Abstract
Axonal morphology is a critical determinant of neuronal connectivity, and perturbation of the rate or extent of axonal growth during development has been linked to neurobehavioral deficits in animal models and humans. We previously demonstrated that the organophosphorus pesticide (OP) chlorpyrifos (CPF) inhibits axonal growth in cultured neurons. In this study, we used a zebrafish model to determine whether CPF, its oxon metabolite (CPFO), or the excreted metabolite trichloro-2-pyridinol (TCPy) alter spatiotemporal patterns of axonal growth in vivo. Static waterborne exposure to CPFO, but not CPF or TCPy, at concentrations ≥ 0.03μM from 24- to 72-h post fertilization significantly inhibited acetylcholinesterase, and high-performance liquid chromatography detected significantly more TCPy in zebrafish exposed to 0.1μM CPFO versus 1.0μM CPF. These data suggest that zebrafish lack the metabolic enzymes to activate CPF during these early developmental stages. Consistent with this, CPFO, but not CPF, significantly inhibited axonal growth of sensory neurons, primary motoneurons, and secondary motoneurons at concentrations ≥ 0.1μM. Secondary motoneurons were the most sensitive to axonal growth inhibition by CPFO, which was observed at concentrations that did not cause mortality, gross developmental defects, or aberrant somatic muscle differentiation. CPFO effects on axonal growth correlated with adverse effects on touch-induced swimming behavior, suggesting the functional relevance of these structural changes. These data suggest that altered patterns of neuronal connectivity contribute to the developmental neurotoxicity of CPF and demonstrate the relevance of zebrafish as a model for studying OP developmental neurotoxicity.
Keywords: axon outgrowth, chlorpyrifos, chlorpyrifos-oxon, developmental neurotoxicology, organophosphorus pesticides, zebrafish
Organophosphorus pesticides (OPs) are currently the most commonly utilized pesticides worldwide (Zaim and Jambulingam, 2009). There is widespread exposure of children to these compounds (Adgate et al., 2001; Barr et al., 2004; Curl et al., 2003; Davis and Ahmed, 1998; Eskenazi et al., 1999; Landrigan et al., 1999; Lu et al., 2001; Whyatt and Barr, 2001), and low-level OP exposure has been linked to behavioral and cognitive problems in infants and school-aged children (Engel et al., 2007; Eskenazi et al., 2007; Jacobson and Jacobson, 2006; Kofman et al., 2006; Lizardi et al., 2008; Rohlman et al., 2005; Rosas and Eskenazi, 2008). Experimental animal data confirm that exposure during critical stages of brain development can cause persistent neurobehavioral deficits (reviewed by Costa, 2006; Eaton et al., 2008; Slotkin, 2004b). These observations underscore the need to better understand the cellular and molecular mechanism(s) of OP developmental neurotoxicity.
One of the OPs most extensively studied in the context of developmental neurotoxicity is chlorpyrifos (CPF), which is also one of the most widely used OPs in both developing and industrialized countries, including the United States (Zaim and Jambulingam, 2009). In animal models, CPF exposure during development causes learning deficits and altered locomotor activity at doses that do not elicit acute cholinergic toxicity or significant downregulation of cholinergic receptors (Jett et al., 2001; Levin et al., 2001, 2002). These findings suggest that developmental neurotoxicity is mediated by mechanisms other than those implicated in acute OP neurotoxicity (Abou-Donia, 2003; Costa, 2006; Ecobichon, 1994). It is postulated that the neurobehavioral deficits associated with developmental CPF exposure reflect altered neuronal connectivity in the developing brain (Bigbee et al., 1999; Brimijoin and Koenigsberger, 1999; Slotkin, 2004a).
A critical determinant of neuronal connectivity is axonal growth, and interference with either temporal or spatial aspects of axonal growth can result in functional deficits (Barone et al., 2000; Berger-Sweeney and Hohmann, 1997; Cremer et al., 1998). In vitro studies demonstrate that CPF and its oxon metabolite (CPFO) inhibit neurite outgrowth in neural cell lines (Das and Barone, 1999; Li and Casida, 1998; Sachana et al., 2001; Song et al., 1998) and axonal outgrowth in primary neuronal cell cultures (Howard et al., 2005; Yang et al., 2008). Perinatal exposure of rats to OPs alters brain morphometry (Veronesi and Pope, 1990) and the ratio of membrane protein to total protein in the brain (Qiao et al., 2003), the latter considered by the authors to be a surrogate measure of axonal outgrowth. Similarly, a recent report suggests that OPs interfere with sensory neuron morphogenesis in zebrafish (Jacobson et al., 2010). However, definitive evidence that OPs interfere with axonal growth in vivo is lacking and whether aberrant axonal growth correlates with OP-induced behavioral deficits has yet to be demonstrated. The objective of this study, therefore, was to utilize a zebrafish model to address these gaps in the database.
The rationale for using zebrafish for developmental neurotoxicity studies derives from the consensus that fundamental processes and mechanisms of neurodevelopment are remarkably conserved across species (Lein et al., 2005). Significant advantages of zebrafish relative to rodent models for studies of axonal growth include: (1) zebrafish embryos develop externally, which eliminates maternal toxicity as a confounding factor and (2) zebrafish are optically transparent, which allows for the use of simple microscopic techniques to resolve individual neuronal cells in vivo across a broad range of developmental stages. Resolution is increased by using transgenic zebrafish that express fluorescent reporter genes in individual neurons of specific lineages. Additional advantages of zebrafish include their small size, rapid embryonic development, and short life cycle (Dodd et al., 2000; Udvadia and Linney, 2003; Wixon, 2000). Similar to observations of CPF developmental neurotoxicity in rodent models, developmental exposure to CPF causes persistent deficits in learning and locomotor activity in zebrafish (Eddins et al., 2010; Levin et al., 2003, 2004). The data reported herein demonstrate that axonal growth is inhibited in zebrafish exposed to CPFO, and this effect correlates with deficits in touch-induced swimming behavior. These data indicate that altered patterns of neuronal connectivity contribute to the developmental neurotoxicity of CPF.
MATERIALS AND METHODS
Fish lines and husbandry.
Four lines of zebrafish were used in this study: the wild-type AB line; Tg(isl1:GFP), referred to as islet-1 from this point forward, which expresses green fluorescent protein (GFP) in secondary motoneurons and their axons; Tg(NBT:MAPT-GFP)zc1, referred to as NBT from this point forward, which expresses GFP in primary motoneurons and their axons; and Tg(-8.4neurog1:GFP), referred to as Neurog-1 from this point forward, which expresses GFP in Rohon-Beard and dorsal root ganglia (DRG) sensory neurons and their axons. All lines were raised on a 14:10 light:dark cycle in polycarbonate tanks on a recirculating system in which the water was maintained at 28°C and a pH of 7.0. Fish care was in accordance with approved Institutional Animal Care and Use Committee protocols at Oregon State University. For all the described experiments, newly fertilized eggs were staged according to previously described methods (Kimmel et al., 1995).
Pesticide exposures.
CPF (O, O-diethyl O-phosphorothionate, 99.5% purity), CPF-oxon (CPFO, 99.5% purity), and 3,4,5-trichloro-2-pyrindinol (TCPy, 99.5% purity) were purchased from Chem Service (West Chester, PA) and stored as recommended by the manufacturer. All embryos were exposed to OPs using a static waterborne method. It was recently reported that CPFO has a half-life of 1 day in fish water at 28°C (Jacobson et al., 2010); therefore, for exposures lasting more than 1 day, pesticides were replaced daily by transferring zebrafish to new plates with fresh pesticide diluted in fish water. Pesticide stock solutions in dimethyl sulfoxide (DMSO) (Fisher Scientific, Fair lawn, NJ) were made up each day and diluted 1:1000 directly into fish water to give final concentrations ranging from 3nM to 1μM. The final concentration of DMSO was 0.1% across all treatment groups. For OP uptake studies, AChE activity assays and morphometric analyses of axonal growth, embryos were exposed to vehicle (DMSO at 0.1%), CPF, CPFO, or TCPy, in 10-ml glass vials sealed with Teflon-lined lids (Fisher Scientific International, Pittsburgh, PA) to prevent losses from volatilization. For behavioral studies, fish were placed in 96-well plates that were sealed after addition of the OP; fish were maintained in these OP-containing solutions during behavioral assessments. With the exception of the OP uptake studies in which a subset of exposures were initiated at 6-h post fertilization (hpf), all exposures were initiated at 24 hpf, which corresponds to the first wave of axonal outgrowth of spinal neurons. Exposures were terminated at 24, 48, or 72 hpf. At the end of the exposure period, embryos were rapidly washed several times with fish water and either snap-frozen (for OP uptake studies or AChE activity assays) or fixed with 4% paraformaldehyde (for morphometric analyses). For the axonal growth recovery assays, zebrafish larvae were washed and transferred to OP-free fish water in which they were maintained until 96 or 120 hpf at which time they were fixed for axonal measurements.
