscopic vascular-related findings that fell within five constructs adapted from a diagnostic coding tool were recorded. Items in the “Maternal Vascular – Disturbance of Integrity” (MV-I) construct, i.e. microscopic evidence of retroplacental blood with adjacent disc disruption/compression, decidual hemorrhage in the basal plate, and decidual hemosiderin-like pigment in the membranes or basal plate, were used to calculate an MV-I score for each woman. Microscopic hemorrhage was defined as the top quintile of MV-I scores based on the distribution of scores among term deliveries with normal MSAFP. This serves as a possible indicator of atypical maternal vessel hemorrhage. This distributional cut-point along a continuum of findings was previously shown to correlate with PTD risk in the POUCH Study.26
Medical record abstraction
Study nurses abstracted subcohort medical records in detail. For all PTDs, an additional brief abstraction was completed by a physician with expertise in obstetrics. A pool of possible placental abruption cases, identified by bleeding near delivery or any mention of placental abruption in the abstracted data, were later reviewed by three clinicians with labor and delivery experience who were unaware of the placental pathology findings recorded by the POUCH Study pathologist (PKS). Placental abruption was defined as (1) documented signs and symptoms consistent with abruption (e.g. vaginal bleeding, pain, increased uterine tone, fetal distress); or (2) retroplacental hematoma visualized on a prenatal ultrasound scan. Disagreements among the reviewers were resolved by discussion with the principal investigator (CBH) and study pathologist (PKS) until a consensus was reached.
Other relevant information abstracted from patient charts included trauma or injuries during pregnancy, episodes of vaginal bleeding during pregnancy and date of occurrence, blood pressure and proteinuria values, and details of the delivery process including timing of membrane rupture, cervical dilatation, and interventions. Hypertension was defined as documented diastolic blood pressure ≥90 mmHg and/or systolic blood pressure ≥140 mmHg on ≥2 days or a documented diagnosis and/or history of hypertension, or anti-hypertensive medication prior to 20 weeks. Preeclampsia, gestational hypertension and chronic hypertension were not considered separately because numbers of each were small. PTD subtypes, including medically indicated, spontaneous labor, and premature rupture of membranes (PPROM) were determined based on chart-level data as previously described.27 Spontaneous PTD included spontaneous preterm labor and PPROM.
Evidence of placental hemorrhage
The four principal PH indicators compared in this study were (1) placental abruption, (2) disc-impacting blood clots, (3) microscopic hemorrhage, and (4) first trimester bleeding (any vs. none, ascertained during enrollment interview). In selected analyses, we replaced first trimester bleeding (any vs. none) with variables that captured more detailed information: (1) first-trimester bleeding from the enrollment interview categorized as heavier than spotting, spotting only or none, and (2) first and second trimester vaginal bleeding (excluding bleeding in the week before delivery) recorded in prenatal charts categorized as first trimester only, second trimester only, both trimesters, or none.
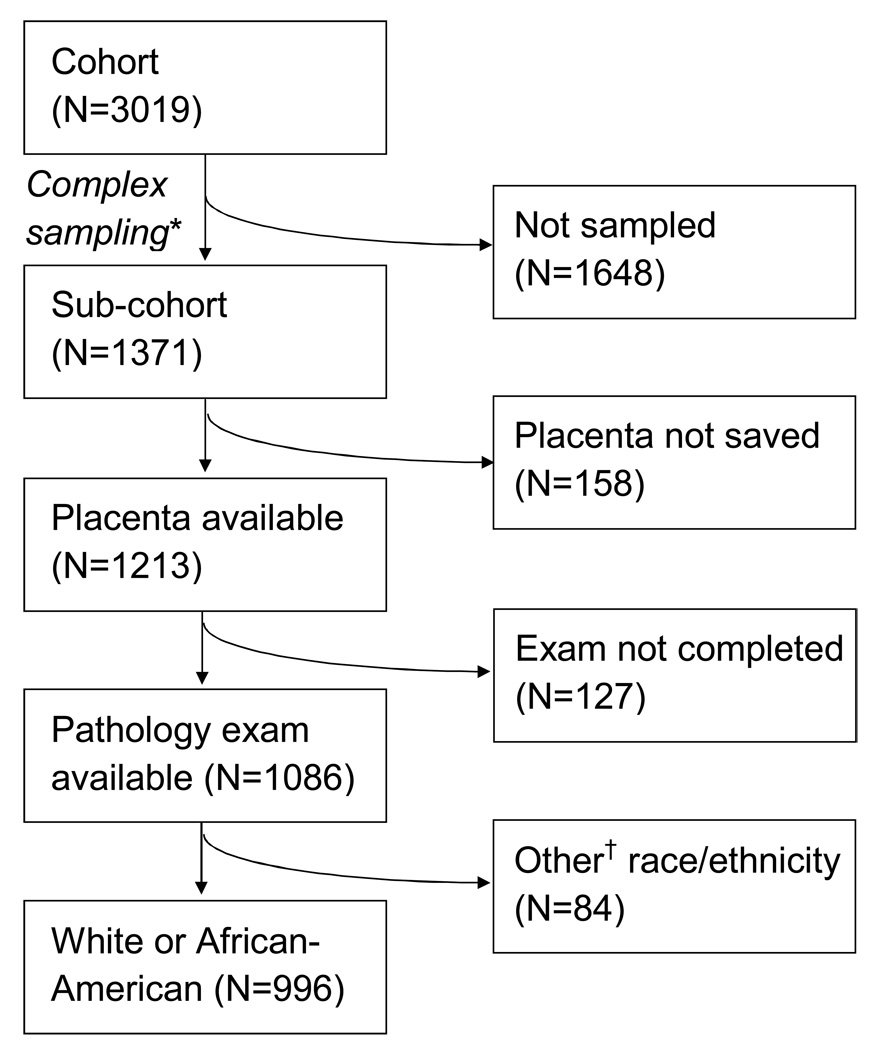
Study Sample
The derivation of the study sample is shown in Figure 1. From the subcohort, we sequentially excluded 158 women whose placentas were not saved, 127 women whose placental exams were not yet complete, 6 women with placenta previa at delivery, and 84 women who identified themselves as any race/ethnic category other than “White or Caucasian” (referred to as “white”) or “Black or African-American” (referred to as “African-American”). After these exclusions, a sample of 996 women remained.
