Abstract
A large group of diseases, termed protein misfolding disorders, share the common feature of the accumulation of misfolded proteins. The possibility of a common mechanism underlying either the pathogenesis or therapy for these diseases is appealing. Thus, there is great interest in the role of protein degradation via autophagy in such conditions where the protein is found in the cytoplasm. Here we review the growing evidence supporting a role for autophagic dysregulation as a contributing factor to protein accumulation and cellular toxicity in certain protein misfolding disorders and discuss the available evidence that upregulation of autophagy may be a valuable therapeutic strategy.
Introduction
Protein misfolding disorders or proteinopathies appear, at the clinical level, to be a diverse group of disorders encompassing many diseases, from late-onset neurodegenerative disorders through to forms of heart failure. These conditions are unified by the common feature of accumulation of misfolded proteins. The specific protein, cell type and cellular localisation of these accumulations vary between the diseases. For example, Parkinson's disease is characterised by the presence of cytoplasmic aggregates of α-synuclein, whereas in the polyglutamine expansion disorders, aggregates are seen predominantly within the nucleus in spinocerebellar ataxia type 1, or in the cytoplasm in adult-onset Huntington's disease. In Alzheimer's disease (AD), both intracellular tau aggregates and extracellular amyloid-β (Aβ) aggregates are seen.
Macroautophagy, which we will refer to as autophagy, is an intracellular process in which cytoplasmic materials are engulfed by double membrane structures, which form autophagosomes. The autophagosomes first fuse with endosomes to form hybrid organelles called amphisomes that later fuse with lysosomes, where the entrapped cytosolic contents are degraded. The mechanisms of autophagy are described elsewhere in this review issue. The process of macroautophagy has been proposed to be important in protein misfolding disorders, both as a contributing factor, through inhibition of the process, and a potential therapeutic strategy, through its upregulation (Figure 1). Here we will discuss the evidence for both these possibilities, concentrating on the neurodegenerative proteinopathies in which misfolded protein accumulation is seen in the central nervous system.
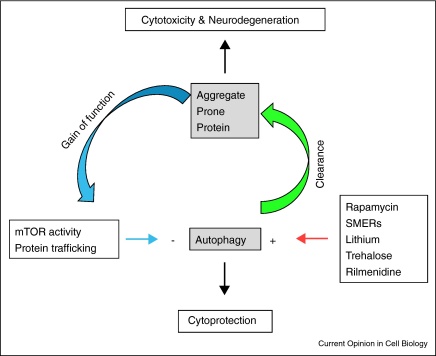
Figure 1.
A model for autophagy regulation in proteinopathies. An interconnected system exists in which autophagy is able to control the levels of intracytoplasmic aggregate-prone proteins (green arrow). However, these proteins are also able to control levels of autophagy (blue arrows). Additionally levels of autophagy are also able to be regulated using drugs (red arrow). The balance between these factors will alter cell survival.
Inhibition of macroautophagy as a contributing factor in proteinopathies
Macroautophagy is induced following various stimuli, the most studied of which is starvation, where upregulation of autophagy acts to provide vital cellular nutrients. In the brain there was thought to be little induction of autophagy following starvation [1], although this view has been challenged by a recent study suggesting profound autophagy upregulation after starvation [2]. Clarification of this issue may require refinement of methods to assess autophagic flux in the brain. In post-mitotic cells, such as neurons, turnover of proteins and organelles is particularly important for cellular quality control, and basal (or constitutive) autophagy appears to be vital to maintain this. Complete knockout of the essential autophagy genes Atg5 or Atg7 in mice causes lethality soon after birth [3,4]. However, selective knockout of these genes in neuronal cells results in a phenotype closely resembling those seen in neurodegenerative diseases, as well as protein aggregation without the expression of a disease-causing protein [5,6].
There is also accumulating evidence for the fact that aggregating, misfolded proteins may have an impact on autophagic function, suggesting that this could be a secondary pathological mechanism in many diseases. This aspect will be discussed below.
Huntington's disease
Huntington's disease (HD) is a hereditary neurodegenerative disease resulting from an expansion of the polyglutamine region of the ubiquitously expressed protein huntingtin (htt) [7]. This mutant protein accumulates inside cells, forming toxic oligomeric species and aggregates. Immunohistochemistry and electron microscopy approaches, either in HD patients or experimental models, have suggested alterations in the autophagic pathway. The earliest evidence comes from observations of increased numbers of autophagic vacuoles across a range of HD models and in patients [8–13]. While it is not clear if this is due to enhanced autophagosome formation or decreased clearance of the vesicles, mTOR is inactivated by cells with mutant huntingtin inclusions, as it is sequestered into aggregates, and this would be compatible with autophagy upregulation [14].
