Abstract
Schizophrenia and related mental disorders are common and devastating conditions for which we have a limited understanding of their origin and mechanisms. Although this apparent lack of progress despite vast research efforts could be due to difficulties in reproducing the disease in animals, animal work is now providing important insight onto possible pathophysiological changes in the brain. Postmortem studies of human brains have provided data indicating altered local inhibitory circuits in the cerebral cortex in schizophrenia and different developmental, pharmacological, and genetic animal models converge in revealing deficits in cortical interneuron function that can be associated with neurophysiological and behavioral alterations resembling aspects of the disease. Schizophrenia pathophysiology has a complex developmental trajectory because overt symptoms become evident during late adolescence despite earlier events contributing to the disease. The late incidence of schizophrenia can be explained by the protracted maturation of brain circuits implicated in the disease, particularly during adolescence. Excitatory and inhibitory processes in cortical circuits are tightly modulated by dopamine (DA), and many aspects of DA function in cortical regions acquire their adult profile during adolescence. This maturation fails to occur or is abnormal in several different rodent models of schizophrenia, yielding a number of functional and behavioral deficits relevant to the disease. Thus, periadolescent changes in cortical inhibitory circuits are a critical developmental stage likely implicated in the transition to schizophrenia. These observations provide the foundation for novel research-based therapeutic approaches and perhaps will even lead to ways to prevent the progression of the disease in predisposed subjects.
Keywords: GABA, interneuron, prefrontal cortex, hippocampus, review, dopamine, adolescence, development
Adolescent Maturation of Cortical Circuits and Schizophrenia
The development of brain circuits during adolescence is of obvious importance for schizophrenia and other major psychiatric disorders including bipolar disorder and major depression. Although there is a clear genetic component and/or early developmental and environmental factors contributing to schizophrenia, symptoms do not manifest fully until late adolescence.1 A possible explanation for this delay is that developmental alterations affecting immature brain systems may not have a strong impact on their function and the behaviors, these systems support until they complete their maturation. The prefrontal cortex (PFC) and hippocampus stand out as major regions affected in schizophrenia, and the transmitters involved include dopamine (DA), glutamate, and gamma amino butyric acid (GABA).2,3 DA is critical for modulating both excitatory glutamate transmission and local inhibition mediated by GABA.4 It has indeed been proposed that the modulation of excitation-inhibition balance in the PFC and hippocampus by DA matures during adolescence.4 Here, I will review recent studies addressing adolescent maturation of cortical circuits and data showing altered maturation in animal models of schizophrenia.
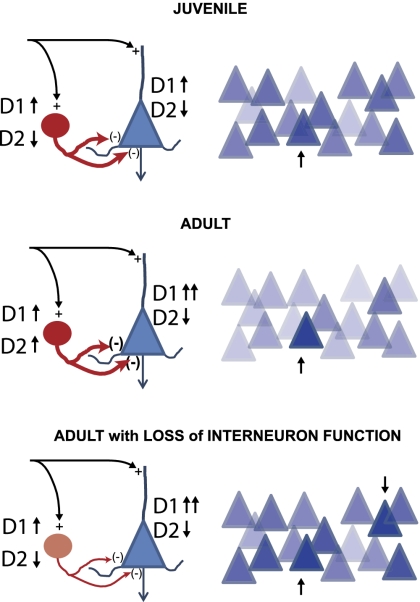
Several groups have identified changes in the DA modulation of GABA interneuron function and pyramidal neuron ability to sustain persistent activity during adolescence. DA receptors reach stable adult levels by the end of the adolescent period,5 and the density of DA fibers in the primate PFC increases during adolescence to be later pruned to adult levels.6 D1 receptors increase their density postnatally to peak during adolescence and decrease later in rodents7 and humans.8 The PFC also shows a delayed anatomical maturation, as evidenced in imaging studies in humans9 and characterized by cortical thinning during adolescence that correlates with intellectual ability.10 Electrophysiologically, D1 agonists can enhance N-methyl-D-aspartate (NMDA) receptor-dependent function in the PFC, an interaction likely relevant to the persistent activity involved in working memory mechanisms. The D1-NMDA interaction in the rodent PFC becomes more robust during adolescence, allowing the emergence of persistent depolarizations in adult brain slices when D1 and NMDA receptors are coactivated.11 This enhanced glutamatergic efficacy in the adult PFC is balanced by an increase in the DA recruitment of local inhibitory processes. In juvenile rats, D1 agonists increase GABA interneuron firing, whereas D2 agonists have either a weak inhibitory effect or no effect.12,13 In the adult PFC, however, D2 agonists become strongly excitatory over fast-spiking interneurons,12 allowing a more efficient attenuation of cortico-cortical synaptic responses in the adult circuit14 (figure 1). Thus, as the control of excitation-inhibition balance within the cortex by DA (and likely by other modulators as well) is refined during adolescence, interneuron activation becomes more critical for cognitive performance in adults. Furthermore, early deficits in cortical glutamate, GABA, and DA interactions would not become apparent until the periadolescent maturation brings these interactions online. This hypothetical scenario is supported by human imaging data indicating behavioral adolescent traits are related to complex developmental trajectories of prefrontal regions and reward systems,15 and it can be directly tested using animal models in which early development is altered and functional and behavioral anomalies emerge during adolescence.