Quantification of CPF, CPFO, and TCPy in zebrafish embryos.
To assess OP uptake and metabolism in embryonic and larval zebrafish, CPF, CPFO, and TCPy levels in extracts of whole fish were quantified by reverse-phase high-performance liquid chromatography (HPLC). Zebrafish were exposed to either CPF at 1μM beginning at 6 hpf and terminating at 24, 48, or 72 hpf or to CPFO at 0.1μM from 24 to 72 hpf. At the end of the exposure period, zebrafish were snap-frozen and stored at −80°C until analyzed.
Frozen tissues were thawed on ice and sonicated in 0.5 ml homogenizing buffer (10% NaCl, 25mM Tris, 2.5mM MgCl2, pH 7.4). Spiked samples were prepared using Coho salmon eggs containing 0.25 or 2.5 μg of CPF, CPFO, and TCPy per sample. Sonicated samples were mixed with hydromatrix and then extracted with ethyl acetate using a Dionex Automated Solvent Extraction system (model ASE300; Dionex, Sunnyvale, CA) programmed as follows: temperature at 60°C, pressure at 1500 psi, 5-min heat cycle, two cycles of 5-min stat cycle, 150% flush volume, 90 s purge time. Extracts were collected and concentrated to approximately 1 ml under ultrapure nitrogen using a Zymark TurboVapII Concentration System (Caliper Life Sciences, Hopkinton, MA). Concentrates were loaded onto an acid-conditioned florisil solid-phase extraction cartridge (500 mg; Waters Corporation, Milford, MA). Sequential elution with ethyl acetate (15 ml) and 50% acetonitrile (2 ml) was performed to collect CPF and CPFO in the ethyl acetate fractions, which were subsequently dried under nitrogen and reconstituted in 1 ml 50% acetonitrile, and TCPy in the acetonitrile fractions, which were further concentrated under nitrogen as necessary.
Chromatographic analysis was performed using a HPLC system (HP1100 series; Agilent, Santa Clara, CA) equipped with a degasser (model G1322A), quarternary pump (model G1311A), autosampler (model G1313A), and photo diode array detector (model G1315A). Sample (50 μl) was injected onto an Ascentis C-18 analytical column (4.6 × 250 mm, 5 μm size particles; Supelco, Bellefonte, PA) equipped with Ascentis C-18 guard column (4.6 × 20 mm, 5 μm size particles; Supelco) and eluted at a flow rate of 1 ml/min with a mobile phase consisting of solvent A (5% acetonitrile and 0.2% H3PO4 in water) and solvent B (100% acetonitrile). The elution gradient was 75% solvent A/25% solvent B for 3 min after injection followed by a 5-min linear gradient to 40% A/60% B, which was held for 10 min and then a 5-min linear gradient to 100% B, which was held for 8 min. Columns were re-equilibrated between samples to initial conditions for 20 min. All HPLC solvents were filtered through a 0.2-μm nylon filter. The eluates were monitored at 290 nm for CPF and CPFO and 300 nm for TCPy. Each analyte was quantified against its corresponding standard calibration curve established with each batch run. For TCPy and CPFO, the calibration curve was from 1.25 to 500 ng per 50 μl; for CPF, it was 2.5 to 500 ng per 50 μl. Two spike samples were included in each batch run, and the overall recovery for TCPy, CPFO, and CPF was 93, 72, and 92%, respectively.
Acetylcholinesterase (AChE, EC 3.1.1.7) activity assay.
At the end of the exposure period (as indicated in Fig. 1), 10 embryos from each experimental group were euthanized by incubation on ice for 5 min. Each embryo was lysed in 120 μl PBS containing 1% Triton X-100 by passing the whole embryo through a 26-gauge needle 10 times. Lysates were snap-frozen and stored at −80°C until analyzed. On the day of analysis, lysates were thawed on ice, aliquoted into triplicate samples, and AChE activity quantified using the standard Ellman assay (Ellman et al., 1961) with 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB) and acetylthiocholine iodide (ASChI) as the substrates (Sigma-Aldrich, St Louis, MO). Assays were run against blanks containing DTNB. After a 5-min equilibration, the reaction was started with the addition of ASChI. ASChI hydrolysis was determined by monitoring the change in absorbance at 405 nm, and AChE activity was normalized using total protein concentration as determined using the BCA assay (Pierce, Rockford, IL). This method accurately measures AChE activity in zebrafish embryos as early as 24 hpf (Behra et al., 2002).
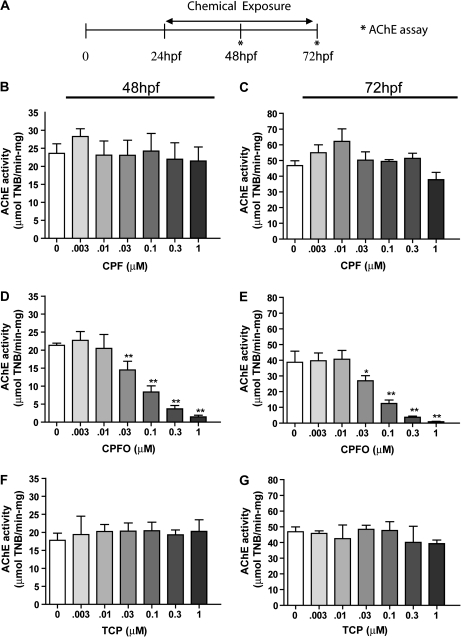
FIG. 1.
CPFO, but not CPF or TCPy, inhibits AChE activity in wild-type zebrafish at 48 and 72 hpf. (A) Zebrafish (AB line) were exposed to varying concentrations of CPF, CPFO, or TCPy beginning 24 hpf, and AChE activity was determined at 48 or 72 hpf using the Ellman assay. Neither the parent compound CPF (B and C) nor the metabolite TCPy (F and G) significantly inhibited AChE activity at 48 (B and F) or 72 hpf (C and G). In contrast, the oxon metabolite CPFO (D and E) significantly inhibited AChE activity in a concentration-dependent manner at both 48 (D) and 72 hpf (E). *Significantly different from vehicle control at p < 0.05; **p < 0.01 (n = 10 per experimental condition).
Teratology screening.
Zebrafish were screened for gross teratogenic responses by observers blinded to treatment using a Nikon dissecting microscope. Zebrafish were scored with respect to the following teratogenic endpoints: (1) absence of eyes; (2) structural malformation of the snout, jaw, otic vesicles, notochord, somite, or fin; (3) edema in the heart, brain, pericardial, and yolk sac region; (4) curvature of the body axis; and (5) reduced pigmentation as previously described (Svoboda et al., 2002).
Assessment of swimming behavior.
Zebrafish (24 per experimental group) were separated into individual wells of 96-well plates to monitor individual fish swimming behavior. Zebrafish were exposed to vehicle or varying concentrations of CPF or CPFO starting at 24 hpf; swimming behavior was evaluated at 72hpf. Because zebrafish larvae at 48–72 hpf are generally inactive with only occasional episodes of spontaneous “burst” swimming (Brustein et al., 2003), we assessed early swimming behavior by touching the caudal trunk region with a fine eyelash probe. Control fish consistently responded to this stimulus by swimming at least twice around the perimeter of the well. Exposure to OPs did not alter the tendency of zebrafish to swim along the wall of the well; however, it did affect swimming distance. Therefore, as a semiquantitative assessment of swimming behavior, an observer blinded to treatment noted the number of circles completed (to the nearest half circle) by each zebrafish in response to touch. This parameter was subsequently converted into distance by multiplying the number of circles completed by the circumference of the well (20 mm).
Morphometric analyses of neuronal development.