More recently, it has been reported that the autophagic turnover of cytoplasmic components is partially impaired in cells expressing mutant huntingtin (Table 1) [15•]. While autophagosomes are able to form and fuse with lysosomes, the authors report that expression of mutant huntingtin results in inefficient cargo loading, although mutant huntingtin is efficiently delivered to the autophagosomes. This preferentially affects organelle sequestration, in particular that of lipid droplets, which are seen to be increased in Huntington's disease, and mitochondria, dysfunction in which have been widely reported in Huntington's disease [16].
Table 1.
Regulation of autophagy in proteinopathies
| Disease | Mutant protein | Autophagy activity | Mechanism |
|---|---|---|---|
| Alzheimer | – | Inhibition (AV formation) | Beclin-1 targeting by HSV protein ICP34.5 |
| – | Inhibition (AV formation) | Beclin-1 inhibition | |
| PS1 | Inhibition (AV maturation) | Lysosome acidification (v-ATPase targeting) | |
| – | Induction (AV formation) | ROS production | |
| Parkinson | α-Synuclein | Inhibition (AV formation) | Rab1 activity; Atg9 localisation |
| LRRK2 | Inhibition (AV maturation) | MVB formation; UPS impairment | |
| PINK1 | Inhibition (AV formation and mitophagy) | PINK1/Beclin-1 interaction; mitochondria targeting | |
| Parkin | Inhibition (mitophagy) | Mitochondria targeting | |
| DJ-1 | Induction (AV formation) | ROS production; mTOR | |
| Huntington | Huntingtin | Inhibition (selectivity) | Cargo recognition |
| Lafora | Laforin | Inhibition (AV formation) | mTOR activation |
| Malin | Unknown | ||
| ALS | Dynein | Inhibition (AV maturation) | Autophagosome transport to lysosome |
| Dynactin | Unknown | ||
| ESCRT-III | Inhibition (AV maturation) | Autophagosome/lysosome fusion | |
| Fig4 | Inhibition | Decreased PI(3,5)P2 levels | |
| FTD3 | ESCRT-III | Inhibition (AV maturation) | Autophagosome/lysosome fusion |
Further evidence for a contribution of autophagy to pathogenesis in HD comes from recent genetic studies, which suggest that the V471A Atg7 polymorphism is associated with earlier age of onset [17]. Whether or not this polymorphism has any effect on autophagic activity has yet to be established.
Alzheimer's disease
The (Aβ) peptide plaques, which characterise AD, are derived from proteolysis of amyloid precursor protein (APP). Mutations in APP and presenilin (PS1), a protein involved in APP to Aβ proteolysis, cause rare autosomal dominant forms of familial Alzheimer disease (FAD) [18–20]. Sporadic AD, which is far more prevalent, presents the same clinical and pathological characteristics, which suggest that factors affecting the APP to Aβ pathway play a significant role in this form of the disease [21].
A prominent feature of AD is the accumulation of autophagosomes, many containing amyloid-β peptide, in massive numbers within affected neurons [22], probably reflecting defective autophagosome clearance [23]. Changes in the autophagic pathway have been linked to AD through diverse mechanisms, however, it is not clear if autophagosome formation is increased or decreased. A decrease in formation is supported by the reduction in levels of the autophagy protein Beclin-1 [24••] or by inhibition of Beclin-1 activity by the HSV1 (Herpes Simplex Virus Type 1) viral protein ICP34.5 (Table 1) [25]. An increase in autophagosome formation is supported by data suggesting that Aβ is able to induce autophagy via the generation of reactive oxygen species [26•].
Lysosome-related pathology, along with neuronal loss and amyloid deposition, is greatly accentuated in FAD due to mutations of PS1 [27]. PS1 appears to regulate proteolysis during autophagy by targeting the v-ATPase to lysosomes [28••]. PS1 in the ER acts as a chaperone to facilitate maturation and targeting of the v-ATPase V0a1 subunit to lysosomes, which is essential for acidification, protease activation, and degradation of autophagic/lysosomal substrates and could account for the accumulation of autophagosomes seen in AD.
Parkinson's disease
Parkinson's disease (PD) is characterised by the presence of intraneuronal cytoplasmic inclusions known as Lewy bodies, of which α-synuclein is a major constituent [29]. Several mutations have been identified in autosomal recessive forms of PD that provide some insight into the pathogenesis of this disease. Recent studies have linked two such genes, PINK1 and Parkin with mitochondrial clearance and autophagy (Table 1). Parkin has been demonstrated to be recruited to damaged mitochondria and promote their clearance by autophagy [30•] in a manner that is dependent on the stabilisation of Pink1 on the mitochondria [31•,32•,33•,34•]. This translocation is disrupted by mutations in Pink1 or Parkin seen in familial PD [31•,32•]. A direct interaction between Pink1 and Beclin-1 has also been demonstrated recently, promoting autophagosome formation [35], further strengthening the link between autophagy and PD.