Fig. 1.
Periadolescent maturation of the dopamine (DA) modulation of prefrontal cortex (PFC) circuits. Top: schematic representation of a juvenile circuit; left: DA receptor effects in pyramidal neurons (D1 receptors increase cell excitability and glutamate responses; D2 receptors decrease cell excitability and glutamate responses) and gamma amino butyric acid (GABA) interneurons (D1 agonists enhance cell excitability and D2 agonists have a weak inhibitory effect). Right: levels of activity in a hypothetical network of PFC pyramidal cells are displayed with darker being higher. In this illustration of a juvenile network, the more strongly activated neuron (arrow) stands out from the rest. Middle: similar schematic representation of an adult PFC circuit. In the adult, D1 receptors increase NMDA responses in pyramidal neurons more effectively and D2 receptors increase GABA interneuron activity. The result is a higher contrast between strongly activated and weakly activated units (right). Bottom: In a circuit in which interneurons fail to be properly activated by DA, such as what is observed in animal models of schizophrenia, the contrast between strongly and weakly activated units is blurred, and there may be even units that are improperly enhanced (downward arrow), thereby the encoding of goals and decision making by the PFC becomes inefficient.
Can We Model Psychiatric Disorders in Rodents?
The pathophysiology of schizophrenia has been an elusive target of research. An important factor in such slow progress has been the difficulty in reproducing many aspects of the disease in animals. Over the past few decades, several attempts at modeling the disease were made using pharmacological approaches,16,17 lesions,18,19 environmental manipulations,20 and more recently genetic manipulations.21–23 Although progress was initially slow, converging findings across models suggest altered development of cortical circuits as a possible key factor in schizophrenia pathophysiology.
What do we look for in an animal model of schizophrenia? The consensus is that such models must have some extent of “face,” “construct,” and “predictive” validity. Face validity is the reproduction of major outcomes of the disease, such as agitation, hypersensitivity to stress, hallucinations, social withdrawal, etc. This is certainly very difficult if not impossible to obtain with the positive symptoms of the disease (ie, hallucinations and delusions) but manageable with negative symptoms (such as social withdrawal and anhedonia) and cognitive symptoms (ie, working memory deficits, altered executive functions, etc.). Because cognitive neuroscience has blossomed with sophisticated tools to analyze the neurobiological underpinnings of complex behaviors, this realm of the disease has become amenable to study in animal models. Predictive validity is usually present when outcomes of a model are treated with drugs that are effective in the disease. Although certainly important for positive symptoms, this approach does not allow advances in our understanding of cognitive deficits because this symptom cluster is poorly treated by typical and atypical antipsychotic drugs.24 Construct validity refers to a model reproducing “etiology” or “mechanisms” possibly associated with the disease, and here is where many models have shown a remarkable convergence of findings that can be related to observations in human subjects. The concept of construct validity is limited in schizophrenia because we do not really know the origin or mechanisms of the disease and, therefore, we cannot reproduce them in an animal model. However, several animal models have been built to test the consequences of known risk factors or hypothesized pathophysiological conditions. This line of research has provided important information regarding functional circuits that would not have been possible with human studies. As a consequence, schizophrenia research is on the verge of a highly significant advance because decades of animal work have changed the meaning of construct validity: animal models have provided a wealth of information suggesting possible pathophysiological scenarios for human mental disease, which can now be directly tested in patients.