Whole-mount immunohistochemistry was performed in fixed zebrafish larvae as previously described (Svoboda et al., 2002) using well-characterized antibodies to visualize specific subsets of neurons and their axons. Primary motoneurons were immunolabeled using znp-1 (Developmental Studies Hybridoma Bank, University of Iowa); secondary motoneurons were immunolabeled using zn-5 (Developmental Studies Hybridoma Bank). Endogenous GFP fluorescence or fluorescence emitted by tagged secondary antibodies used in whole-mount immunohistochemistry was visualized using a Spot digital camera and Spot image acquisition software (Diagnostic Instruments Inc., Sterling Heights, MI). An observer blinded to the experimental conditions performed all morphometric analyses. To calculate innervation frequency, a minimum of six segments (segments 7–14) were analyzed in the trunk region of each spinal cord spanning the yolk sac extension. Innervation frequency was expressed as the number of segments exhibiting znp-1 or zn-5 immunopositive axons relative to the total number of segments analyzed. Axonal length was quantified using Spot image analysis software, and the average axonal length from each fish was normalized to its body width. To determine body width, a straight line was drawn across the trunk region above the end of the yolk extension.
Immunohistochemical staining of axial muscle.
To evaluate differentiation of axial musculature and organization of myofibers, the muscle-specific F59 and F310 antibodies (Developmental Studies Hybridoma Bank, University of Iowa) were used for whole animal immunohistochemistry at a 1:10 dilution as previously described (Behra et al., 2002; Birely et al., 2005). Rhodamine-conjugated α-bungarotoxin (BTX; 1:1000 dilution; Molecular Probes, Eugene, OR) was used to label neuromuscular junctions as previously described (Behra et al., 2002).
Statistical analysis.
All data are presented as the mean ± SEM unless stated otherwise. The chi-squared (X)2 test followed by Fisher’s exact test was used to determine statistically significant differences between treatment groups with respect to the frequency of segmental innervations and the percentage of developmental defects. All other data were analyzed by one-way ANOVA for treatment effects, and if significant treatment effects were identified (p < 0.05), Tukey’s multiple comparison test was used post hoc to identify statistically significant differences between treatment groups.
RESULTS
Uptake of CPF and CPFO in Zebrafish Following Static Waterborne Exposure
To determine uptake of CPF and CPFO into zebrafish following static waterborne exposure, we analyzed the concentrations of CPF, CPFO, and TCPy in homogenates of whole wild-type (AB line) zebrafish following incubation for varying periods of time in fish water supplemented with CPF or CPFO. As shown in Table 1, exposure of zebrafish to CPF at 1μM resulted in detectable tissue levels of CPF and these levels increased with increasing exposure time. CPFO was not detected in CPF-exposed zebrafish, whereas TCPy was detected but at very low levels, ranging from 0.4 to 0.8% of CPF tissue levels at 24 and 72 hpf, respectively. CPFO was also not detected in zebrafish embryos exposed directly to CPFO at 0.1μM for 48 h. However, zebrafish exposed to CPFO did have detectable levels of TCPy that were twice the highest levels of TCPy detected in zebrafish exposed to CPF (Table 1).
TABLE 1.
Levels of CPF, CPFO, and TCPy Detected in Zebrafish Exposed to Either CPF or CPFO During Early Developmental Stages
| Exposure | Exposure duration (hpf) | CPF (ng/embryo) | CPFO (ng/embryo) | TCPy (ng/embryo) | TCPy (ng/mg)a |
| CPF (1μM) | 6–24 | 11.06 | BDL | 0.04 | 0.044 |
| CPF (1μM) | 6–48 | 32.48 | BDL | 0.16 | 0.18 |
| CPF (1μM) | 6–72 | 36.86 | BDL | 0.29 | 0.32 |
| Vehicle | 24–72 | — | BDL | BDL | BDL |
| CPFO (0.1μM) | 24–72 | — | BDL | 0.60 | 0.67 |
Note. BDL, below the detection limit.
Weight wet.
To further assess the bioavailability of CPF and CPFO following static waterborne exposure, AChE activity was measured in wild-type zebrafish exposed to varying concentrations (3nM–1μM) of CPF, CPFO, and TCPy for 24 or 48 h. OP exposures were initiated at 24 hpf and zebrafish were collected at 48 or 72 hpf for AChE activity assays (Fig. 1A). AChE activity was significantly inhibited in a concentration-dependent manner in zebrafish exposed to CPFO for 24 or 48 h (Figs.1D and E). In contrast, exposure to either CPF (Figs. 1B and C) or TCPy (Figs. 1F and G) over the same concentration range and exposure periods had no significant effect on AChE activity.
OP Exposures and Zebrafish Teratology
To screen for gross teratogenic effects, wild-type zebrafish exposed from 24 to 72 hpf to CPF, CPFO, or TCPy at concentrations ranging from 0.01 to 1μM were scored for (1) absence of eyes; (2) structural malformation of the snout, jaw, otic vesicles, notochord, somite, or fin; (3) edema in the heart, brain, pericardial, and yolk sac region; (4) curvature of the body axis; and (5) reduced pigmentation as previously described (Svoboda et al., 2002). Of these, the only teratogenic effects observed under these exposure conditions were pericardial edema, curvature of the body axis, and pigmentation (Table 2). Significant increases in the percentage of zebrafish exhibiting defects in these endpoints relative to vehicle controls were only observed in the group exposed to CPFO at 1μM (Table 2). None of the exposures, including 1μM CPFO, significantly increased mortality relative to vehicle controls (Table 2).
TABLE 2.
Developmental Abnormalities Observed Among Zebrafish Exposed to OPs From 24 to 72 hpf
| OP (μM) | Mortalityab | Pericardial edemab | Curvature of body axisb | Reduced pigmentationb | |
| CPF | |||||
| 0 | 1 | 1 | 0 | 0 | |
| 0.01 | 0 | 1 | 0 | 0 | |
| 0.1 | 2 | 1 | 1 | 0 | |
| 1 | 0 | 0 | 0 | 0 | |
| CPFO | |||||
| 0 | 2 | 2 | 2 | 0 | |
| 0.01 | 7 | 6 | 2 | 0 | |
| 0.1 | 5 | 6 | 6 | 0 | |
| 1 | 13 | 16* | 74*** | 42*** | |
| TCPy | |||||
| 0 | 0 | 0 | 0 | 0 | |
| 0.01 | 3 | 0 | 0 | 0 | |
| 0.1 | 1 | 0 | 3 | 0 | |
| 1 | 1 | 0 | 0 | 0 | |
Zebrafish were scored as dead if there was no sign of a visibly beating heart and the fish failed to move in response to touch.
Values represent the % of zebrafish exhibiting the defect (n = 43–54 fish per group).
*p < 0.05, ***p < 0.001 relative to vehicle control as determined using X2 test followed by Fisher’s exact test.
CPFO but not CPF or TCPy Alters Swimming Behavior
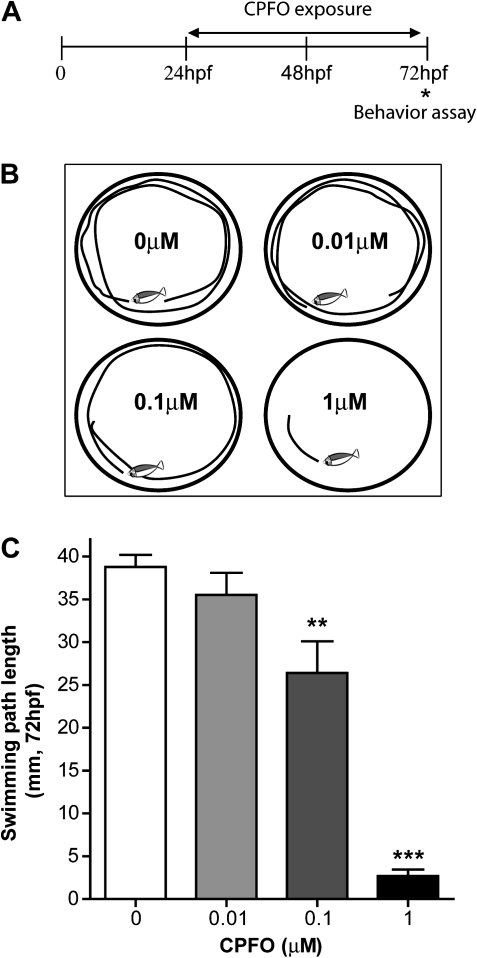
Motor activity develops in a stereotypic sequence in developing zebrafish and consists of an early period of transient spontaneous coiling contractions, followed by twitching responses to touch beginning at 27 hpf, and a vigorous swimming response to tactile stimulation by 36–42 hpf (Granato and Nusslein-Volhard, 1996; Ribera and Nusslein-Volhard, 1998; Saint-Amant and Drapeau, 1998). Thus, to determine whether developmental exposures to OPs alter swimming behavior, wild-type zebrafish were exposed from 24 to 72 hpf to CPF, CPFO, or TCPy at concentrations ranging from 0.01 to 1μM. At the end of the exposure period, touch-induced swimming behavior was assessed by quantifying the distance swam by individual zebrafish in response to tactile simulation (Fig. 2B). CPFO exposure caused a concentration-dependent decrease in swimming path length with significant effects observed at concentrations ≥ 0.1μM (Fig. 2C). In contrast, exposure to either CPF or TCPy over the same concentration range and exposure period had no significant effect on touch-induced swimming behavior (data not shown).