Mitochondrial dysfunction has also been linked to mutations of DJ-1 (PARK7), another autosomal recessive PD gene [36]. Its loss, which can be rescued by the expression of Pink1 and Parkin, leads to increased susceptibility of neurons to oxidative stress and death. Interestingly, DJ1-deficiency leads to increased autophagic activity [36], probably with the aim of clearing dysfunctional mitochondria, by a mechanism that remains to be established, but could involve ROS production, mTOR signalling or direct interaction with Pink1/Parkin pathway [36].
Autosomal dominant mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common genetic cause of PD [37,38]. LRRK2 loss causes phenotypes that may be due to impairment of the autophagy-lysosomal pathway, like the accumulation of α-synuclein and apoptotic cell death in aged mice [39]. The effect of LRRK2 on autophagy still remains to be elucidated but might involve the formation of multivesicular bodies (MVB) or the inhibition of the UPS system following the accumulation of α-synuclein (Table 1).
Mutations in the α-synuclein gene, including point mutations and multiplications of the entire locus, have been shown to cause autosomal dominant forms of PD, although the mechanism still remains obscure. In yeast, overexpression of α-synuclein perturbs the secretory pathway by inhibiting Rab1 activity [40]. We recently found that α-synuclein overexpression causes autophagy inhibition by inhibiting Rab1a [41•].
Dementia and amyotrophic lateral sclerosis
A group of neurodegenerative proteinopathies, such as motor neuron diseases (MND), are associated with defects in autophagosome trafficking. Disruption of retrograde axonal transport of cargo by overexpression or depletion of dynein complex components in transgenic mice results in the progressive degeneration of motor neurons and the formation of inclusions, mimicking the phenotype in some MND patients [42–44]. As dynein activity is crucial for microtubule-based delivery of autophagosomes to lysosomes, mutations in the dynein machinery impair autophagosome clearance. Indeed, an increase in autophagosome number and LC3-II levels can be observed in mice with dynein mutations [45,46]. Further research will be needed to clarify the relative importance of autophagy dysfunction in MND, particularly in forms not due to primary mutations of the dynein machinery, but where axonal transport deficiencies have been reported.
Another group of diseases that manifest impaired autophagic flux are due to mutations in the ESCRT complex machinery, which has been implicated in neurodegenerative disorders, such as frontotemporal dementia linked to chromosome 3 (FTD3) [47] and amyotrophic lateral sclerosis (ALS) [48,49]. Expression of a deletion mutant of CHMP2B, a subunit of the ESCRT-III complex, in cell and fly models, increased LC3-II levels and caused an accumulation of autophagosomes [50]. Experimental characterisation of CHMP2B indicates that the proper dissociation of the ESCRT-III complex is crucial to both autophagosome maturation and proper fusion of autophagosomes with lysosomes [51].
Further evidence suggesting a perturbation of autophagy may contribute to pathogenesis of ALS and other associated disorders comes from studies of the lipid phosphatase Fig4. Mutations in this gene are responsible for Charcot-Marie-Tooth disease type 4 and a variant has also been described in ALS patients [52]. A decrease in PI(3,5)P2 levels mice lacking Fig4 has been reported, and this is associated with alterations in autophagic markers consistent with a decrease in autophagy in these animals [53].
Lafora disease
Lafora disease (LD) is an autosomal recessive myoclonus epilepsy. Its pathological hallmark is the accumulation of polyglucosan inclusions, called Lafora bodies, in the cytoplasm of cells in many organs. The majority of mutations causing LD occur in two genes: EPM2A, which encodes laforin, and EPM2B, which codes for malin [54]. It has recently been demonstrated that a deficiency in autophagy may contribute to the accumulation of Lafora bodies [55•]. Data obtained in patient cells, laforin knockout mice and in cell culture systems showed that laforin is a positive regulator of autophagy (Table 1). Loss of laforin resulted in an increase in the activity of the negative regulator of autophagy, mTOR. The exact mechanism for the laforin effect on autophagy is still elusive, as specific laforin substrates relevant to autophagy have yet to be identified.
Protection by autophagy induction in protein misfolding disorders
While progress has been made in understanding the molecular pathology of protein misfolding disorders, exactly how these proteins cause cellular toxicity still remains to be elucidated. However, extensive data suggest that toxicity is mediated primarily via gain-of-function mechanisms (Figure 1). Disease severity appears to correlate with the level of expression of the mutant protein, while loss-of-function mutants often show phenotypes distinct from the disease. Whether it is the aggregated protein, the soluble protein, or an intermediate oligomeric form that confers this toxic gain-of-function is a matter of controversy. However, the large aggregates visible by light microscopy may not be the most toxic species [56]. Regardless of the exact toxic species, the fact these diseases result from a toxic gain-of-function means that the efficiency of the removal of the mutant proteins from the cell is likely to be an important factor in their toxicity.