Figure 1.
Flow diagram showing derivation of study sample from the Pregnancy Outcomes and Community Health (POUCH) Study cohort. *Sampling scheme was stratified by race, MSAFP, and delivery timing (term/preterm); see text for details. †”Other” race includes Asian, Hispanic, Native American, and others.
Descriptive statistics
As a first step, we calculated the prevalence of the four PH indicators and maternal characteristics overall and stratified by delivery timing (term/preterm). Next, we calculated the prevalence of each PH indicator among women with the other three indicators, and calculated bivariate odds ratios (OR) and 95% confidence intervals (CI) for each pair of PH indicators to examine their co-occurrence. Finally, we estimated the prevalence of each indicator according to selected maternal characteristics, and tested for differences in proportions using modified Rao-Scott χ2 tests for complex survey designs.28
Modeling strategy
We used weighted logistic regression models to assess relations between the four PH indicators and PTD. We calculated ORs for each PH indicator individually, then entered all four variables into the same model (i.e., a “mutually adjusted” model). Because the PH indicators were not highly concordant, it was possible to include all four in the same model without introducing multicollinearity problems. Next, we added maternal characteristics to the model, and retained those that changed ORs for any of the PH indicators by ≥5% from the mutually adjusted estimates. Potential confounders considered were those associated with PTD or any of the PH indicators in univariate analyses but not directly in the pathway between PH indicators and PTD. Finally, we added hypertension separately to examine its effect on other variables in the model. Hypertension may have a direct, proximate effect on placental abruption, placental pathology findings, and delivery timing.
Weighted polytomous logistic regression models were used to assess the relations among PH indicators and PTD subtypes defined by timing (35–36 weeks, <35 weeks, term [referent]) or clinical circumstances (spontaneous, medically indicated, term [referent]; and spontaneous labor, PPROM, medically indicated, term [referent]). All statistical analyses were conducted using SAS 9.1.3 (Statistical Analysis Software, Cary, NC).
Results
Descriptive statistics
Characteristics of the subcohort sample are detailed in Table 1, overall and stratified by delivery timing. In weighted analyses, 10.7% of pregnancies ended in PTD. In the total sample, prevalence of the four PH indicators ranged from 2.0% for placental abruption and 5.6% for disc-impacting blood clots to about 20% for microscopic hemorrhage and first trimester bleeding. All four PH indicators were more prevalent among PTDs than term deliveries. Medicaid insurance, lack of high school education, spending > 1 hour per week in a smoky room, hypertension and parity/prior PTD were also significantly associated with PTD in these univariate analyses. Although the relation between PTD and BMI categories was not statistically significant overall (p=0.13), preterm deliveries had larger proportions of both underweight (6.1%) and obese (30.1%) women than term deliveries (3.3% and 25.8%, respectively).
Table 1.
Indicators of placental hemorrhage, maternal characteristics and risk factors, overall and by preterm delivery status
| Total | Term | Preterm | P† | ||||
|---|---|---|---|---|---|---|---|
| N | %* | N | %* | N | %* | ||
| Total | 996 | 758 | 89.3 | 238 | 10.7 | ||
| Indicators of Placental hemorrhage (not mutually exclusive) | |||||||
| Placental abruption | 31 | 2.0 | 12 | 1.4 | 17 | 7.3 | <.0001 |
| Cut surface clot impacting adjacent tissue | 62 | 5.6 | 36 | 5.0 | 26 | 10.4 | .005 |
| Microscopic hemorrhage | 215 | 20.4 | 143 | 19.3 | 72 | 29.4 | .002 |
| First Trimester Vaginal Bleeding | 220 | 19.2 | 154 | 18.2 | 66 | 27.8 | .005 |
| Maternal characteristics | |||||||
| African-American | 416 | 24.6 | 340 | 23.6 | 76 | 32.9 | ‡ |
| Medicaid-insured | 536 | 45.6 | 411 | 44.8 | 125 | 52.7 | .03 |
| Education: did not complete high school | 203 | 16.3 | 153 | 15.7 | 50 | 21.6 | .03 |
| Smoked during pregnancy | 282 | 26.8 | 209 | 26.3 | 73 | 30.6 | .23 |
| Smoked prior to pregnancy | 343 | 33.1 | 250 | 32.4 | 93 | 38.5 | .10 |
| Smoky room > 1 hr/wk | 479 | 44.9 | 349 | 43.6 | 130 | 55.6 | .002 |
| Cocaine use (ever) | 82 | 8.5 | 25 | 10.7 | 57 | 8.3 | .29 |
| Trauma/injury noted in medical record | 116 | 10.7 | 89 | 10.6 | 27 | 11.8 | .62 |
| Physical abuse (previous 6 months) | 105 | 9.1 | 83 | 9.1 | 22 | 8.8 | .89 |
| Hypertensive disorder (includes preeclampsia, pregnancy-induced hypertension, and chronic hypertension) | 99 | 8.6 | 61 | 7.7 | 38 | 16.3 | <.01 |
| Cesarean delivery | 267 | 26.6 | 194 | 26.2 | 73 | 30.0 | 0.28 |
| Age | |||||||
| <20 | 160 | 13.3 | 122 | 12.9 | 38 | 16.3 | 0.36 |
| 20–34 | 765 | 78.6 | 583 | 78.8 | 182 | 77.0 | |
| 35+ | 71 | 8.1 | 53 | 8.3 | 18 | 6.7 | |
| Parity/prior preterm birth | |||||||
| Nulliparous | 407 | 40.8 | 304 | 40.5 | 103 | 43.6 | <0.0001 |
| Parous/no prior preterm birth | 449 | 49.2 | 380 | 51.7 | 69 | 28.6 | |
| Prior preterm birth | 139 | 9.9 | 74 | 7.8 | 65 | 27.7 | |
| Prepregnancy Body Mass Index | |||||||
| <18.5 | 44 | 3.6 | 30 | 3.3 | 14 | 6.1 | 0.13 |
| 18.5 – 24.9 | 438 | 45.4 | 336 | 45.8 | 102 | 42.5 | |
| 25–29.9 | 228 | 24.7 | 179 | 25.1 | 49 | 21.3 | |
| ≥30 | 286 | 26.3 | 213 | 25.8 | 73 | 30.1 | |
Percentages have been weighted (inverse of sampling probability) to reflect distribution in cohort.