FIG. 2.
CPFO decreases the touch-induced swimming burst in wild-type zebrafish. (A) Swimming behavior was assessed in zebrafish (AB line) exposed to varying concentrations of CPFO for 48 h beginning at 24 hpf. Swimming behavior was assessed at 72 hpf. (B) Representative traces of the swim path in response to touch in zebrafish exposed to vehicle (0μM) or varying concentrations of CPFO. (C) Quantification of the swim path length confirms that CPFO causes a concentration-dependent decrease in the distance swam. **Significantly different from vehicle control at p < 0.01; ***p < 0.001 (n = 24 per experimental condition).
CPFO, but not CPF, Alters Axonal Growth in Neuronal Cell Types That Regulate Touch-Induced Swimming Behavior
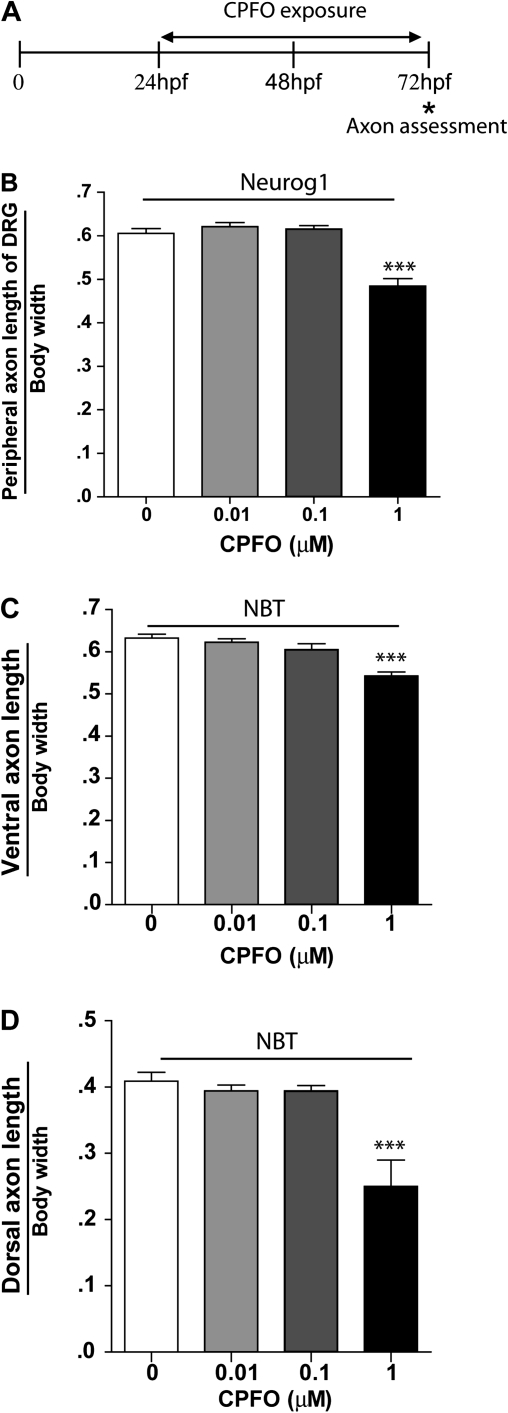
The deficits in touch-induced swimming behavior in zebrafish treated with CPFO suggested a problem with neuromusculature function and/or perception of touch stimuli. Rohon-Beard and DRG sensory neurons are early sensory neurons that detect touch stimuli and initiate an escape response in developing zebrafish (Clarke et al., 1984). Because we previously demonstrated that CPF and CPFO interfere with axonal outgrowth of sensory neurons cultured from embryonic rat DRG (Yang et al., 2008), we first examined whether exposure to CPF or CPFO from 24 to 72 hpf altered patterns of axonal growth in transgenic Neurog1 zebrafish that express GFP in Rohon-Beard and DRG sensory neurons. CPF at concentrations ranging from 0.01 to 1μM had no effect on either the length or the trajectory of GFP-positive axons in Neurog1 zebrafish (data not shown). In contrast, CPFO at 1μM significantly decreased the length of GFP-positive axons in these transgenic zebrafish (Fig. 3B).
FIG. 3.
CPFO inhibits axonal growth in sensory neurons and primary motoneurons. (A) Transgenic Neurog1 zebrafish that express GFP in Rohon-Beard and DRG sensory neurons and transgenic NBT zebrafish that express GFP in primary motoneurons were exposed to varying doses of CPFO from 24 to 72 hpf. Zebrafish were fixed at 72 hpf for subsequent morphometric analyses. (B) Quantification of the length of the peripheral axon of GFP-positive sensory neurons indicated that CPFO at 1μM significantly decreased axonal length in Neurog1 zebrafish. CPFO at 1μM similarly inhibited axonal outgrowth in GFP-positive primary motoneurons in NBT zebrafish, as indicated by a significant decrease in the length of znp-1 immunoreactive ventral (C) and dorsal (D) axons. ***Significantly different from vehicle control at p < 0.001 (n = 12–13 per experimental condition).
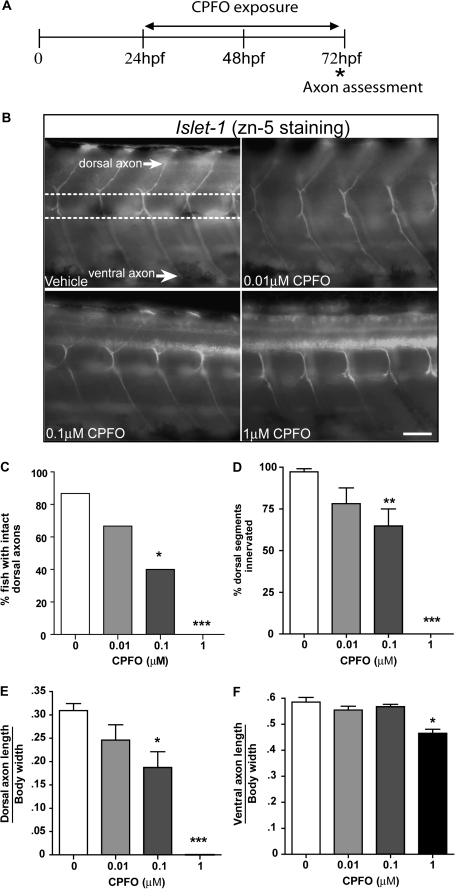
Deficits in touch-induced swimming behavior were observed at CPFO concentrations less than 1μM; therefore, we next analyzed effects of CPF and CPFO on the axonal growth of motoneurons to determine whether these neuronal cell types were more sensitive than sensory neurons to the axon inhibiting effects of CPFO. Transgenic zebrafish expressing GFP under the control of either the NBT or the islet-1 promoter were used to assess axonal growth patterns in primary and secondary motoneurons, respectively. As observed in sensory neurons, exposure from 24 to 72 hpf to CPF at concentrations ranging from 0.01 to 1μM had no effect on GFP-positive axons in either NBT or islet-1 transgenic zebrafish (data not shown). However, CPFO inhibited axonal growth of GFP-positive neurons in both transgenic lines. Significantly decreased lengths of both ventral and dorsal neurons of primary motoneurons were observed in NBT zebrafish exposed to CPFO at 1μM (Figs. 3C and D). In islet-1 zebrafish, CPFO similarly inhibited axonal growth (Figs. 4E and F), but significant effects were observed at the lower concentration of 0.1μM, corresponding to the same concentration range observed to impair touch-induced swimming behavior (Fig. 2). To confirm that CPFO exposure was not interfering with GFP expression, islet-1 zebrafish exposed to CPFO from 24 to 72 hpf were immunostained for zn-5, which is a marker of secondary motoneurons axons (Beattie et al., 1997; Fashena and Westerfield, 1999). The growth of zn-5-immunopositive axons was significantly inhibited in islet-1 zebrafish exposed to CPFO at either 0.1 or 1μM (Fig. 4). Dorsal axons were more susceptible to this effect than were ventral axons as determined by both a shift in the concentration-effect relationship to the left for dorsal versus ventral axons and a more dramatic effect on dorsal relative to ventral axons at 1μM CPFO (Fig. 4).