In general, intracytoplasmic aggregate-prone proteins are good autophagy substrates [57,58], although there are some exceptions to this [59]. Clearance by autophagy has been demonstrated for both wild-type and mutant forms of tau [58], mutant forms of α-synuclein that cause familial Parkinson's disease [60] and a range of polyglutamine-expanded proteins [14,57,58,61]. Interestingly, the non-aggregate-prone species of many of these proteins (e.g. huntingtin and α-synuclein) show a much lower dependency on autophagy for their clearance, compared to their mutant counterparts [60,62–64]. This may have additional benefits in certain neurodegenerative diseases, allowing preferential clearance of the mutant/aggregate-prone protein, without affecting wild-type protein levels.
The importance of autophagy in aggregate-prone protein clearance is probably increased due to the proteasome being unable to degrade oligomeric species, as they cannot be unfolded to enter the narrow barrel of the proteasome [65]. Additionally, in the case of polyglutamine proteins, the proteasome may be unable to cleave within the polyglutamine tract [65,66]. By contrast, autophagosomes are able to engulf oligomeric species. This does not mean that autophagy clears purely aggregated species, as its upregulation results in a decrease in the levels of both soluble and aggregated protein forms [60]. Equally, it is not known if autophagy can clear large aggregates directly or if a decrease in aggregates is seen due to a decrease in the soluble levels of the protein [67,68], although aggregates visible by light microscopy are not membrane-bound, suggesting that earlier and smaller oligomeric structures are the autophagy substrates.
Therapeutic implications for upregulation of autophagy
Evidence for the importance of autophagy in the degradation of mutant, misfolded proteins has wide therapeutic implications in the treatment of proteinopathies. We initially demonstrated that upregulation of autophagy, using the mTOR inhibitor rapamycin or its water-soluble ester CCI-779, reduced levels of soluble and aggregated mutant huntingtin and was protective in cell, Drosophila and mouse models of Huntington's disease [14]. Further to this it has been demonstrated that genetic inhibition of mTOR, by overexpression of TSC1 and TSC2, conferred neuroprotective effects in a Drosophila model of Huntington's disease [69]. Indeed, the therapeutic scope for upregulation of autophagy by mTOR inhibitors extends beyond Huntington's disease, as CCI-779 treatment also demonstrated protective effects in a mouse model of spinocerebellar type 3 [70].
As the most common neurodegenerative disease, possible treatments for AD are of great interest, and there is now evidence that a rapamycin treatment may be beneficial. Aβ levels were decreased and cognitive defects were prevented by rapamycin treatment in two different AD mouse models [71•,72•]. Further evidence for a potential protective role of autophagy upregulation in AD comes from genetic studies. Overexpression of Beclin-1, an autophagy regulating gene, in AD mice models reduced intracellular accumulation of Aβ and extracellular deposition of Aβ plaques [24••]. This strategy of autophagy upregulation has also proved beneficial in a mouse model of Parkinson's disease overexpressing α-synuclein [73].
While mTOR inhibitors show some potential as therapeutic agents, mTOR has multiple roles in cellular homeostasis, and thus mTOR inhibitors are likely to have side-effects due to modulations in processes other than autophagy. It has therefore become a priority to identify other drugs that induce autophagy via mTOR-independent mechanisms. Screening of United States Food and Drug Administration-approved drugs with this aim in mind, identified a number of drugs that were able to upregulate autophagy and protect against toxicity in cell, Drosophila and zebrafish models of Huntington's disease [74]. One of these drugs, rilmenidine, a centrally acting anti-hypertensive, has subsequently been shown to be protective in a mouse model of Huntington's disease [75•].
Lithium induces autophagy by lowering intracellular inositol or inositol 1,4,5-trisphosphate (IP3) levels in an mTOR-independent manner [76], and it has been shown to delay disease progression in a mouse model of ALS overexpressing a mutant form of SOD1 and also in a small trial with ALS patients [77]. Cellular studies have previously demonstrated the requirement of autophagy for the clearance of SOD1 [78], and more recently for TDP-43, another aggregate-prone protein, mutations in which cause ALS [79,80] suggesting that there may be therapeutic potential for the upregulation of autophagy in ALS. Further support for this comes from evidence that XBP-1 deficiency protects against neurodegeneration in mice overexpressing mutant SOD1 [81]. XBP-1 is a transcription factor required for induction of the unfolded protein response (UPR), and it was therefore predicted that its loss would increase the toxicity of mutant SOD in a mouse model. However, deficiency of XBP-1 markedly attenuated development of disease signs, as it resulted in an increase in autophagy and decreased levels of SOD1 aggregation [81].
Autophagy can also be upregulated in an mTOR-independent manner by the disaccharide trehalose [64]. Treatment of a mouse model of tauopathy with parkinsonism, in which mutant tau is overexpressed along with a parkin deletion, resulted in a decrease in the levels of phosphorylated tau and protection against loss of dopaminergic neurons [82•]. Trehalose has previously been shown to have neuroprotective effects, which were attributed to its activity as a chemical chaperone (see [83] for review). However, in this mouse model, an upregulation of autophagy was seen, confirming the potential for dual mechanisms of protection by trehalose.