P-value for preterm vs. term comparison by Rao-Scott chi-square test (SAS PROC SURVEYFREQ).
P-value can not be calculated in univariate analyses because exposure and outcome are sampling strata.
Table 2 shows pairwise associations among the four PH indicators. Placental abruption was associated with all three other PH indicators (versus disc-impacting blood clot, OR=5.5 (1.7, 17.3); versus microscopic hemorrhage, OR=2.3 (1.0, 5.5); versus first trimester bleeding, OR=3.4 (1.4, 8.5). However, for each pairwise comparison, less than 50% of placental abruption cases had one of the other PH indicators (23.0%, 37.0%, and 43.8%, respectively). Disc-impacting blood clot and microscopic hemorrhage were strongly associated with one another (OR=4.6 (2.3, 8.9)), but first trimester bleeding was not associated with disc-impacting blood clot or microscopic hemorrhage (ORs 1.1 and 0.9).
Table 2.
Prevalence of four indicators of placental hemorrhage overall and among women with other indicators of placental hemorrhage (weighted row percent), and odds ratios for bivariate comparisons
| Placental abruption | Disc-impacting blood clots |
Microscopic hemorrhage |
First trimester bleeding |
|||||
|---|---|---|---|---|---|---|---|---|
| % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) | |
| Among all women | 2.0 | 5.6 | 20.4 | 19.2 | ||||
| Among women with placental abruption (n=31) | - | 23.0 | 5.5 (1.7, 17.3) | 37.0 | 2.3 (1.0, 5.5) | 43.8 | 3.4 (1.4, 8.5) | |
| Among women with disc-impacting blood clots (n=62) | 8.4 | 5.5 (1.7, 17.3) | - | 51.1 | 4.6 (2.3, 8.9) | 20.7 | 1.1 (0.5, 2.4) | |
| Among women with microscopic hemorrhage (n=215) | 3.7 | 2.3 (1.0, 5.5) | 13.9 | 4.6 (2.3, 8.9) | - | 18.5 | 0.9 (0.6, 1.5) | |
| Among women with first trimester bleeding (n=220) | 4.6 | 3.4 (1.4, 8.5) | 6.0 | 1.1 (0.5, 2.4) | 19.6 | 0.9 (0.6, 1.5) | - | |
Figure 2 shows the number of women with all combinations of the four PH indicators. A total of 413 women (38.9% weighted) had at least one PH indicator, and 321 women (31.9% weighted) had only one PH indicator. Nine of 31 placental abruption cases (35.2% weighted) had no other PH indicators.
Figure 2.
Venn diagram showing 15 possible combinations of the 4 indicators of placental hemorrhage. Disc-impacting blood clot = oval, double line, microscopic hemorrhage = rectangle, double line; first trimester bleeding = rectangle, single line; placental abruption = oval, single line. Note: 583 women with none of the 4 types of evidence are not represented in the figure.
Maternal characteristics had few statistically significant associations with evidence of PH (Table 3). Women with <12 years of education had a lower prevalence of first trimester bleeding than women with more education. Smokers had a lower prevalence of microscopic hemorrhage than non-smokers. Hypertensive women had a higher prevalence of disc-impacting blood clots than non-hypertensive women. Nulliparous women had a lower prevalence of all four PH indicators than parous women with and without prior PTD, although only the association with placental abruption was statistically significant. Seven percent of underweight women had placental abruption, but this was not statistically significantly higher than the proportions in women in other BMI categories (range 1.5 to 2.1 percent).
Table 3.