FIG. 4.
CPFO inhibits axonal growth in secondary motoneurons. (A) Transgenic islet1 zebrafish that express GFP in secondary motoneurons were exposed to varying concentrations of CPFO from 24 to 72 hpf. Zebrafish were fixed at 72 hpf for subsequent morphometric analyses. (B) Representative photomicrographs illustrating zn-5 immunopositive axons in dorsal and ventral trunk regions. The dotted lines indicate the common path along which secondary motoneurons migrate; the upper dotted line indicates the origin of both the dorsal and the ventral axon. Innervation frequency and the length of both ventral and dorsal axons were quantified in the trunk region (a minimum of six segments). CPFO exposure caused a concentration-dependent decrease in axonal outgrowth of secondary motoneurons as evidenced by a significantly decreased percentage of zebrafish larvae with intact dorsal axons (C), a decreased percentage of dorsal segments innervated by secondary motoneuron axons in any given zebrafish larva (D), and a significant decrease in the length of both dorsal (E) and ventral (F) axons. *Significantly different from vehicle control at p < 0.05; **p < 0.01; ***p < 0.001 (n = 15 per experimental condition). Bar = 50 μm.
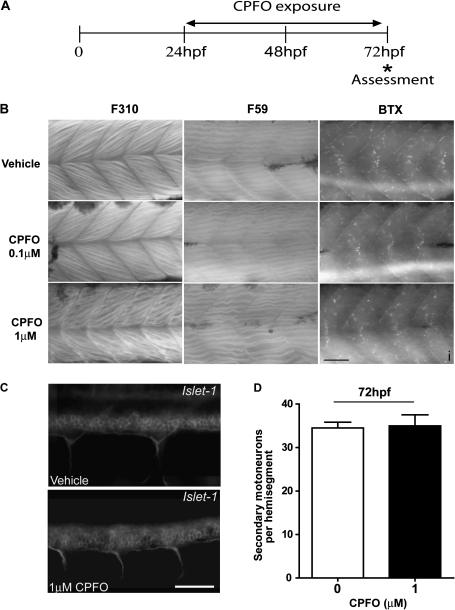
CPFO Effects on Somatic Muscle Development and Number of Secondary Motoneurons
Signals derived from somatic muscle influence axonal growth of motoneurons during embryonic development (Beattie, 2000; Zeller et al., 2002); therefore, we investigated the effects of CPFO on the differentiation of the axial musculature by immunostaining islet-1 zebrafish exposed to CPFO with antibody F59, which predominantly recognizes slow muscle myosin fibers (Behra et al., 2002), and antibody F310, which specifically recognizes the myosin heavy chain of fast muscle fibers (Birely et al., 2005). Subtle alterations in the arrangement and integrity of myofibers as determined by F59 and F310 immunostaining were apparent in zebrafish exposed to CPFO at 1μM from 24 to 72 hpf; however, in zebrafish exposed to CPFO at 0.1μM over the same developmental time frame, the pattern of F59 and F310 immunoreactivity was comparable with that observed in vehicle controls (Fig. 5B). To determine whether CPFO alters the development of neuromuscular junctions, somatic muscle was labeled with rhodamine-conjugated α-BTX, which specifically binds to the nicotinic acetylcholine receptor (nAChR). Exposure to CPFO at either 0.1 or 1μM had no obvious effect on the number, size, or distribution of nAChR relative to vehicle controls (Fig. 5B).
FIG. 5.
CPFO has minimal effect on axial muscle development and number of secondary motoneurons. (A) Transgenic islet1 zebrafish were exposed to varying concentrations of CPFO from 24 to 72 hpf. (B) Representative photomicrographs of somatic muscle stained with antibody F310 (fast muscle fiber marker), F59 (slow muscle fiber marker), or BTX (neuromuscular junction marker). Expression patterns of fast and slow muscle myosin are not noticeably altered by exposure to CPFO at 0.1μM relative to controls, but subtle changes are apparent in zebrafish exposed to CPFO at 1μM. Exposure to CPFO at either concentration does not cause significant changes in either the distribution or the density of nicotinic neuromuscular junctions in the same trunk region. Comparable data were observed in four of four zebrafish examined per treatment group. (C) Representative photomicrographs of endogenous GFP fluorescence in the rostral spinal cord of islet1 zebrafish exposed to vehicle or 1μM CPFO. (D) CPFO exposure did not affect the average number of secondary motoneurons in segments 7–12 of the spinal cord region (n = 4 fish per experimental condition). Bar = 50 μm.
To distinguish between the possibilities that CPFO generally depressed secondary motoneuron development versus specifically inhibited axonal growth, we assessed the effect of CPFO on the number of GFP-positive neuronal cell bodies in the rostral spinal cord of islet-1 zebrafish. As illustrated in Figure 5C, exposure from 24 to 72 hpf to CPFO at 1μM had no effect on the apparent density of secondary motoneuron cell bodies. Quantification of the number of GFP-positive neurons per hemi-segment confirmed that CPFO did not alter the density of secondary motoneurons in the rostral spinal cord (Fig. 5D).
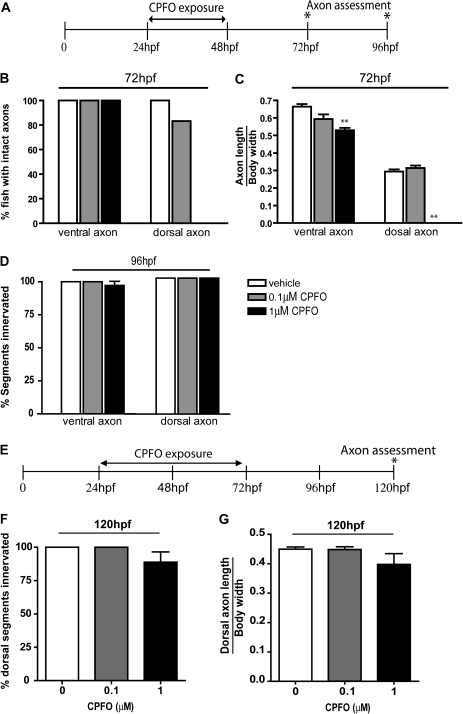
CPFO Effects on Axonal Growth of Secondary Motoneurons Are Reversible
To determine whether shorter exposures to CPFO inhibit axonal growth and whether the inhibitory effects are transient, we examined axonal growth of GFP-positive neurons in islet zebrafish that were exposed to CPFO at 0.1 or 1μM for either 24 or 48 h and then transferred to fresh water not supplemented with CPFO for an additional 24 or 48 h prior to fixation for morphometric analyses. Exposure to CPFO at 0.1 and 1.0μM from 24 to 48 hpf for a total exposure time of 24 h was sufficient to significantly decrease the percentage of zebrafish with intact dorsal axons (Fig. 6B) as well as the length of both ventral and dorsal axons (Fig. 6C) when measured at 72 hpf. As observed, following a 48-h exposure to CPFO (Fig. 4), the dorsal axons of secondary motoneurons were impacted to a greater extent than ventral axons following a 24-h exposure to CPFO (Fig. 6C). By 96 hpf (48 h after CPFO exposure ended), axonal growth had completely recovered to vehicle control levels (Fig. 6D). Similarly, the effects of a 48-h exposure to CPFO at 0.1 or 1μM on the percentage of dorsal segment innervated (Fig. 6F), and the length of dorsal axons (Fig. 6G) was largely reversed by 48 h after the end of CPFO exposure.
FIG. 6.
The inhibitory effects of CPFO on axonal growth are reversible. (A) Transgenic islet1 zebrafish were exposed to varying concentrations of CPFO from 24 to 48 hpf. At 48 hpf, zebrafish were transferred to fresh fish water containing no CPFO. A subset of these fish was fixed at 72 hpf and the remainder at 96 hpf for morphometric analyses. Analyses of the percentage of segments with intact axons in GFP-positive neurons (B) and the length of zn-5 immunopositive axons at 72 hpf (C) indicated that the inhibitory effects of CPFO on axonal outgrowth in secondary motoneurons persisted 24-h post-exposure in zebrafish exposed to CPFO at 1 but not 0.1μM. By 48-h post-exposure (96 hpf), the percentage of segments innervated by either ventral or dorsal axons of secondary motoneurons did not differ between treatment groups (D). (E) Islet zebrafish were exposed to varying concentrations of CPFO from 24 to 72 hpf and then transferred to fresh CPFO-free fish water for 48 h prior to being fixed for morphometric analyses at 120 hpf. No statistically significant differences were noted between treatment groups with respect to either the percentage of dorsal segments innervated by zn-5 immunopositive axons (F) or the length of the dorsal axons of secondary motoneurons (G). *Significantly different from vehicle control at p < 0.05; **p < 0.01; ***p < 0.001 (n = 15 per experimental condition).