Future perspectives
For many diseases, the upregulation of autophagy is a promising therapeutic target. Combining knowledge of the potential mechanisms of autophagy compromise in neurodegenerative proteinopathies with knowledge of the range of signalling pathways and drugs available to control autophagy may make the development of therapeutics based on this process possible.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Work in DCRs lab on autophagy and neurodegenerative disease is funded by the Wellcome Trust (Senior Fellowship for DCR), an MRC programme Grant, and the NIHR Biomedical Research Centre at Addenbrooke's Hospital.
References
- 1.Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alirezaei M., Kemball C.C., Flynn C.T., Wood M.R., Whitton J.L., Kiosses W.B. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6:702–710. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 4.Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 6.Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 7.Imarisio S., Carmichael J., Korolchuk V., Chen C.W., Saiki S., Rose C., Krishna G., Davies J.E., Ttofi E., Underwood B.R. Huntington's disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- 8.Sapp E., Schwarz C., Chase K., Bhide P.G., Young A.B., Penney J., Vonsattel J.P., Aronin N., DiFiglia M. Huntingtin localization in brains of normal and Huntington's disease patients. Ann Neurol. 1997;42:604–612. doi: 10.1002/ana.410420411. [DOI] [PubMed] [Google Scholar]
- 9.Davies S.W., Turmaine M., Cozens B.A., DiFiglia M., Sharp A.H., Ross C.A., Scherzinger E., Wanker E.E., Mangiarini L., Bates G.P. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 10.Heng M.Y., Duong D.K., Albin R.L., Tallaksen-Greene S.J., Hunter J.M., Lesort M.J., Osmand A., Paulson H.L., Detloff P.J. Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet. 2010;19:3702–3720. doi: 10.1093/hmg/ddq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M., Lee H.S., LaForet G., McIntyre C., Martin E.J., Chang P., Kim T.W., Williams M., Reddy P.H., Tagle D. Mutant huntingtin expression in clonal striatal cells: dissociation of inclusion formation and neuronal survival by caspase inhibition. J Neurosci. 1999;19:964–973. doi: 10.1523/JNEUROSCI.19-03-00964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kegel K.B., Kim M., Sapp E., McIntyre C., Castano J.G., Aronin N., DiFiglia M. Huntingtin expression stimulates endosomal–lysosomal activity, endosome tubulation, and autophagy. J Neurosci. 2000;20:7268–7278. doi: 10.1523/JNEUROSCI.20-19-07268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata E., Sawa A., Ross C.A., Snyder S.H. Autophagosome-like vacuole formation in Huntington's disease lymphoblasts. Neuroreport. 2004;15:1325–1328. doi: 10.1097/01.wnr.0000127073.66692.8f. [DOI] [PubMed] [Google Scholar]
- 14.Ravikumar B., Vacher C., Berger Z., Davies J.E., Luo S., Oroz L.G., Scaravilli F., Easton D.F., Duden R., O’Kane C.J. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 15•.Martinez-Vicente M., Talloczy Z., Wong E., Tang G., Koga H., Kaushik S., de Vries R., Arias E., Harris S., Sulzer D. Cargo recognition failure is responsible for inefficient autophagy in Huntington's disease. Nat Neurosci. 2010;13:567–576. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a novel role for huntingtin in the loading of cargo into autophagosomes, and suggests a novel mechanism that may contribute to disease.
- 16.Browne S.E. Mitochondria and Huntington's disease pathogenesis: insight from genetic and chemical models. Ann N Y Acad Sci. 2008;1147:358–382. doi: 10.1196/annals.1427.018. [DOI] [PubMed] [Google Scholar]
- 17.Metzger S., Saukko M., Van Che H., Tong L., Puder Y., Riess O., Nguyen H.P. Age at onset in Huntington's disease is modified by the autophagy pathway: implication of the V471A polymorphism in Atg7. Hum Genet. 2010;128:453–459. doi: 10.1007/s00439-010-0873-9. [DOI] [PubMed] [Google Scholar]
- 18.Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 19.Levy-Lahad E., Wasco W., Poorkaj P., Romano D.M., Oshima J., Pettingell W.H., Yu C.E., Jondro P.D., Schmidt S.D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 20.Goate A., Chartier-Harlin M.C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 21.Hardy J. The Alzheimer family of diseases: many etiologies, one pathogenesis? Proc Natl Acad Sci U S A. 1997;94:2095–2097. doi: 10.1073/pnas.94.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu W.H., Cuervo A.M., Kumar A., Peterhoff C.M., Schmidt S.D., Lee J.H., Mohan P.S., Mercken M., Farmery M.R., Tjernberg L.O. Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boland B., Kumar A., Lee S., Platt F.M., Wegiel J., Yu W.H., Nixon R.A. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci. 2008;28:6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P.A., Small S., Spencer B., Rockenstein E., Levine B. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that Beclin-1 levels are decreased in the brains of Alzheimer's disease patients, and that reduction of Beclin-1 in mice causes neurodegeneration. Further to this, they demonstrate that in mice overexpressing human APP concomitant increases in levels of Beclin-1 expression confers neuroprotection.