Prevalence (weighted row percent) of four indicators placental hemorrhage according to selected maternal characteristics
| Placental abruption |
Disc- impacting blood clot |
Microscopic hemorrhage |
First trimester bleeding |
|
|---|---|---|---|---|
| Overall | 2.0 | 5.6 | 20.4 | 19.2 |
| Race/ethnicity | ||||
| African-American | 3.2 | 5.9 | 18.9 | 18.2 |
| White | 1.7 | 5.4 | 20.9 | 19.5 |
| Medicaid-insured | ||||
| Yes | 2.2 | 5.7 | 17.7 | 16.9 |
| No | 1.9 | 5.4 | 22.7 | 21.1 |
| Education | ||||
| <12 years | 2.9 | 6.0 | 16.6 | 13.4* |
| ≥12 years | 1.9 | 5.5 | 21.1 | 20.3 |
| Smoked during pregnancy | ||||
| Yes | 1.1 | 4.6 | 15.0* | 15.8 |
| No | 2.4 | 5.9 | 22.4 | 20.5 |
| Smoked 6 months before pregnancy | ||||
| Yes | 1.3 | 4.5 | 16.7 | 15.5 |
| No | 2.4 | 6.1 | 22.3 | 21.0 |
| Time spent in smoky room | ||||
| ≥1 hr/wk | 2.3 | 5.5 | 19.2 | 19.2 |
| <1 hr/wk | 1.8 | 5.6 | 21.4 | 19.2 |
| Ever used cocaine | ||||
| Yes | 2.0 | 4.7 | 13.4 | 16.1 |
| No | 2.0 | 5.6 | 21.1 | 19.5 |
| Trauma/injury noted in medial record | ||||
| Yes | 5.4 | 3.6 | 17.5 | 17.0 |
| No | 1.6 | 5.8 | 20.8 | 19.5 |
| Physical abuse (previous 6 months) | ||||
| Yes | 1.5 | 8.1 | 23.0 | 21.7 |
| No | 2.1 | 5.3 | 20.1 | 18.9 |
| Hypertensive disease | ||||
| Yes | 4.0 | 15.3* | 24.3 | 19.3 |
| No | 1.9 | 4.6 | 20.0 | 1.92 |
| Age | ||||
| <20 | 2.1 | 5.4 | 20.7 | 14.5 |
| 20–34 | 2.1 | 5.4 | 20.3 | 19.8 |
| 35+ | 1.4 | 7.0 | 20.7 | 21.1 |
| Parity/prior preterm birth | ||||
| Nulliparous | 0.8* | 4.4 | 18.5 | 17.6 |
| Parous/no prior preterm birth | 2.7 | 6.1 | 20.6 | 19.3 |
| Prior preterm birth | 3.9 | 7.3 | 27.3 | 25.6 |
| Prepregnancy Body Mass Index | ||||
| <18.5 | 7.1 | 7.7 | 16.3 | 20.1 |
| 18.5 – 24.9 | 2.1 | 4.5 | 21.7 | 21.3 |
| 25–29.9 | 1.7 | 6.6 | 20.7 | 20.6 |
| ≥30 | 1.5 | 6.0 | 18.5 | 14.1 |
P<.05 from Rao-Scott modified chi-square test.
Model Results
For PTD <37 weeks, there were elevated odds for each PH indicator in unadjusted analyses (Table 4). In a mutually adjusted model (i.e., a model that included all four PH indicators), all four ORs were attenuated, and disc-impacting blood clot lost statistical significance. Only minor changes occurred after adding maternal characteristics (maternal race, marital status, and BMI) to the model. Adding hypertension to the model slightly attenuated the ORs for placental abruption and disc-impacting blood clot but had no impact on the other two PH indicators. When we considered two alternative specifications of vaginal bleeding during pregnancy in these models, we found that heavier bleeding had a stronger association with PTD than spotting, and bleeding in both first and second trimesters (from patient charts) had a stronger association with PTD than bleeding confined to either the first or second trimester in unadjusted but not adjusted analyses (not shown). However, neither of these alternative specifications changed the interpretations for the other three PH indicators, thus all further analyses use first trimester bleeding (any vs. none) from the maternal interviews.
Table 4.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between four indicators of placental hemorrhage and preterm delivery (PTD), overall and stratified by delivery timing (N=996).
| PTD (n) |
Unadjusted | Mutually Adjusted* |
Adjusted for maternal characteristics‡ |
Adjusted for maternal characteristics and hypertension‡ |
|||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Preterm Delivery Overall | |||||||||
| <37 weeks§ | 238 | ||||||||
| Placental abruption | 19 | 5.5 | 2.4, 12.8 | 4.2 | 1.8, 9.8 | 4.0 | 1.7, 9.7 | 3.8 | 1.5, 9.5 |
| Disc-impacting blood clot | 26 | 2.2 | 1.3, 4.0 | 1.7 | 0.9, 3.1 | 1.8 | 1.0, 3.2 | 1.5 | 0.8, 2.9 |
| Microscopic Hemorrhage (top quintile) | 72 | 1.7 | 1.2, 2.5 | 1.6 | 1.1, 2.3 | 1.6 | 1.1, 2.4 | 1.6 | 1.1, 2.3 |
| First Trimester Bleeding | 66 | 1.7 | 1.2, 2.5 | 1.6 | 1.1, 2.4 | 1.7 | 1.2, 2.5 | 1.7 | 1.2, 2.5 |
| Preterm Delivery Stratified by Timing | |||||||||
| <35 Weeks║ | 77 | ||||||||
| Placental abruption | 11 | 10.2 | 3.9, 26.5 | 6.4 | 2.4, 17.4 | 5.5 | 1.7, 17.2 | 5.3 | 1.7, 17 |
| Disc-impacting blood clot | 14 | 4.2 | 2.0, 8.5 | 2.6 | 1.2, 5.5 | 2.8 | 1.3, 6.0 | 2.4 | 1.1, 5.3 |
| Microscopic Hemorrhage (top quintile) | 33 | 3.0 | 1.8, 4.9 | 2.4 | 1.4, 4.1 | 2.5 | 1.4, 4.3 | 2.5 | 1.4, 4.3 |
| First Trimester Bleeding | 25 | 2.1 | 1.2, 3.5 | 1.8 | 1.0, 3.2 | 1.9 | 1.1, 3.5 | 1.9 | 1.0, 3.5 |
| 35–36 Weeks║ | 161 | ||||||||
| Placental abruption | 8 | 3.5 | 1.3, 9.6 | 3.0 | 1.1, 8.4 | 3.1 | 1.1, 8.8 | 2.9 | 1.0, 8.5 |
| Disc-impacting blood clot | 12 | 1.4 | 0.7, 2.9 | 1.2 | 0.6, 2.7 | 1.3 | 0.6, 2.7 | 1.1 | 0.5, 2.5 |
| Microscopic Hemorrhage (top quintile) | 39 | 1.3 | 0.8, 2.0 | 1.2 | 0.8, 1.9 | 1.3 | 0.8, 2.0 | 1.3 | 0.8, 2.0 |
| First Trimester Bleeding | 41 | 1.6 | 1.0, 2.4 | 1.5 | 1.0, 2.4 | 1.6 | 1.1, 2.5 | 1.6 | 1.1, 2.5 |
Model includes all four types of evidence of placental hemorrhage
Model includes all four types of evidence of placental hemorrhage, maternal race, marital status, and BMI (categorical)
Model includes all four types of evidence of placental hemorrhage, maternal race, marital status, BMI (categorical), and hypertension.