DISCUSSION
We previously reported that CPF and CPFO inhibit axonal growth in primary neurons in vitro (Howard et al., 2005; Yang et al., 2008). In this study, we provide novel data demonstrating similar effects on axonal growth in vivo coincident with neurobehavioral deficits. Specifically, in zebrafish exposed to CPFO from 24 to 72 hpf, axonal growth was significantly decreased in sensory neurons and primary motoneurons at 1.0μM and in secondary motoneurons at ≥ 0.1μM, and touch-induced swimming response was significantly attenuated at concentrations ≥ 0.1μM.
Our observations of inhibited axonal growth are consistent with recently published photomicrographs illustrating aberrant axonal projection pathways of Rohon-Beard neurons in zebrafish exposed to CPFO at 0.3μM from 3 to 72 hpf (Jacobson et al., 2010). Our quantitative analyses of CPFO effects on axon outgrowth confirm and extend these earlier quantitative data to indicate that secondary motoneurons are more susceptible to axon growth inhibition by CPFO than either sensory neurons or primary motoneurons as evidenced by a shift in the concentration-effect relationship to the left for secondary motoneurons relative to sensory neurons and primary neurons. Among secondary motoneurons, dorsal axons were more susceptible to inhibition than ventral axons, as seen by not only a shift in the concentration-effect curve to the left but also a more robust inhibition of their growth relative to that of ventral axons. The differential concentration-effect relationship between neuronal cell types and between processes within a specific neuronal cell population suggests that CPFO inhibition of axonal growth is not due to a general cytotoxic effect on neurons. In support of this conclusion, CPFO inhibited axonal growth at concentrations that did not cause mortality, gross developmental defects, or reduced numbers of secondary motoneurons, and the effects on axonal growth were reversible upon termination of CPFO exposure. Collectively, these observations suggest that CPFO targets specific molecular cues that regulate axonal growth.
Interactions with target tissue influence axonal growth of sensory neurons and motoneurons during embryonic development (Beattie, 2000; Zeller et al., 2002); therefore, we determined whether exposure to CPFO interfered with development of somatic muscle, which is a major target of the neuronal cell types whose axonal growth was altered by CPFO. Immunohistochemical analyses indicated that although 1.0μM CPFO did cause subtle changes in the myofibril organization of axial somatic muscle, 0.1μM CPFO did not cause notable changes relative to vehicle controls. Moreover, the distribution and density of nAChR were not altered by any of the concentrations of CPFO used in these studies. These observations are consistent with previous reports that zebrafish exposed to CPFO at 0.3μM from 3 to 72 hpf exhibited normal muscle fiber formation as analyzed by birefringence and normal nAChR cluster formation as determined by quantitative analyses of α-BTX staining (Jacobson et al., 2010). Collectively, these data suggest that inhibition of axonal growth is not an indirect effect of CPFO disruption of muscle development but rather CPFO targets neurons directly to inhibit axonal growth. This conclusion is supported by our previous studies demonstrating that CPFO inhibits axonal growth of cultured neurons (Howard et al., 2005; Yang et al., 2008).
Our earlier studies of axonal growth inhibition by CPF and CPFO in primary neuronal cell cultures derived from embryonic rat pups suggested that these OPs interfere with the morphogenic activity of AChE (Howard et al., 2005; Yang et al., 2008). This conclusion was based in part on observations that axonal growth was inhibited at concentrations of CPF and CPFO that did not inhibit the enzymatic activity of AChE. In contrast, in zebrafish, axonal growth was inhibited only at CPFO concentrations that also inhibited the enzymatic activity of AChE. Acetylcholine is a morphogen (Lauder and Schambra, 1999), so it is possible that in zebrafish, the inhibitory effects of CPFO on axonal growth are mediated by increased levels of acetylcholine secondary to inhibition of the enzymatic activity of AChE. Such a mechanism would be consistent with reports that (1) transient exposure to nicotine alters axonal pathfinding of secondary motoneurons in embryonic zebrafish (Svoboda et al., 2002) and (2) expression of an ache mutation that completely abolishes AChE enzymatic activity in the zebrafish embryo causes abnormal targeting of motoneuron axons, and this phenotype is rescued by homozygous expression of a loss-of-function allele of the α-subunit of the nAChR (Behra et al., 2002). However, the effects of transient nicotine exposure on secondary motoneuron axons were persistent (Svoboda et al., 2002), in contrast to the reversible effects of transient CPFO exposure on axonal growth, and the inactivation of nAChR in the ache mutant zebrafish did not rescue morphogenic defects in sensory neurons (Behra et al., 2002). These observations suggest that mechanisms other than inhibition of AChE enzymatic activity contribute to axonal growth inhibition by CPFO in zebrafish.
An alternate explanation that would be consistent with our current findings in zebrafish and previous evidence demonstrating noncholinergic mechanisms of CPF developmental neurotoxicity (Costa, 2006; Slotkin, 2004a), including our previous observations of cultured rat neurons (Howard et al., 2005; Yang et al., 2008), is that CPFO inhibits axonal growth by interfering with the morphogenic activity of AChE but that the relative dose-response relationships for inhibition of the morphogenic versus enzymatic activities of AChE overlap in zebrafish but not in rat-derived neuronal cell cultures. Several lines of evidence support this possibility. First, there is evidence that AChE is an axonal morphogen in zebrafish: AChE is expressed in motor neurons and sensory neurons of zebrafish during early embryonic stages, and AChE is required for normal axonal projections of these neuronal cell types (Behra et al., 2002). Second, interactions between the acylation site and peripheral anionic site are thought to influence the morphogenic activity of AChE (Sternfeld et al., 1998; Yang et al., 2008). Various OPs, including CPF and CPFO, bind to not only the acylation site but also the peripheral anionic site, and ligand binding to either site influences functionally significant conformational interactions between these sites (De Ferrari et al., 2001; Kousba et al., 2004; Rosenfeld and Sultatos, 2006). These interactions may have different kinetics in zebrafish versus rodents: Species-specific differences have been documented in the amino acid sequence and protein structure of AChE (Bertrand et al., 2001) as well as in the kinetics of OP inhibition of AChE enzymatic activity (Wang and Murphy, 1982; Johnson and Wallace, 1987). Although further testing is required to confirm that CPFO inhibits axonal growth in zebrafish by interfering with the morphogenic activity of AChE, these findings suggest the novel hypothesis that age-related differences in AChE function contribute to the age-related vulnerability to OP neurotoxicity.
An interesting finding of this study is that CPF has no effect on either axonal growth or touch-induced swimming response in zebrafish at 72 hpf. HPLC analyses indicated that the lack of effect is not due to inability of zebrafish to take up CPF from the water. An alternative explanation is that at early developmental stages, zebrafish lack the metabolic enzymes needed to activate CPF. In support of this possibility, Linney and colleagues have reported that CPF does not significantly inhibit AChE in zebrafish during the first 72 hpf, although this activity is manifested by 96 hpf (Aschner et al., 2010). Similarly, we observed that CPFO but not CPF inhibits AChE enzymatic activity in zebrafish at developmental ages < 72 hpf. To explore this further, we used HPLC to quantify CPFO and TCPy levels in zebrafish exposed to the parent or oxon OP. CPFO was not detected in zebrafish exposed to CPF; however, CPFO was also not detected in zebrafish exposed to CPFO. These findings are consistent with previous studies of animals treated with these compounds (Barron et al., 1991; Drevenkar et al., 1993; Hunter et al., 1999; Ivey, 1979; Mann et al., 1973; McKellar et al., 1976) and likely reflect the highly reactive nature of CPFO. An alternative approach to assessing CPF metabolism is to measure the inactive metabolite TCPy. However, TCPy is generated not only by paraoxonase-mediated detoxification of CPFO but also by cytochrome P450-mediated dearylation of CPF (Foxenberg et al., 2007). To distinguish between these possibilities, we compared levels of TCPy in zebrafish exposed to CPF at 1.0μM for 24, 48, or 72 hpf versus zebrafish exposed to CPFO at 0.1μM from 24 to 72 hpf. If zebrafish were capable of metabolically activating CPF, then exposure to CPF at a concentration 10 times that of CPFO would be expected to result in TCPy tissue levels in the CPF-exposed fish that were significantly greater than the levels observed in the CPFO-exposed fish. However, our findings were contrary to this prediction: TCPy levels in CPFO-exposed zebrafish were more than two times higher than those detected in CPF-exposed zebrafish. Collectively, these data indicate that at < 72 hpf, zebrafish lack the metabolic capacity to activate CPF.
