Abstract
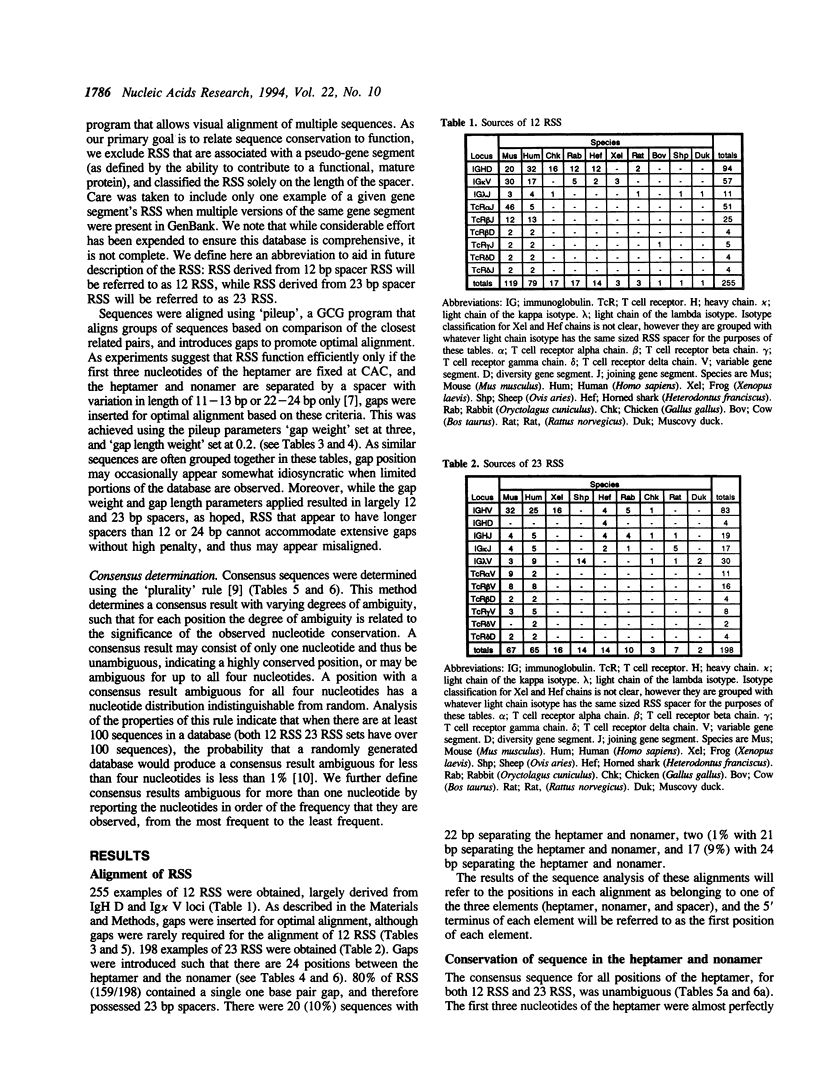