OR and 95% CI calculated using weighted logistic regression models.
OR and 95% CI for PTD in both time intervals calculated simultaneously using weighted polytomous logistic regression models.
NOTE: Boldface denotes P<0.05.
When we stratified PTD by gestational week, all four PH indicators had stronger associations with PTDs at <35 weeks than with PTDs at 35–36 weeks in all models (Table 4). All four PH indicators were more strongly associated with PTD <35 weeks than PTD at 35–36 weeks.
When we stratified PTD by delivery circumstances, ORs did not differ meaningfully between spontaneous and medically indicated PTDs in unadjusted, mutually adjusted, or maternal characteristics-adjusted models (Table 5). After adding hypertension, the OR of 2.2 for disc-impacting blood clot and medically indicated PTD observed in the maternal characteristics-adjusted model was reduced to 1.1. This finding may be attributable to the expected strong association between hypertension and medically indicated PTDs (OR=9.9, unweighted) in conjunction with a more modest association between hypertension and disc-impacting blood clot among term (OR=3.0 unweighted) and indicated preterm (OR=2.6 unweighted) deliveries. However, small numbers in some cells (i.e. only 9 women with indicated PTD had disc-impacting blood clots, and 6 of these had hypertension) warrant caution in interpreting this finding.
Table 5.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between four indicators of placental hemorrhage and spontaneous or medically indicated preterm delivery (PTD)
| PTD (n) |
Unadjusted | Mutually Adjusted* |
Adjusted for maternal characteristics† |
Adjusted for maternal characteristics and hypertension‡ |
|||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Preterm Delivery, spontaneous§ | 169 | ||||||||
| Placental abruption | 13 | 5.4 | 2.2, 13.2 | 4.3 | 1.7, 10.6 | 4.0 | 1.6, 10.1 | 3.9 | 1.5, 10.0 |
| Disc-impacting Blood Clot | 17 | 2.0 | 1.0, 3.8 | 1.5 | 0.8, 3.0 | 1.6 | 0.8, 3.1 | 1.7 | 0.9, 3.4 |
| Microscopic Hemorrhage, top quintile | 51 | 1.7 | 1.1, 2.5 | 1.6 | 1.0, 2.4 | 1.6 | 1.1, 2.5 | 1.6 | 1.1, 2.5 |
| First Trimester Bleeding | 44 | 1.6 | 1.1, 2.4 | 1.5 | 1.0, 2.3 | 1.6 | 1.0, 2.5 | 1.6 | 1.1, 2.5 |
| Preterm Delivery, indicated§ | 69 | ||||||||
| Placental abruption | 6 | 5.9 | 2.0, 18.1 | 4.1 | 1.3, 13.1 | 4.0 | 1.2, 13.9 | 3.0 | 0.6, 14.6 |
| Disc-impacting Blood Clot | 9 | 2.9 | 1.3, 6.6 | 2.2 | 0.9, 5.2 | 2.2 | 0.9, 5.3 | 1.1 | 0.4, 3.4 |
| Microscopic Hemorrhage, top quintile | 21 | 1.8 | 1.0, 3.2 | 1.6 | 0.9, 2.8 | 1.6 | 0.9, 3.0 | 1.7 | 0.8, 3.2 |
| First Trimester Bleeding | 22 | 2.0 | 1.2, 3.6 | 1.9 | 1.1, 3.4 | 2.1 | 1.2, 3.9 | 2.2 | 1.1, 4.3 |
Model includes all four types of evidence of placental hemorrhage
Model includes all four types of evidence of placental hemorrhage, maternal race, marital status, and BMI (categorical)
Model includes all four types of evidence of placental hemorrhage, maternal race, marital status, BMI (categorical), and hypertension.
OR and 95% CI for both PTD subtypes calculated simultaneously using weighted polytomous logistic regression models.
NOTE: Boldface denotes P<0.05.
Although numbers were very small in some cells, we further subdivided spontaneous PTDs into spontaneous preterm labor and PPROM. Only microscopic hemorrhage was associated with PPROM (adjusted OR = 2.0, 95% CI 1.1, 3.5); the other three measures had stronger associations with spontaneous preterm labor (not shown).
Finally, we considered the possibility that mode of delivery may impact placental pathology findings. Thus we repeated the analyses in Table 4 and Table 5 after excluding 73 preterm and 194 term cesarean deliveries. Results for disc-impacting blood clot and microscopic hemorrhage were not meaningfully different, and the associations between placental abruption and PTD subtypes were of a greater magnitude. We also repeated the analyses after excluding women who reported taking aspirin (n=45), having an amniocentesis (n=21), or having chorionic villus sampling (n=6) during the index pregnancy, and results were not meaningfully different.
Discussion
A key finding of this study was that evidence of PH identified through objective gross and microscopic placental pathology exams (i.e. disc-impacting blood clot and microscopic hemorrhage) were associated with PTD at <35 weeks even after accounting for clinically evident bleeding (i.e. placental abruption and first trimester bleeding). Previously, Salafia et. al. found that hemosiderin in the decidua or extraplacental membranes was more common in very preterm (<32 weeks’ gestation) deliveries than in a sample of uncomplicated, healthy term deliveries in an unadjusted analysis11. Associations between early pregnancy vaginal bleeding and subsequent PTD2–4, 29 or placental abruption3, 8, 12 have previously been identified. Our study builds on these prior findings by considering these clinically evident manifestations of PH in conjunction with subclinical placental pathology findings.