Our data suggest that changes in axonal growth contribute to the motor deficits observed in zebrafish exposed to CPFO. The critical target appears to be secondary motoneurons because the concentration-effect relationship for CPFO inhibition of axonal growth in secondary motoneurons was comparable with that for CPFO attenuation of the touch-induced swim response. Studies in rodent models have shown that experimental manipulations that delay but do not prevent cortical axons from innervating target tissue cause persistent behavioral deficits (Berger-Sweeney and Hohmann, 1997). So even though adverse effects on axonal growth were reversible upon removal of CPFO, the transient inhibition of axonal growth could be a mechanism contributing to the persistent neurobehavioral deficits observed in fish and rodent models following developmental exposure to CPF (Jett et al., 2001; Levin et al., 2001, 2002, 2003, 2004). Transient changes in axonal growth resulting in persistent changes in neuronal connectivity could also provide a unifying mechanism to explain the neurochemical changes across multiple neurotransmitter systems that have been documented in various animal models of developmental CPF exposure by Slotkin and colleagues (Slotkin and Seidler, 2007, 2008; Slotkin et al., 2001, 2002, 2004, 2008).
In summary, our data suggest that altered patterns of neuronal connectivity contribute to the developmental neurotoxicity of CPF and demonstrate the relevance of zebrafish as a model for studying OP developmental neurotoxicity.
FUNDING
Johns Hopkins University Center for Alternatives to Animal Testing (project number 2007-27 to P.J.L. and R.L.T.); National Institute of Environmental Health Sciences (ES016308 to W.K.A. and P.J.L. and ES00210 to R.L.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health sciences or the National Institutes of Health.
References
- Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch. Environ. Health. 2003;58:484–497. doi: 10.3200/AEOH.58.8.484-497. [DOI] [PubMed] [Google Scholar]
- Adgate JL, Barr DB, Clayton CA, Eberly LE, Freeman NC, Lioy PJ, Needham LL, Pellizzari ED, Quackenboss JJ, Roy A, et al. Measurement of children’s exposure to pesticides: analysis of urinary metabolite levels in a probability-based sample. Environ. Health Perspect. 2001;109:583–590. doi: 10.1289/ehp.01109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Levin ED, Sunol C, Olopade JO, Helmcke KJ, Avila DS, Sledge D, Ali RH, Upchurch L, Donerly S, et al. Gene-environment interactions: neurodegeneration in non-mammals and mammals. Neurotoxicology. 2010;31:582–588. doi: 10.1016/j.neuro.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone S, Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology. 2000;21:15–36. [PubMed] [Google Scholar]
- Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Jr, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ, et al. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ. Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MG, Plakas SM, Wilga PC. Chlorpyrifos pharmacokinetics and metabolism following intravascular and dietary administration in channel catfish. Toxicol. Appl. Pharmacol. 1991;108:474–482. doi: 10.1016/0041-008x(91)90093-t. [DOI] [PubMed] [Google Scholar]
- Beattie CE. Control of motor axon guidance in the zebrafish embryo. Brain Res. Bull. 2000;53:489–500. doi: 10.1016/s0361-9230(00)00382-8. [DOI] [PubMed] [Google Scholar]
- Beattie CE, Hatta K, Halpern ME, Liu H, Eisen JS, Kimmel CB. Temporal separation in the specification of primary and secondary motoneurons in zebrafish. Dev. Biol. 1997;187:171–182. doi: 10.1006/dbio.1997.8604. [DOI] [PubMed] [Google Scholar]
- Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, Strahle U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 2002;5:111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Hohmann CF. Behavioral consequences of abnormal cortical development: insights into developmental disabilities. Behav. Brain Res. 1997;86:121–142. doi: 10.1016/s0166-4328(96)02251-6. [DOI] [PubMed] [Google Scholar]
- Bertrand C, Chatonnet A, Takke C, Yan YL, Postlethwait J, Toutant JP, Cousin X. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7. Gene structure and polymorphism; molecular forms and expression pattern during development. J. Biol. Chem. 2001;276:464–474. doi: 10.1074/jbc.M006308200. [DOI] [PubMed] [Google Scholar]
- Bigbee JW, Sharma KV, Gupta JJ, Dupree JL. Morphogenic role for acetylcholinesterase in axonal outgrowth during neural development. Environ. Health Perspect. 1999;107(Suppl. 1):81–87. doi: 10.1289/ehp.99107s181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birely J, Schneider VA, Santana E, Dosch R, Wagner DS, Mullins MC, Granato M. Genetic screens for genes controlling motor nerve-muscle development and interactions. Dev. Biol. 2005;280:162–176. doi: 10.1016/j.ydbio.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Brimijoin S, Koenigsberger C. Cholinesterases in neural development: new findings and toxicologic implications. Environ. Health Perspect. 1999;107(Suppl. 1):59–64. doi: 10.1289/ehp.99107s159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustein E, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Drapeau P. Steps during the development of the zebrafish locomotor network. J. Physiol. Paris. 2003;97:77–86. doi: 10.1016/j.jphysparis.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J. Physiol. 1984;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin. Chim. Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Carleton A, Goridis C, Vincent JD, Lledo PM. Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl CL, Fenske RA, Elgethun K. Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ. Health Perspect. 2003;111:377–382. doi: 10.1289/ehp.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol. Appl. Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- Davis DL, Ahmed AK. Exposures from indoor spraying of chlorpyrifos pose greater health risks to children than currently estimated. Environ. Health Perspect. 1998;106:299–301. doi: 10.1289/ehp.98106299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Mallender WD, Inestrosa NC, Rosenberry TL. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J. Biolog. Chem. 2001;276:23282–23287. doi: 10.1074/jbc.M009596200. [DOI] [PubMed] [Google Scholar]
- Dodd A, Curtis PM, Williams LC, Love DR. Zebrafish: bridging the gap between development and disease. Hum. Mol. Genet. 2000;9:2443–2449. doi: 10.1093/hmg/9.16.2443. [DOI] [PubMed] [Google Scholar]
- Drevenkar V, Vasilic Z, Stengl B, Frobe Z, Rumenjak V. Chlorpyrifos metabolites in serum and urine of poisoned persons. Chem. Biol. Interact. 1993;87:315–322. doi: 10.1016/0009-2797(93)90059-8. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008;38(Suppl. 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Ecobichon DJ. Organophosphorus ester insecticides. In: Ecobichon DJ, Joy RM, editors. Pesticides and Neurological Diseases. Boca Raton, FL: CRC Press; 1994. pp. 171–249. [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol. Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr., Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. Journal Epidemiol. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ. Health Perspect. 1999;107(Suppl. 3):409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fashena D, Westerfield M. Secondary motoneuron axons localize DM-GRASP on their fasciculated segments. J. Comp. Neurol. 1999;406:415–424. doi: 10.1002/(sici)1096-9861(19990412)406:3<415::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR. Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos. Drug Metab. Dispos. 2007;35:189–193. doi: 10.1124/dmd.106.012427. [DOI] [PubMed] [Google Scholar]
- Granato M, Nusslein-Volhard C. Fishing for genes controlling development. Curr. Opin. Genet. Dev. 1996;6:461–468. doi: 10.1016/s0959-437x(96)80068-2. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol. Appl. Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Hunter DL, Lassiter TL, Padilla S. Gestational exposure to chlorpyrifos: comparative distribution of trichloropyridinol in the fetus and dam. Toxicol. Appl. Pharmacol. 1999;158:16–23. doi: 10.1006/taap.1999.8689. [DOI] [PubMed] [Google Scholar]
- Ivey MC. Chlorpyrifos and 3,5,6,-trichloro-2-pyridinol: residues in the body tissues of cattle wearing chlorpyrifos-impregnated plastic ear tags. J. Econ. Entomol. 1979;72:909–911. doi: 10.1093/jee/72.6.909. [DOI] [PubMed] [Google Scholar]