An important consideration for all animal models is the timing of appearance of anomalies. Because the onset of florid symptoms typically occurs during late adolescence, it is critical that schizophrenia-relevant phenomena emerge at a similar developmental stage in animal models. Adolescence is a well-characterized period in rodents. Several studies revealed behavioral and neural changes occurring during and after the hormonal changes that characterize puberty. In short, gonadal maturation takes place by the end of the first month of life in rats, and the second month (adolescence) is characterized by risk-taking and impulsive behaviors,25 as well as many immature anatomical and physiological features.4 Because this time period is brief and amenable to studies, the knowledge gained in recent years on different aspects of rodent adolescent maturation is proving very important for the development of models relevant to schizophrenia.
The Early Days: Pharmacological Models
In the 70’s and 80’s, the primary approach to modeling complex psychiatric conditions was to use pharmacological tools known to induce psychosis in humans. Amphetamine and least significant difference are capable to induce psychotic episodes, but their characteristics differ from what is seen in the disease.26,27 When used in animal research, these agents have been important for elucidating several aspects of monoamine involvement in psychosis. Indeed, current effective therapeutic approaches involve blockade of DA D2 receptors, and therefore, the main thinking about schizophrenia pathophysiology focused on altered monoaminergic transmission for several decades. But the development of the DA hypothesis has not been a planned discovery process. In fact, antipsychotic drugs were discovered by accident due to the efficacy of agents designed as antialergic28; the clinical efficacy of these drugs was later shown to correlate with their affinity for D2 receptors.29 There is no doubt DA systems are affected in schizophrenia and are perhaps responsible for positive symptoms but no pathophysiological scenario can put all the pieces of the schizophrenia puzzle together based on an etiological process involving a DA alteration. Other pharmacological tools have been more illuminating in this regard. Phencyclidine (PCP) and its congeners (eg, ketamine) are noncompeting NMDA receptor antagonists, and they have been shown not only to induce psychotic states in normal subjects but also to bring back symptoms in schizophrenia patients in remission.30,31 These agents have been used extensively in imaging studies to elucidate brain regions affected in the disease. They are also the best approach at bridging human and animal studies because they can be used in parallel to assess behavioral and neurophysiological outcomes and their relationship with neurobiological mechanisms. Noncompeting NMDA antagonists have provided us with a possible scenario in which DA alterations are secondary to abnormal information processing in cortical regions.
Animal work using NMDA antagonists reveals a disinhibited cerebral cortex. The pioneering work of Bita Moghaddam showed increases in rat PFC pyramidal cell firing and decreases in fast-spiking interneuron firing32 as well as increase in glutamate levels33 with these agents, suggesting their effect may be located primarily in NMDA receptors of local inhibitory interneurons. A preferential effect of NMDA agonists on GABA interneurons is easy to explain. NMDA receptors are voltage-sensitive ligand gated ion channels that are blocked by Mg++ at the negative membrane potential most pyramidal neurons exhibit at rest. It takes a significant depolarization (around 20 mV) to remove the Mg++ blockade. GABA interneurons, on the other hand, are continuously depolarized and exhibit high levels of firing,12 therefore likely to escape Mg++ blockade. NMDA antagonists can bind to all NMDA receptors, but their impact will be felt on those that are active, primarily those located in inhibitory interneurons. Indeed, PFC interneurons exhibit NMDA receptors with higher proportion of NR2B subunits,34 but they also express NR2C and 2D subunits, which yield NMDA receptors with a lower degree of Mg++ blockade.35 Although these reports of high-density NMDA receptors in PFC interneurons contrast with recent studies showing negligible NMDA currents in this cell population,36,37 it is possible that either noncompeting NMDA antagonists affect a subpopulation of interneurons or the slice preparation loses a critical input that drives cortical interneurons and does not reflect well the in vivo condition. The consequence of NMDA antagonism on PFC circuits will likely be a reduced local inhibition and altered excitation-inhibition balance in the cortex that can result in a noisy pattern of activity in cortical ensembles. Thus, interneurons are a critical element affected by NMDA blockade, and the excitation-inhibition imbalance thereby produced could be an important factor in the psychotomimetic effect of NMDA antagonists.