Prior studies have shown that pathology findings consistent with placental abruption are more common than clinical diagnoses.5, 30 Some have suggested that placental pathology-based abruption-related findings may be inconsequential in the absence of clinical suspicion, given that most pregnancies with such findings have unremarkable outcomes6. In the POUCH Study, two types of evidence of PH from pathology exams – disc-impacting blood clot (prevalence 5.6%) and microscopic hemorrhage (“prevalence” 20.4%, based on a distributional cut-point) – were clearly more common than placental abruption cases (prevalence 2.0%), and the majority of women with disc-impacting blood clots and high microscopic hemorrhage scores delivered at term without placental abruption. However, after accounting for placental abruption cases in multivariable models, we identified excess PTD risk associated with both of these placental pathology findings, particularly for PTDs occurring at <35 weeks. Few studies have empirically demonstrated an association between subclinical evidence of hemorrhage in the delivered placenta and PTD by comparing preterm and term placentas. In prior work from the POUCH Study,26 the microscopic hemorrhage construct was found to be associated with both spontaneous and medically indicated PTDs occurring at <35 weeks. However, information on prior vaginal bleeding episodes and placental abruption were not considered in that study, thus it was unknown whether the observed results were primarily attributable to clinically evident hemorrhage.
Since retroplacental clots are sometimes employed in placental abruption clinical diagnoses, and have been required to confirm placental abruption cases in some epidemiologic studies,31–33 the limited association between placental abruption and disc-impacting blood clots is noteworthy. It is important to distinguish the disc-impacting blood clot construct captured by the POUCH Study pathologist from adherent retroplacental clots visualized on a freshly delivered placenta by an attending clinician. Data on the latter is not uniformly recorded in patient charts, thus we relied on a gross placental pathology protocol to infer presence of a clot prior to delivery. Collected blood not resulting from hemorrhage can become sequestered in the space between the membranes and disc and become firm with formalin fixation, resembling a true clot from hemorrhage. Thus, the pathologist identified clots associated with disc tissue changes that would support a significant retroplacental clot on gross examination alone. Disc-impacting blood clots undiagnosed as placental abruption may signal concealed (possibly intraplacental or dissecting) hemorrhage, a less severe clinical picture, or a case with a low index of suspicion for placental abruption. Placental abruption diagnoses in the absence of disc-impacting blood clots could occur in very acute abruptions in which clots do not have time to organize and cause tissue reactions prior to delivery.34
We found that microscopic hemorrhage was modestly associated with PTD <37 weeks (mutually adjusted OR=1.6) and PTD <35 weeks (mutually adjusted OR=2.4), and these associations remained after maternal demographic characteristics and hypertension were added to the models. While it is possible that findings in this construct represent intermediate steps on a pathway that sometimes leads to overt placental abruption, most cases of placental abruption (63%, see Table 2) did not have high scores on this construct. We hypothesized that maternal-reported gestational vaginal bleeding remote from delivery, which has been linked to placental abruption and PTD in this and other studies,2–4, 8, 12, 35 might signal chronic, slow hemorrhage, and would thus be associated with high microscopic hemorrhage scores, but this was not the case. Microscopic hemorrhage was associated with PPROM, while the three other manifestations of placental hemorrhage were not. Possible mechanisms linking specific items included in our microscopic hemorrhage construct to spontaneous PTD and PPROM have previously been suggested: thrombin generated in response to decidual hemorrhage may cause cervical ripening, contractions, and membrane degradation resulting in preterm labor or PPROM;36 and tissue hemosiderin, an iron compound produced during the breakdown of blood, may exert tissue irritant and pro-inflammatory effects that contribute to preterm labor.37 Additional research is needed to discover the antecedents of high microscopic hemorrhage scores.
The strengths and limitations of this study primarily derive from the prospective cohort design and the placental pathology protocol. Statistical power to detect significant associations differed among the four PH indicators given their widely varying prevalence, which ranged from 2.0% to 20.4%. Sample size limitations precluded assessing risk of very early PTDs (i.e. <32 weeks). However, by performing the pathology examination on a large sample of term and preterm deliveries we were able to empirically evaluate the relations among PTD and four PH indicators. Like most studies that have investigated gestational vaginal bleeding in relation to pregnancy outcome, we cannot discern the actual origin of the blood – some may be from the placenta, while other reported bleeding may have originated in the cervix or vaginal tract. Data on first trimester bleeding and substance use were ascertained prospectively; therefore this information was not subject to differential reporting based on mother’s or clinician’s knowledge of pregnancy outcome.
The study population was drawn from a well-characterized cohort, which was very similar to community women giving birth in the study years based on race-specific birth certificate comparisons.38 The incidence of placental abruption observed in this study (2.0%) is higher than recent population-based estimates using administrative data and vital records in the United States. 7, 39 Birth certificates and hospital discharge data have been shown to have limited sensitivity for ascertainment of maternal morbidity compared with medical chart review and application of standardized definitions, 40 the method used in the POUCH Study. Furthermore, the POUCH Study included a relatively high proportion of African-American women, and this demographic group is known to have a higher rate of abruption diagnoses compared with whites. 39 The higher than expected incidence of placental abruption was not otherwise attributable to the sampling scheme, because appropriate weighted analyses were used to account for oversampling of PTD and women with high MSAFP.
Each of the four manifestations of PH considered in this study contributed information regarding PTD risk. Some of the PH indicators were associated with one another, but others were not. Many placental abruption cases did not show strong evidence of PH in their placental pathology examinations. The PH indicators diverged somewhat with respect to their relations with maternal characteristics and PTD subtypes. Taken together, these findings suggest potential heterogeneity in the hypothesized PH “iceberg.” In the future, it may be helpful to consider both clinical and subclinical manifestations of PH in relation to biomarker data (e.g. gene polymorphisms,41 anti-angiogenic factors) in order to gain insight into broader pathways to PTD that may involve disrupted uteroplacental vascular integrity.
Acknowledgements
The authors would like to acknowledge the work contributed by the histotechnicians in the MSU Investigative HistoPathology Laboratory. The authors would also like to thank Dr Bertha Bullen, project director, the Prematurity Study Group, and Drs Joseph Marshall and Judith Suess, physician abstractors, and Drs Nazish Siddiqi and Judith Suess and Ms Lynn Thelen for reviewing suspected cases of placental abruption.