- Jacobson SM, Birkholz DA, McNamara ML, Bharate SB, George KM. Subacute developmental exposure of zebrafish to the organophosphate pesticide metabolite, chlorpyrifos-oxon, results in defects in Rohon-Beard sensory neuron development. Aquat. Toxicol. 2010;100:101–111. doi: 10.1016/j.aquatox.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL. New evidence of effects of organophosphate pesticides on neurodevelopment in children: commentary on the article by Kofman et al. on page 88. Pediatr. Res. 2006;60:22–23. doi: 10.1203/01.pdr.0000220353.05515.d4. [DOI] [PubMed] [Google Scholar]
- Jett DA, Navoa RV, Beckles RA, McLemore GL. Cognitive function and cholinergic neurochemistry in weanling rats exposed to chlorpyrifos. Toxicol. Appl. Pharmacol. 2001;174:89–98. doi: 10.1006/taap.2001.9198. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Wallace KB. Species-related differences in the inhibition of brain acetylcholinesterase by paraoxon and malaoxon. Toxicol. Appl. Pharmacol. 1987;88:234–241. doi: 10.1016/0041-008x(87)90009-3. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kofman O, Berger A, Massarwa A, Friedman A, Jaffar AA. Motor inhibition and learning impairments in school-aged children following exposure to organophosphate pesticides in infancy. Pediatr. Res. 2006;60:88–92. doi: 10.1203/01.pdr.0000219467.47013.35. [DOI] [PubMed] [Google Scholar]
- Kousba AA, Sultatos LG, Poet TS, Timchalk C. Comparison of chlorpyrifos-oxon and paraoxon acetylcholinesterase inhibition dynamics: potential role of a peripheral binding site. Toxicol. Sci. 2004;80:239–248. doi: 10.1093/toxsci/kfh163. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Godbold JH, et al. Pesticides and inner-city children: exposures, risks, and prevention. Environ. Health Perspect. 1999;107(Suppl. 3):431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ. Health Perspect. 1999;107(Suppl. 1):65–69. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein P, Silbergeld EK, Locke P, Goldberg AM. In vitro and other alternative approaches to developmental neurotoxicity testing (DNT) Environ. Toxicol. Pharmacol. 2005;19:735–744. doi: 10.1016/j.etap.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol. Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Brain Res. Dev. Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol. Teratol. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol. Teratol. 2004;26:719–723. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Li W, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol. Lett. 1998;98:139–146. doi: 10.1016/s0378-4274(98)00116-7. [DOI] [PubMed] [Google Scholar]
- Lizardi PS, O'Rourke MK, Morris RJ. The effects of organophosphate pesticide exposure on Hispanic children’s cognitive and behavioral functioning. J. Pediatr. Psychol. 2008;33:91–101. doi: 10.1093/jpepsy/jsm047. [DOI] [PubMed] [Google Scholar]
- Lu C, Knutson DE, Fisker-Andersen J, Fenske RA. Biological monitoring survey of organophosphorus pesticide exposure among pre-school children in the Seattle metropolitan area. Environ. Health Perspect. 2001;109:299–303. doi: 10.1289/ehp.01109299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann HD, Ivey MC, Kunz SE, Hogan BF. Chlorpyrifos, its oxygen analogue and 3,5,6-trichloro-2-pyridinol: residues in the body tissue of turkeys confined in pens on treated soil. J. Econ. Entomol. 1973;66:715–717. doi: 10.1093/jee/66.3.715. [DOI] [PubMed] [Google Scholar]
- McKellar RL, Dishburger HJ, Rice JR, Craig LF, Pennington J. Residues of chlorpyrifos, its oxygen analogue, and 3,5,6-trichloro-2- pyridinol in milk and cream from cows fed chlorpyrifos. J. Agricult. Food Chem. 1976;24:283–286. doi: 10.1021/jf60204a047. [DOI] [PubMed] [Google Scholar]
- Qiao D, Nikitina LA, Buznikov GA, Lauder JM, Seidler FJ, Slotkin TA. The sea urchin embryo as a model for mammalian developmental neurotoxicity: ontogenesis of the high-affinity choline transporter and its role in cholinergic trophic activity. Environ. Health Perspect. 2003;111:1730–1735. doi: 10.1289/ehp.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera AB, Nusslein-Volhard C. Zebrafish touch-insensitive mutants reveal an essential role for the developmental regulation of sodium current. J. Neurosci. 1998;18:9181–9191. doi: 10.1523/JNEUROSCI.18-22-09181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Arcury TA, Quandt SA, Lasarev M, Rothlein J, Travers R, Tamulinas A, Scherer J, Early J, Marin A, et al. Neurobehavioral performance in preschool children from agricultural and non-agricultural communities in Oregon and North Carolina. Neurotoxicology. 2005;26:589–598. doi: 10.1016/j.neuro.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CA, Sultatos LG. Concentration-dependent kinetics of acetylcholinesterase inhibition by the organophosphate paraoxon. Toxicol. Sci. 2006;90:460–469. doi: 10.1093/toxsci/kfj094. [DOI] [PubMed] [Google Scholar]
- Rosas LG, Eskenazi B. Pesticides and child neurodevelopment. Curr. Opin. Pediatr. 2008;20:191–197. doi: 10.1097/MOP.0b013e3282f60a7d. [DOI] [PubMed] [Google Scholar]
- Sachana M, Flaskos J, Alexaki E, Glynn P, Hargreaves AJ. The toxicity of chlorpyrifos towards differentiating mouse N2a neuroblastoma cells. Toxicol. In Vitro. 2001;15:369–372. doi: 10.1016/s0887-2333(01)00038-8. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004a;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Guidelines for developmental neurotoxicity and their impact on organophosphate pesticides: a personal view from an academic perspective. Neurotoxicology. 2004b;25:631–640. doi: 10.1016/S0161-813X(03)00050-0. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod. Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol. Appl. Pharmacol. 2008;233:211–219. doi: 10.1016/j.taap.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Ryde IT, Yanai J. Developmental neurotoxic effects of chlorpyrifos on acetylcholine and serotonin pathways in an avian model. Neurotoxicol. Teratol. 2008;30:433–439. doi: 10.1016/j.ntt.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Southard MC, Adam SJ, Cousins MM, Seidler FJ. Alpha7 nicotinic acetylcholine receptors targeted by cholinergic developmental neurotoxicants: nicotine and chlorpyrifos. Brain Res. Bull. 2004;64:227–235. doi: 10.1016/j.brainresbull.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Brain Res. Dev. Brain Res. 2002;133:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol. Appl. Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Sternfeld M, Ming G, Song H, Sela K, Timberg R, Poo M, Soreq H. Acetylcholinesterase enhances neurite growth and synapse development through alternative contributions of its hydrolytic capacity, core protein, and variable C termini. J. Neurosci. 1998;18:1240–1249. doi: 10.1523/JNEUROSCI.18-04-01240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Vijayaraghavan S, Tanguay RL. Nicotinic receptors mediate changes in spinal motoneuron development and axonal pathfinding in embryonic zebrafish exposed to nicotine. J. Neurosci. 2002;22:10731–10741. doi: 10.1523/JNEUROSCI.22-24-10731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvadia AJ, Linney E. Windows into development: historic, current, and future perspectives on transgenic zebrafish. Dev. Biol. 2003;256:1–17. doi: 10.1016/s0012-1606(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Veronesi B, Pope C. The neurotoxicity of parathion-induced acetylcholinesterase inhibition in neonatal rats. Neurotoxicology. 1990;11:465–482. [PubMed] [Google Scholar]
- Wang C, Murphy SD. Kinetic analysis of species difference in acetylcholinesterase sensitivity to organophosphate insecticides. Toxicol. Appl. Pharmacol. 1982;66:409–419. doi: 10.1016/0041-008x(82)90307-6. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Barr DB. Measurement of organophosphate metabolites in postpartum meconium as a potential biomarker of prenatal exposure: a validation study. Environ. Health Perspect. 2001;109:417–420. doi: 10.1289/ehp.01109417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixon J. Featured organism: Danio rerio, the zebrafish. Yeast. 2000;17:225–231. doi: 10.1002/1097-0061(20000930)17:3<225::AID-YEA34>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol. Appl. Pharmacol. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaim M, Jambulingam P. Global Insecticide Use for Vector-Borne Disease Control. Geneva, Switzerland: World Health Organization; 2009. p. 91. [Google Scholar]
- Zeller J, Schneider V, Malayaman S, Higashijima S, Okamoto H, Gui J, Lin S, Granato M. Migration of zebrafish spinal motor nerves into the periphery requires multiple myotome-derived cues. Dev. Biol. 2002;252:241–256. doi: 10.1006/dbio.2002.0852. [DOI] [PubMed] [Google Scholar]