- 25.Orvedahl A., Alexander D., Talloczy Z., Sun Q., Wei Y., Zhang W., Burns D., Leib D.A., Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26•.Lipinski M.M., Zheng B., Lu T., Yan Z., Py B.F., Ng A., Xavier R.J., Li C., Yankner B.A., Scherzer C.R. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:14164–14169. doi: 10.1073/pnas.1009485107. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study implicates reactive oxygen species as important regulators of type III PI3 kinase activity and therefore autophagy, in response to APP. They further describe a transcriptional upregulation of autophagy in Alzheimer's disease brain compared to normal aged brains.
- 27.Cataldo A.M., Peterhoff C.M., Schmidt S.D., Terio N.B., Duff K., Beard M., Mathews P.M., Nixon R.A. Presenilin mutations in familial Alzheimer disease and transgenic mouse models accelerate neuronal lysosomal pathology. J Neuropathol Exp Neurol. 2004;63:821–830. doi: 10.1093/jnen/63.8.821. [DOI] [PubMed] [Google Scholar]
- 28••.Lee J.H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study the authors show that PS1 is required for targeting v-ATPase to the lysosomes and that without it, lysosomal acidification and hence proteolysis is inhibited.
- 29.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 30•.Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides the first evidence that genes involved in Parkinson's disease may be associated with mitophagy.
- 31•.Narendra D.P., Jin S.M., Tanaka A., Suen D.F., Gautier C.A., Shen J., Cookson M.R., Youle R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here it is demonstrated that Pink1 accumulates on damaged mitochondria and recruits Parkin. This process is disrupted by disease causing mutations in these proteins.
- 32•.Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C.A., Sou Y.S., Saiki S., Kawajiri S., Sato F. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]; Theses authors show Pink1 accumulates on depolarised mitochondria and recruits Parkin and activates its E3 ligase activity.
- 33•.Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R.L., Kim J., May J., Tocilescu M.A., Liu W., Ko H.S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper suggests that Pink1 and Parkin modulate mitochondrial trafficking and thus their clearance by autophagy.
- 34•.Geisler S., Holmstrom K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]; Here it is reported that Parkin forms ubiquitin chains at the mitochondria resulting in their targeting for autophagic degradation by the adapter protein p62.
- 35.Michiorri S., Gelmetti V., Giarda E., Lombardi F., Romano F., Marongiu R., Nerini-Molteni S., Sale P., Vago R., Arena G. The Parkinson-associated protein PINK1 interacts with Beclin1 and promotes autophagy. Cell Death Differ. 2010;17:962–974. doi: 10.1038/cdd.2009.200. [DOI] [PubMed] [Google Scholar]
- 36.Irrcher I., Aleyasin H., Seifert E.L., Hewitt S.J., Chhabra S., Phillips M., Lutz A.K., Rousseaux M.W., Bevilacqua L., Jahani-Asl A. Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 37.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Paisan-Ruiz C., Jain S., Evans E.W., Gilks W.P., Simon J., van der Brug M., Lopez de Munain A., Aparicio S., Gil A.M., Khan N. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Tong Y., Yamaguchi H., Giaime E., Boyle S., Kopan R., Kelleher R.J., 3rd, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B., Liu K., Xu K., Strathearn K.E., Liu F. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Winslow A.R., Chen C.W., Corrochano S., Acevedo-Arozena A., Gordon D.E., Peden A.A., Lichtenberg M., Menzies F.M., Ravikumar B., Imarisio S. alpha-Synuclein impairs macroautophagy: implications for Parkinson's disease. J Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]; α-Synuclein accumulates in sporadic PD, and duplications of the α-synuclein gene are sufficient to cause autosomal dominant PD. This paper shows that excess α-synuclein inhibits autophagy via Rab1a.