Developmental Models Reveal Loss of Interneuron Function
More recently, a variety of models were created to assess whether developmental alterations could yield phenomena relevant to schizophrenia. The best characterized among these models include the prenatal administration of the antimitotic methylazoxymethanol (MAM) acetate and the neonatal ventral hippocampal lesion (NVHL).19,38 Markers of interneuron function were found abnormal in practically every developmental model tested. Whether it is loss of parvalbumin (PV) immunoreactivity or electrophysiological deficits in interneurons or interneuron-dependent functions, the data indicate inhibitory cortical interneurons are a vulnerable population that could be affected at many different developmental stages with a variety of manipulations.
The NVHL model is the most extensively studied with nearly 100 published articles and more than 20 laboratories using it. Behaviorally, adult rats with a NVHL show hyperlocomotion,39,40 increased response to novelty,41 abnormal social behaviors,41 exaggerated responses to stress, stimulants and NMDA antagonists,39,42 and increased liability for addictive behaviors.43,44 Several studies identified cognitive deficits in this model, including working memory deficits,45–47 perseveration,46 sensorimotor gating deficits,48 and object recognition deficits in primates with a similar lesion.49 Thus, this model produces anomalies that could be related to all symptom domains of schizophrenia. Recent work has focused on identifying pathophysiological changes that may be relevant to the behavioral deficits in the NVHL model. Neurons in the medial PFC and nucleus accumbens in anesthetized NVHL rats respond with exaggerated firing to stimulation of the ventral tegmental area (VTA),50,51 the source of dopaminergic projections to these regions. Recordings in brain slices revealed increased excitability of pyramidal neurons52 and a deficient activation of fast-spiking GABA interneurons by D2 agonists53 in the PFC of NVHL rats. Many electrophysiological and behavioral features of the NVHL model can be ameliorated with antipsychotic drug treatment.51,54 The critical role of altered PFC information processing in this model is further shown by deficits being reversed by an adult PFC lesion.55 As with the human condition, most alterations observed in NVHL rats emerge during adolescence. An important caveat in this model is the presence of a (small) lesion, which is not observed in the brains of schizophrenia patients. Because most of the behavioral and physiological data relate to PFC function, the NVHL is not likely to model hippocampal pathology in schizophrenia, but the deleterious effects of affecting hippocampal activity at a critical stage in development, which ultimately can yield subtle alterations in PFC circuitry. In summary, the data obtained with NVHL rats indicate that impaired activation of PFC interneurons by DA is a critical feature of this model, and the functional consequences of such impairment emerge during adolescence. Thus, it is likely that although circuit anomalies were driven early in development by the neonatal lesion, deficits only emerge when PFC circuits would mature during adolescence. Therefore, what this and other models may have in common is a failure in the periadolescent maturation of circuits responsible for excitation-inhibition balance in the PFC and perhaps other cortical regions.