Funding
This work was supported by Perinatal Epidemiological Research Initiative Program Grant from the March of Dimes Foundation [Grants 20FY01-38 and 20-FY04-37], the National Institute of Child Health and Human Development and the National Institute of Nursing Research [Grant R01 HD34543], the Thrasher Research Foundation [Grant 02816-7], and the Centers for Disease Control and Prevention [Grant U01 DP000143-01].
Footnotes
Disclosure of interests
None
Contribution to authorship
J Gargano: concept, design and drafting of manuscript, data analysis and interpretation, final approval of version to be published
C Holzman: concept, design of manuscript, data acquisition, analysis and interpretation, critical revision for intellectual content, and final approval of version to be published
P Senagore: Acquisition of data, performance of placental pathology examinations, critical revision for intellectual content, and final approval of version to be published
M Reuss: data interpretation, critical revision for intellectual content, and final approval of version to be published
D Pathak: data analysis and interpretation, critical revision for intellectual content, and final approval of version to be published
M Williams: data interpretation, critical revision for intellectual content, and final approval of version to be published
R Fisher: data interpretation, review of manuscript, and final approval of version to be published
Details of Ethics Approval
The POUCH study has maintained IRB approval from all participating institutions since the Study’s inception.
References
- 1.Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193(3 Pt 1):626–635. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 2.Hossain R, Harris T, Lohsoonthorn V, Williams MA. Risk of preterm delivery in relation to vaginal bleeding in early pregnancy. Eur J Obstet Gynecol Reprod Biol. 2007;135(2):158–163. doi: 10.1016/j.ejogrb.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss JL, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, et al. Threatened abortion: A risk factor for poor pregnancy outcome, a population-based screening study. Am J Obstet Gynecol. 2004;190(3):745–750. doi: 10.1016/j.ajog.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Hartmann KE, Savitz DA, Herring AH, Dole N, Olshan AF, et al. Vaginal bleeding during pregnancy and preterm birth. Am J Epidemiol. 2004;160(2):118–125. doi: 10.1093/aje/kwh180. [DOI] [PubMed] [Google Scholar]
- 5.Fox H. Pathology of the Placenta. 2nd ed. London: W. B. Saunders Company Ltd; 1997. Macroscopic abnormalities of the placenta; pp. 102–150. [Google Scholar]
- 6.Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol. 2006;108(4):1005–1116. doi: 10.1097/01.AOG.0000239439.04364.9a. [DOI] [PubMed] [Google Scholar]
- 7.Ananth CV, Getahun D, Peltier MR, Smulian JC. Placental abruption in term and preterm gestations: evidence for heterogeneity in clinical pathways. Obstet Gynecol. 2006;107(4):785–792. doi: 10.1097/01.AOG.0000207560.41604.19. [DOI] [PubMed] [Google Scholar]
- 8.Tikkanen M, Nuutila M, Hiilesmaa V, Paavonen J, Ylikorkala O. Clinical presentation and risk factors of placental abruption. Acta Obstet Gynecol Scand. 2006;85(6):700–705. doi: 10.1080/00016340500449915. [DOI] [PubMed] [Google Scholar]
- 9.Ball RH, Ade CM, Schoenborn JA, Crane JP. The clinical significance of ultransonographically detected subchorionic hemorrhages. Am J Obstet Gynecol. 1996;174(3):996–1002. doi: 10.1016/s0002-9378(96)70339-3. [DOI] [PubMed] [Google Scholar]
- 10.Nagy S, Bush M, Stone J, Lapinski RH, Gardo S. Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet Gynecol. 2003;102(1):94–100. doi: 10.1016/s0029-7844(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 11.Salafia CM, Lopezzeno JA, Sherer DM, Whittington SS, Minior VK, Vintzileos AM. Histologic Evidence of Old Intrauterine Bleeding Is More Frequent in Prematurity. Am J Obstet Gynecol. 1995;173(4):1065–1070. doi: 10.1016/0002-9378(95)91327-0. [DOI] [PubMed] [Google Scholar]
- 12.Ananth CV, Oyelese Y, Prasad V, Getahun D, Smulian JC. Evidence of placental abruption as a chronic process: associations with vaginal bleeding early in pregnancy and placental lesions. Eur J Obstet Gynecol Reprod Biol. 2006;128(1–2):15–21. doi: 10.1016/j.ejogrb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Ananth CV, Peedicayil A, Savitz DA. Effect of hypertensive diseases in pregnancy on birthweight, gestational duration, and small-for-gestational-age births. Epidemiology. 1995;6(4):391–395. doi: 10.1097/00001648-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Ananth CV, Savitz DA, Bowes WA, Jr, Luther ER. Influence of hypertensive disorders and cigarette smoking on placental abruption and uterine bleeding during pregnancy. BJOG. 1997;104(5):572–578. doi: 10.1111/j.1471-0528.1997.tb11535.x. [DOI] [PubMed] [Google Scholar]
- 15.Samadi AR, Mayberry RM. Maternal hypertension and spontaneous preterm births among black women. Obstet Gynecol. 1998;91(6):899–904. doi: 10.1016/s0029-7844(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 16.Shen TT, DeFranco EA, Stamilio DM, Chang JJ, Muglia LJ. A population-based study of race-specific risk for placental abruption. BMC Pregnancy Childbirth. 2008;8:43. doi: 10.1186/1471-2393-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Savitz DA. Preterm birth subtypes among blacks and whites. Epidemiology. 1992;3(5):428–433. doi: 10.1097/00001648-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kyrklund-Blomberg NB, Cnattingius S. Preterm birth and maternal smoking: risks related to gestational age and onset of delivery. Am J Obstet Gynecol. 1998;179(4):1051–1055. doi: 10.1016/s0002-9378(98)70214-5. [DOI] [PubMed] [Google Scholar]