- 42.Puls I., Jonnakuty C., LaMonte B.H., Holzbaur E.L., Tokito M., Mann E., Floeter M.K., Bidus K., Drayna D., Oh S.J. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 43.LaMonte B.H., Wallace K.E., Holloway B.A., Shelly S.S., Ascano J., Tokito M., Van Winkle T., Howland D.S., Holzbaur E.L. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 44.Hafezparast M., Ahmad-Annuar A., Hummerich H., Shah P., Ford M., Baker C., Bowen S., Martin J.E., Fisher E.M. Paradigms for the identification of new genes in motor neuron degeneration. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:249–257. doi: 10.1080/14660820310016084. [DOI] [PubMed] [Google Scholar]
- 45.Ravikumar B., Acevedo-Arozena A., Imarisio S., Berger Z., Vacher C., O’Kane C.J., Brown S.D., Rubinsztein D.C. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37:771–776. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- 46.Laird F.M., Farah M.H., Ackerley S., Hoke A., Maragakis N., Rothstein J.D., Griffin J., Price D.L., Martin L.J., Wong P.C. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J Neurosci. 2008;28:1997–2005. doi: 10.1523/JNEUROSCI.4231-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skibinski G., Parkinson N.J., Brown J.M., Chakrabarti L., Lloyd S.L., Hummerich H., Nielsen J.E., Hodges J.R., Spillantini M.G., Thusgaard T. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 48.Parkinson N., Ince P.G., Smith M.O., Highley R., Skibinski G., Andersen P.M., Morrison K.E., Pall H.S., Hardiman O., Collinge J. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B) Neurology. 2006;67:1074–1077. doi: 10.1212/01.wnl.0000231510.89311.8b. [DOI] [PubMed] [Google Scholar]
- 49.Momeni P., Schymick J., Jain S., Cookson M.R., Cairns N.J., Greggio E., Greenway M.J., Berger S., Pickering-Brown S., Chio A. Analysis of IFT74 as a candidate gene for chromosome 9p-linked ALS-FTD. BMC Neurol. 2006;6:44. doi: 10.1186/1471-2377-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J.A., Beigneux A., Ahmad S.T., Young S.G., Gao F.B. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol. 2007;17:1561–1567. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Nara A., Mizushima N., Yamamoto A., Kabeya Y., Ohsumi Y., Yoshimori T. SKD1 AAA ATPase-dependent endosomal transport is involved in autolysosome formation. Cell Struct Funct. 2002;27:29–37. doi: 10.1247/csf.27.29. [DOI] [PubMed] [Google Scholar]
- 52.Chow C.Y., Landers J.E., Bergren S.K., Sapp P.C., Grant A.E., Jones J.M., Everett L., Lenk G.M., McKenna-Yasek D.M., Weisman L.S. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferguson C.J., Lenk G.M., Meisler M.H. Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum Mol Genet. 2009;18:4868–4878. doi: 10.1093/hmg/ddp460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahwan A., Farrell M., Delanty N. Progressive myoclonic epilepsies: a review of genetic and therapeutic aspects. Lancet Neurol. 2005;4:239–248. doi: 10.1016/S1474-4422(05)70043-0. [DOI] [PubMed] [Google Scholar]
- 55•.Aguado C., Sarkar S., Korolchuk V.I., Criado O., Vernia S., Boya P., Sanz P., de Cordoba S.R., Knecht E., Rubinsztein D.C. Laforin, the most common protein mutated in Lafora disease, regulates autophagy. Hum Mol Genet. 2010;19:2867–2876. doi: 10.1093/hmg/ddq190. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper adds Lafora disease to the range of diseases in which alterations in autophagy may be part of the pathogenic mechanisms by demonstrating that loss of the most prevalent protein mutated in Lafora disease decreases autophagy via mTOR pathway activation.
- 56.Arrasate M., Mitra S., Schweitzer E.S., Segal M.R., Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 57.Ravikumar B., Duden R., Rubinsztein D.C. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 58.Berger Z., Ravikumar B., Menzies F.M., Oroz L.G., Underwood B.R., Pangalos M.N., Schmitt I., Wullner U., Evert B.O., O’Kane C.J. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 59.Wong E.S., Tan J.M., Soong W.E., Hussein K., Nukina N., Dawson V.L., Dawson T.M., Cuervo A.M., Lim K.L. Autophagy-mediated clearance of aggresomes is not a universal phenomenon. Hum Mol Genet. 2008;17:2570–2582. doi: 10.1093/hmg/ddn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webb J.L., Ravikumar B., Atkins J., Skepper J.N., Rubinsztein D.C. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 61.Montie H.L., Cho M.S., Holder L., Liu Y., Tsvetkov A.S., Finkbeiner S., Merry D.E. Cytoplasmic retention of polyglutamine-expanded androgen receptor ameliorates disease via autophagy in a mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2009;18:1937–1950. doi: 10.1093/hmg/ddp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin Z.H., Wang Y., Kegel K.B., Kazantsev A., Apostol B.L., Thompson L.M., Yoder J., Aronin N., DiFiglia M. Autophagy regulates the processing of amino terminal huntingtin fragments. Hum Mol Genet. 2003;12:3231–3244. doi: 10.1093/hmg/ddg346. [DOI] [PubMed] [Google Scholar]