Other models have relied on perinatal manipulations to reproduce environmental factors that could confer a predisposition for the disease such as perinatal stress and maternal infection. Several groups have used prenatal exposure to viral particles or bacteria-associated endotoxins to induce behavioral changes in rats with some face validity for schizophrenia.56 Injection of the bacterial endotoxin lipopolysaccharide in the ventral hippocampus of rat pups causes electrophysiological deficits in PV interneurons in the adult PFC, characterized by loss of their activation by DA D2 receptors,57 a finding identical to what was observed in NVHL rats. This result indicates that in order to obtain loss of adult PV interneuron function by an early hippocampal manipulation, a lesion is not required, suggesting that the lesion component in the NVHL model is not critical for its outcome. It is worth noting that the NVHL procedure is effective during a limited critical window (postnatal day [PD] 6–9), and preliminary observations using DiI injections in the ventral hippocampus indicate that this is the time hippocampal afferents are arriving into the PFC (Calhoon and O'Donnell, unpublished observation). Thus, interfering with hippocampal function during that critical period could result in altered development of PFC circuits. Furthermore, the prenatal administration of MAM yields adult rats with fewer interneurons expressing PV and loss of high-frequency oscillations.38 An interesting approach combining NMDA antagonism with a developmental time frame showed that early postnatal PCP reduces the number of adult PV+ neurons in the PFC.58 Another developmental model that addresses environmental factors is rearing animals in social isolation.59 The hippocampus of isolation-reared rats shows reduced number of PV+ cells60 and recognition memory deficits are alleviated by a metabotropic glutamate 2/3 (mGluR2/3) agonist,61 which reduces excess glutamate release. It is therefore possible that during development, the role of interneurons on cortical circuitry can be affected by several factors, and if disrupted could lead to abnormal behaviors and other measures related to the disease in the adult animal.
Emerging Genetic Models Also Reveal Loss of Interneuron Function
Because schizophrenia has a clear genetic component, a large number of candidate genes have been identified as conferring predisposition for the disease. Several mouse models have been developed to express gene variants associated with the human condition. Although this line of work is still in its early stages, in many instances, interneuron function was found altered in adult mice expressing those gene variants. Furthermore, genes associated with risk for schizophrenia are critical for cortical interneuron development. For example, neuregulin and its receptor ErbB4, both implicated in schizophrenia,62 are important for the development of chandelier cell synapses onto pyramidal neurons63 and for excitatory synapses onto GABAergic interneurons.64 ErbB4 knock-out mice exhibit a reduction in PV interneurons and loss of power of kainate-induced gamma oscillations.65 The disrupted-in-schizophrenia 1 (DISC1) gene is truncated in a family with high incidence of major psychiatric disorders.66 Mice with a dominant negative DISC1 present reduced PV in the medial PFC.67 In-utero injections of sh-RNA that transiently knock down DISC1 yield altered adult, not juvenile PFC function.22 In that study, we observed abnormal DA modulation of adult PFC circuits compatible with impaired interneuron function.22 Thus, several genetic manipulations converge in revealing abnormal interneuron function that emerges during late adolescence.
Cortical Disinhibition as a Common Final Pathophysiological Condition for Schizophrenia Models
What would be the consequences of altered interneuron function in all these models? PFC interneurons are critical for cognitive functions and they contribute to high-frequency oscillations detected in electroencephalographic and field potential studies.68 Loss of interneuron function, in particular of the PV+ population, would yield a desynchronized and disinhibited cortex, which could result in exaggerated and inefficient activity such as what is found in imaging studies.69 But the most dramatic effect of interneuron dysfunction would be evident in epochs during which their activity should be enhanced by modulators and other inputs. For example, VTA stimulation silences most rat PFC pyramidal neurons,70 likely via activation of fast-spiking interneurons.71 In awake behaving rats, high-frequency oscillations in the PFC emerge during decision-making epochs in which VTA DA neurons are activated.72 This finding suggests that phasic DA release in the PFC engages local inhibitory processes. Loss of interneuron function would yield a poor recruitment of inhibitory processes by DA, excessive pyramidal cell firing, and impaired response selection. In NVHL rats, we have recently shown loss of transient increase in beta oscillations and excessive pyramidal cell firing during the decision instance of a choice task.72 In that study, NVHL rats showed deficits in reversal learning, a process that correlated with the increase in beta power in controls. Furthermore, reducing glutamate levels with a mGluR2/3 agonist improved performance in NVHL rats.72 Thus, a rodent model that causes altered DA activation of interneurons in the adult PFC exhibits cognitive deficits that can be associated with disinhibition.