- 19.Addis A, Moretti ME, Ahmed Syed F, Einarson TR, Koren G. Fetal effects of cocaine: an updated meta-analysis. Reprod Toxicol. 2001;15(4):341–369. doi: 10.1016/s0890-6238(01)00136-8. [DOI] [PubMed] [Google Scholar]
- 20.Simhan HN, Bodnar LM. Prepregnancy body mass index, vaginal inflammation, and the racial disparity in preterm birth. Am J Epidemiol. 2006;163(5):459–466. doi: 10.1093/aje/kwj053. [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Lieberman E, Mittendorf R, Monson RR, Schoenbaum SC. Risk factors for abruptio placentae. Am J Epidemiol. 1991;134(9):965–972. doi: 10.1093/oxfordjournals.aje.a116181. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Savitz DA, Dole N, Hartmann KE, Herring AH, Olshan AF, et al. Predictors of vaginal bleeding during the first two trimesters of pregnancy. Paediatr Perinat Epidemiol. 2005;19(4):276–283. doi: 10.1111/j.1365-3016.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 23.Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol. 2006;108(4):1005–1016. doi: 10.1097/01.AOG.0000239439.04364.9a. [DOI] [PubMed] [Google Scholar]
- 24.Holzman C, Bullen B, Fisher R, Paneth N, Reuss L. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr Perinat Epidemiol. 2001;15 Suppl 2:136–158. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 25.Holzman C, Lin X, Senagore P, Chung H. Histologic chorioamnionitis and preterm delivery. Am J Epidemiol. 2007;166(7):786–794. doi: 10.1093/aje/kwm168. [DOI] [PubMed] [Google Scholar]
- 26.Kelly R, Holzman C, Senagore P, Wang J, Tian Y, Rahbar MH, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009;170(2):148–158. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiedje L, Holzman CB, De Vos E, Jia X, Korzeniewski S, Rahbar MH, et al. Hostility and anomie: links to preterm delivery subtypes and ambulatory blood pressure at mid-pregnancy. Soc Sci Med. 2008;66(6):1310–1321. doi: 10.1016/j.socscimed.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao JNK, Scott AJ. The analysis of categorical data from complex sample surveys: chi-squared tests for goodness of fit and independence in two-way tables. J Am Stat Assoc. 1981;76:221–230. [Google Scholar]
- 29.Ananth CV, Savitz DA. Vaginal bleeding and adverse reproductive outcomes: a meta-analysis. Paediatr Perinat Epidemiol. 1994;8(1):62–78. doi: 10.1111/j.1365-3016.1994.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 30.Benirschke K, Gille J. Placental Pathology and Asphyxia. In: Gluck L, editor. Intrauterine Asphyxia and the Developing Fetal Brain. Chicago: Year Book Medical Publishers; 1977. pp. 117–136. [Google Scholar]
- 31.Jaaskelainen E, Keski-Nisula L, Toivonen S, Paattiniemi EL, Helisalmi S, Punnonen K, et al. Polymorphism of the interleukin 1 receptor antagonist (IL1Ra) gene and placental abruption. J Reprod Immunol. 2008;79(1):58–62. doi: 10.1016/j.jri.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Naeye RL. Coitus and antepartum haemorrhage. BJOG. 1981;88(7):765–770. doi: 10.1111/j.1471-0528.1981.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XQ, Craven C, Nelson L, Varner MW, Ward KJ. Placental abruption is more frequent in women with the angiotensinogen Thr235 mutation. Placenta. 2007;28(7):616–619. doi: 10.1016/j.placenta.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Benirschke K, Kaufmann P, Baergen R. Maternal Diseases Complicating Pregnancy: Diabetes, Tumors, Preeclampsia, Lupus Anticoagulant. In: Benirschke K, Kaufmann P, Baergen RN, editors. Pathology of the Human Placenta. New York: Springer; 2006. pp. 596–656. [Google Scholar]
- 35.Johns J, Jauniaux E. Threatened miscarriage as a predictor of obstetric outcome. Obstet Gynecol. 2006;107(4):845–850. doi: 10.1097/01.AOG.0000206186.91335.9a. [DOI] [PubMed] [Google Scholar]
- 36.Lockwood CJ. Testing for risk of preterm delivery. Clin Lab Med. 2003;23(2):345–360. doi: 10.1016/s0272-2712(03)00029-5. [DOI] [PubMed] [Google Scholar]
- 37.Salafia CM, Lopez-Zeno JA, Sherer DM, Whittington SS, Minior VK, Vintzileos AM. Histologic evidence of old intrauterine bleeding is more frequent in prematurity. Am J Obstet Gynecol. 1995;173(4):1065–1070. doi: 10.1016/0002-9378(95)91327-0. [DOI] [PubMed] [Google Scholar]
- 38.Holzman C, Eyster J, Tiedje LB, Roman LA, Seagull E, Rahbar MH. A life course perspective on depressive symptoms in mid-pregnancy. Matern Child Health J. 2006;10(2):127–138. doi: 10.1007/s10995-005-0044-0. [DOI] [PubMed] [Google Scholar]
- 39.Ananth CV, Oyelese Y, Yeo L, Pradhan A, Vintzileos AM. Placental abruption in the United States, 1979 through 2001: temporal trends and potential determinants. Am J Obstet Gynecol. 2005;192(1):191–198. doi: 10.1016/j.ajog.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 40.Lydon-Rochelle MT, Holt VL, Nelson JC, Cardenas V, Gardella C, Easterling TR, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol. 2005;19(6):460–471. doi: 10.1111/j.1365-3016.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 41.Gargano JW, Holzman CB, Senagore PK, Reuss ML, Pathak DR, Friderici KH, et al. Polymorphisms in thrombophilia and renin-angiotensin system pathways, preterm delivery, and evidence of placental hemorrhage. Am J Obstet Gynecol. 2009;201(3):317, e1–e9. doi: 10.1016/j.ajog.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]