- 63.Shibata M., Lu T., Furuya T., Degterev A., Mizushima N., Yoshimori T., MacDonald M., Yankner B., Yuan J. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281:14474–14485. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 64.Sarkar S., Davies J.E., Huang Z., Tunnacliffe A., Rubinsztein D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 65.Verhoef L.G., Lindsten K., Masucci M.G., Dantuma N.P. Aggregate formation inhibits proteasomal degradation of polyglutamine proteins. Hum Mol Genet. 2002;11:2689–2700. doi: 10.1093/hmg/11.22.2689. [DOI] [PubMed] [Google Scholar]
- 66.Venkatraman P., Wetzel R., Tanaka M., Nukina N., Goldberg A.L. Eukaryotic proteasomes cannot digest polyglutamine sequences and release them during degradation of polyglutamine-containing proteins. Mol Cell. 2004;14:95–104. doi: 10.1016/s1097-2765(04)00151-0. [DOI] [PubMed] [Google Scholar]
- 67.Iwata A., Christianson J.C., Bucci M., Ellerby L.M., Nukina N., Forno L.S., Kopito R.R. Increased susceptibility of cytoplasmic over nuclear polyglutamine aggregates to autophagic degradation. Proc Natl Acad Sci U S A. 2005;102:13135–13140. doi: 10.1073/pnas.0505801102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamoto A., Cremona M.L., Rothman J.E. Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol. 2006;172:719–731. doi: 10.1083/jcb.200510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang T., Lao U., Edgar B.A. TOR-mediated autophagy regulates cell death in Drosophila neurodegenerative disease. J Cell Biol. 2009;186:703–711. doi: 10.1083/jcb.200904090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Menzies F.M., Huebener J., Renna M., Bonin M., Riess O., Rubinsztein D.C. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain. 2010;133:93–104. doi: 10.1093/brain/awp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Spilman P., Podlutskaya N., Hart M.J., Debnath J., Gorostiza O., Bredesen D., Richardson A., Strong R., Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rapamycin treatment in a mouse model of Alzheimer's disease overexpressing human APP results in increased survival and a slowing of disease progression.
- 72•.Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that treatment of a mouse model of Alzheimer's disease with rapamycin results in rescue of the cognitive deficits and a reduction in the Aβ and tau pathology seen in these animals.
- 73.Spencer B., Potkar R., Trejo M., Rockenstein E., Patrick C., Gindi R., Adame A., Wyss-Coray T., Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams A., Sarkar S., Cuddon P., Ttofi E.K., Saiki S., Siddiqi F.H., Jahreiss L., Fleming A., Pask D., Goldsmith P. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Rose C., Menzies F.M., Renna M., Acevedo-Arozena A., Corrochano S., Sadiq O., Brown S.D., Rubinsztein D.C. Rilmenidine attenuates toxicity of polyglutamine expansions in a mouse model of Huntington's disease. Hum Mol Genet. 2010;19:2144–2153. doi: 10.1093/hmg/ddq093. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that treatment of a mouse model of Huntingtin's disease with rilmenidine, a drug that is able to upregulate autophagy in neurons, decreases levels of mutant Huntingtin and protects against disease signs. Rilmemidine is a safe, well-tolerated FDA approved drug designed for chronic use.
- 76.Sarkar S., Floto R.A., Berger Z., Imarisio S., Cordenier A., Pasco M., Cook L.J., Rubinsztein D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fornai F., Longone P., Cafaro L., Kastsiuchenka O., Ferrucci M., Manca M.L., Lazzeri G., Spalloni A., Bellio N., Lenzi P. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kabuta T., Suzuki Y., Wada K. Degradation of amyotrophic lateral sclerosis-linked mutant Cu, Zn-superoxide dismutase proteins by macroautophagy and the proteasome. J Biol Chem. 2006;281:30524–30533. doi: 10.1074/jbc.M603337200. [DOI] [PubMed] [Google Scholar]
- 79.Wang X., Fan H., Ying Z., Li B., Wang H., Wang G. Degradation of TDP-43 and its pathogenic form by autophagy and the ubiquitin-proteasome system. Neurosci Lett. 2010;469:112–116. doi: 10.1016/j.neulet.2009.11.055. [DOI] [PubMed] [Google Scholar]
- 80.Urushitani M., Sato T., Bamba H., Hisa Y., Tooyama I. Synergistic effect between proteasome and autophagosome in the clearance of polyubiquitinated TDP-43. J Neurosci Res. 2010;88:784–797. doi: 10.1002/jnr.22243. [DOI] [PubMed] [Google Scholar]
- 81.Hetz C., Thielen P., Matus S., Nassif M., Court F., Kiffin R., Martinez G., Cuervo A.M., Brown R.H., Glimcher L.H. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Rodriguez-Navarro J.A., Rodriguez L., Casarejos M.J., Solano R.M., Gomez A., Perucho J., Cuervo A.M., Garcia de Yebenes J., Mena M.A. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis. 2010;39:423–438. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]; This paper demonstrates that treatment with a drug that activates autophagy in an mTOR-independent fashion is able to decrease levels of tau and protect against neurodegeneration in a mouse model of tauopathy with parkinsonism.
- 83.Sarkar S., Rubinsztein D.C. Small molecule enhancers of autophagy for neurodegenerative diseases. Mol Biosyst. 2008;4:895–901. doi: 10.1039/b804606a. [DOI] [PubMed] [Google Scholar]