If interneurons are a primary site of alteration in schizophrenia, manipulations that selectively impair interneurons in the PFC and/or hippocampus should yield schizophrenia-related phenotypes. Indeed, mice in which NR1 subunits of the NMDA receptor were knocked out selectively in PV+ cortical interneurons yield sensorimotor gating deficits, working memory deficits, hyperlocomotion, enhanced response to stimulants, and many other schizophrenia-related phenomena.23 Pharmacological blockade of GABA-A receptors within the PFC in rats also yields cognitive anomalies that are related to schizophrenia,73 indicating proper inhibitory function within the PFC is required for cognitive functions. Thus, multiple etiologies could produce a common set of symptoms by affecting the development of excitatory-inhibitory balance in cortical circuits.
Conclusions
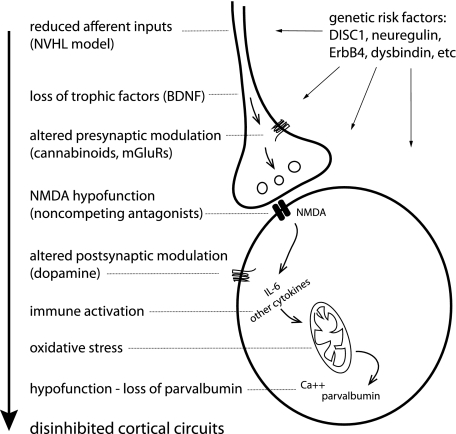
A disinhibited cortex seems to be a central feature of many different animal models of schizophrenia. Loss of adult PV interneuron function is a common observation that can be produced with several manipulations produced at various developmental times. Thus, the diverse model approaches currently in use converge in ultimately affecting cortical GABA interneurons. The diverse set of possible causes of interneuron dysfunction may have relevance to schizophrenia because the disease likely involves multiple etiologies. Why are cortical GABA interneurons affected by such diverse manipulations? Their increased vulnerability compared with pyramidal neurons may stem out of their increased baseline activity and firing. Indeed, fast-spiking PV positive interneurons present high firing rates and their membrane potential sits at a relatively depolarized value. This increased electrical activity may result in enhanced metabolic demand, which could yield to the higher vulnerability. Recent work by Margarita Behrens yielded interesting information regarding cellular processes that may affect interneuron function. Noncompeting NMDA antagonists affect inhibitory interneuron function by altering redox mechanisms,74 an effect that depends on interleukin 6 and other cytokines.75 Thus, blockade of NMDA function in interneurons could elicit changes similar to those reproduced in immune activation models and similar to the loss of glutamatergic inputs that may be occurring in the NVHL model, yielding interneurons affected by oxidative stress (figure 2). As interneuron recruitment by DA is not efficient in juvenile stages (prior to PD45 in rats)12 and in vivo inhibitory processes in prefrontal regions are weak at that age,76 the presence of “sick” interneurons at early developmental stages would not have dramatic functional consequences. Perhaps altered preadolescent PFC circuits could yield “prodromal” cognitive deficits, which are consistent but relatively mild and not too different from what some unaffected children may show. It is during the periadolescent period, when interneurons acquire the strong modulation by DA reviewed above, that an altered interneuron population would yield overtly abnormal physiology and behavior. The ongoing changes in cortical inhibitory circuits during adolescence are also likely to provide vulnerability to the deleterious effects of stress and other environmental influences that could trigger pathological changes in a vulnerable circuit. Human neurophysiological studies are consistent with this idea of a vulnerable period during adolescence, as neural synchrony (a neurophysiological readout of interneuron function) during a Gestalt perception task was transiently reduced during late adolescence, an effect that correlated with loss of performance.77 The maturation of cortical circuits and local inhibitory processes during adolescence is therefore susceptible to environmental influences that can put into evidence preexisting developmental anomalies.
Fig. 2.
Cartoon a cortical interneuron and a representative glutamatergic afferent indicating where vulnerability factors can affect interneuron function during development, and possible events occurring in animal models that ultimately yield disinhibited cortical circuits. There may be many ways to alter interneuron function that yield disinhibited cortical circuits. Some manipulations affect NMDA receptor function in GABAergic interneurons, and these could be driven by NMDA antagonists, reduced inputs, trophic factors, etc. Reduced NMDA activation in this cell population induces cytokine expression and redox alterations, and in the end, the reduced activity of these neurons yields lower levels of parvalbumin. Genetic risk factors may contribute to this scenario both at the presynaptic and postsynaptic level.
The strong data suggesting disinhibition in animal models of schizophrenia parallels the highly replicated observation of interneuron deficits in schizophrenia patients detected in postmortem studies.78 These observations have led to the development of novel therapeutic approaches aimed at restoring excitation-inhibition balance in cortical circuits. A promising initial observation is the efficacy of a mGluR2/3 agonist, which by acting on presynaptic receptors reduces glutamate release, in a clinical trial.79 Remarkably, benzodiazepines are heavily used as adjuvant treatment in schizophrenia,80 supporting a beneficial role of enhancing GABA-A receptor activity. The possible role of oxidative stress in schizophrenia has led to testing antioxidants as adjunct treatment, with some success.81 All these efforts are still on their early stages; allosteric modulators for metabotropic glutamate or GABA-A receptors, partial agonists for DA D1 or D2 receptors, and redox modulators will likely provide greater benefits when they become available. Also, the notion that cortical circuits are abnormal during development may warrant the search of adequate biomarkers that, combined with epidemiological information, can help identify an at-risk population to be treated preventively. The identification of cellular, synaptic, and circuit elements that are responsible for the adolescent maturation of cortical circuits should provide important information that could help identifying possible targets to alleviate or even prevent the conversion to the disease in vulnerable subjects. These are all promising possibilities that have opened up thanks to animal studies. Further work is obviously needed to precisely indentify novel targets and approaches. The answer to the question of whether we can model schizophrenia in rodents may be “no” if one expects reproducing all symptoms of the disease in animals, but it is clear that the available models have advanced the views of pathophysiological mechanisms to a point in which new intervention strategies can be developed with a strong rationale behind.
Funding
National Institutes of Mental Health (R01 MH60131).
Acknowledgments
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 3.Coyle JT. 2Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18:306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- 5.Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 7.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weickert CS, Webster MJ, Gondipalli P, et al. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 11.Tseng KY, O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- 12.Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 14.Tseng KY, O'Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Javitt DC, Zukin SR. Recent advances in the phenciclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 17.Olney JW, Farber NB. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology. 1995;13:335–345. doi: 10.1016/0893-133X(95)00079-S. [DOI] [PubMed] [Google Scholar]
- 18.Lipska BK, Weinberger DR. Subchronic treatment with haloperidol and clozapine in rats with neonatal excitotoxic hippocampal damage. Neuropsychopharmacology. 1994;10:199–205. doi: 10.1038/npp.1994.22. [DOI] [PubMed] [Google Scholar]
- 19.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- 21.Cabungcal JH, Nicolas D, Kraftsik R, Cuenod M, Do KQ, Hornung JP. Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: relevance to schizophrenia. Neurobiol Dis. 2006;22:624–637. doi: 10.1016/j.nbd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Niwa M, Kamiya A, Murai R, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- 25.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 26.Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. J Psychiatr Res. 1974;11:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- 27.Sanfilipo M, Wolkin A, Angrist B, et al. Amphetamine and negative symptoms of schizophrenia. Psychopharmacology. 1996;123:211–214. doi: 10.1007/BF02246180. [DOI] [PubMed] [Google Scholar]
- 28.Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. Int Rec Med Gen Pract Clin. 1955;168:318–326. [PubMed] [Google Scholar]
- 29.Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- 30.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelly R. Study of a new schizophrenomimetic drug—Sernyl. Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 31.Davies BM, Beech HR. The effect of l-arylcyclohexylamine (Sernyl) on twelve normal volunteers. J Ment Sci. 1960;106:912–924. doi: 10.1192/bjp.106.444.912. [DOI] [PubMed] [Google Scholar]
- 32.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xi D, Keeler B, Zhang W, Houle JD, Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: application of laser microdissection technique. J Neurosci Methods. 2009;176:172–181. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HX, Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol. 2010;588:2823–2838. doi: 10.1113/jphysiol.2010.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;90:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 40.Wan RQ, Giovanni A, Kafka SH, Corbett R. Neonatal hippocampal lesions induced hyperresponsiveness to amphetamine: behavioral and in vivo microdialysis studies. Behav Brain Res. 1996;78:211–223. doi: 10.1016/0166-4328(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 41.Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behavior in adulthood. Psychopharmacology. 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow NR, Halim N, Hanlon FM, Platten A, Auerbach PP. Lesion size and amphetamine hyperlocomotion after neonatal ventral hippocampal lesions: more is less. Brain Res Bull. 2001;55:71–77. doi: 10.1016/s0361-9230(01)00492-0. [DOI] [PubMed] [Google Scholar]
- 43.Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brady AM, McCallum SE, Glick SD, O'Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology (Berl) 2008;200:205–215. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- 46.Marquis JP, Goulet S, Dore FY. Neonatal ventral hippocampus lesions disrupt extra-dimensional shift and alter dendritic spine density in the medial prefrontal cortex of juvenile rats. Neurobiol Learn Mem. 2008;90:339–346. doi: 10.1016/j.nlm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- 48.Swerdlow NR, Lipska BK, Weinberger DR, Braff DL, Jaskiw GE, Geyer MA. Increased sensitivity to the sensorimotor gating-disruptive effects of apomorphine after lesions of medial prefrontal cortex or ventral hippocampus in adult rats. Psychopharmacology (Berl) 1995;122:27–34. doi: 10.1007/BF02246438. [DOI] [PubMed] [Google Scholar]
- 49.Bachevalier J, Alvarado MC, Malkova L. Memory and socioemotional behavior in monkeys after hippocampal damage incurred in infancy or in adulthood. Biol Psychiatry. 1999;46:329–339. doi: 10.1016/s0006-3223(99)00123-7. [DOI] [PubMed] [Google Scholar]
- 50.O'Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- 51.Goto Y, O'Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. J Neurosci. 2002;22:9070–9077. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tseng KY, Lewis BL, Lipska BK, O'Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tseng KY, Lewis BL, Hashimoto T, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14:135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 55.Goto Y, O'Donnell P. Prefrontal lesion reverses abnormal mesoaccumbens response in an animal model of schizophrenia. Biol Psychiatry. 2004;55:172–176. doi: 10.1016/s0006-3223(03)00783-2. [DOI] [PubMed] [Google Scholar]
- 56.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 57.Feleder C, Tseng KY, Calhoon GG, O'Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biol Psychiatry. 2010;67:386–392. doi: 10.1016/j.biopsych.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- 59.Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- 61.Jones CA, Brown AM, Auer DP, Fone KC. The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology (Berl) 2010;214:269–283. doi: 10.1007/s00213-010-1931-7. [DOI] [PubMed] [Google Scholar]
- 62.Law AJ, Lipska BK, Weickert CS, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fazzari P, Paternain AV, Valiente M, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 64.Ting AK, Chen Y, Wen L, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31:15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millar JK, Wilson-Annan JC, Anderson S, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 67.Hikida T, Jaaro-Peled H, Seshadri S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 70.Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential “up” states in pyramidal neurons via D1 dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- 71.Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O'Donnell P. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gruber AJ, Calhoon GG, Shusterman IS, Schoenbaum G, Roesch MR, O'Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2010;69:431–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 74.Behrens MM, Ali SS, Dao DN, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 75.Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sturman DA, Moghaddam B. Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. J Neurosci. 2011;31:1471–1478. doi: 10.1523/JNEUROSCI.4210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maldonado-Aviles JG, Curley AA, Hashimoto T, et al. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 80.Guidotti A, Auta J, Davis JM, et al. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- 81.Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]