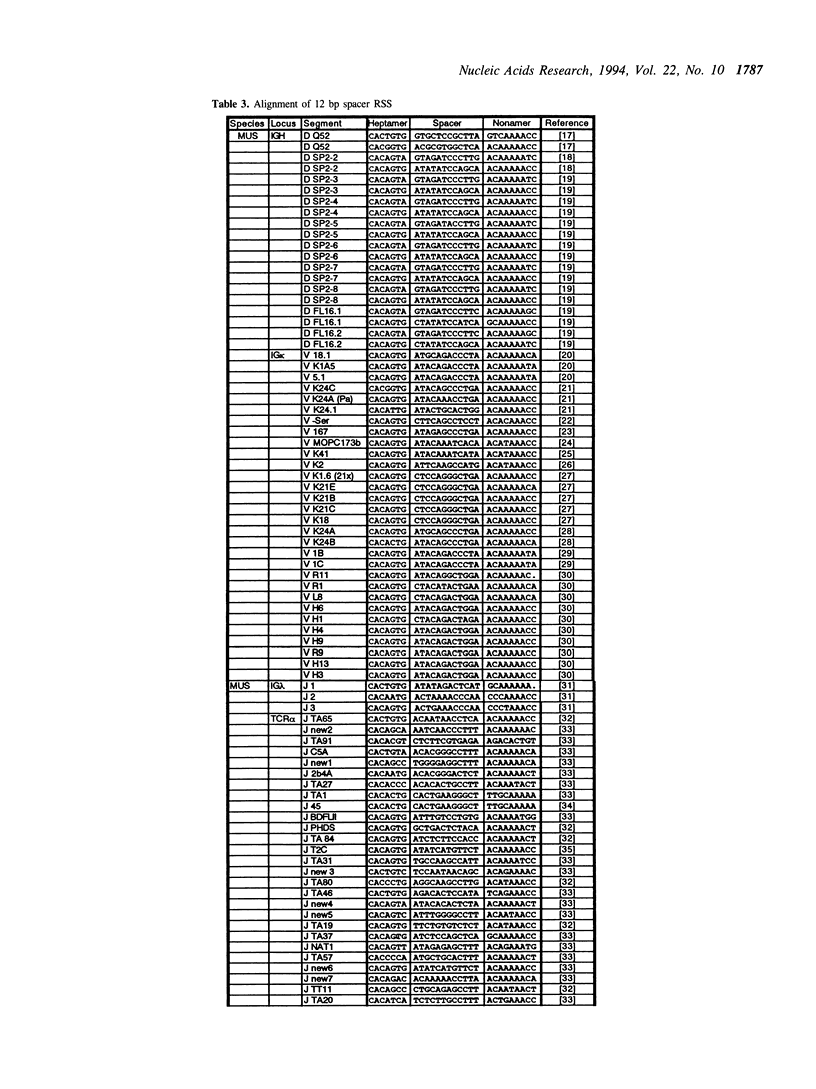
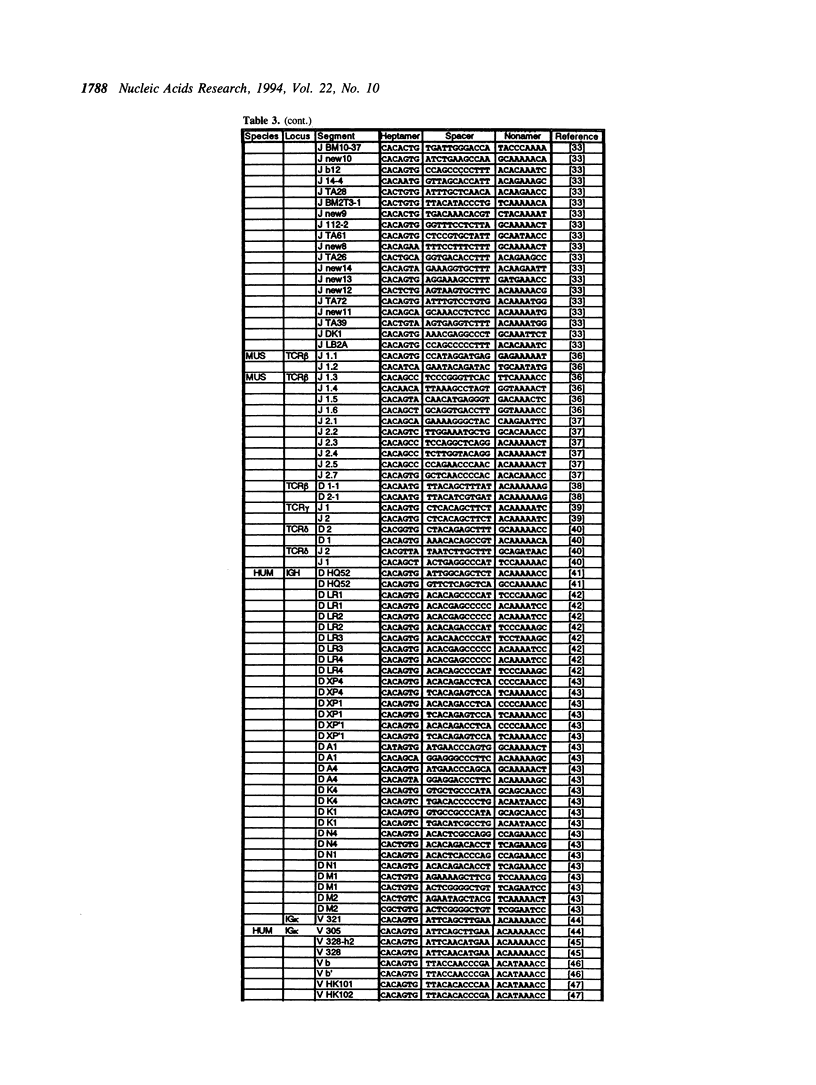
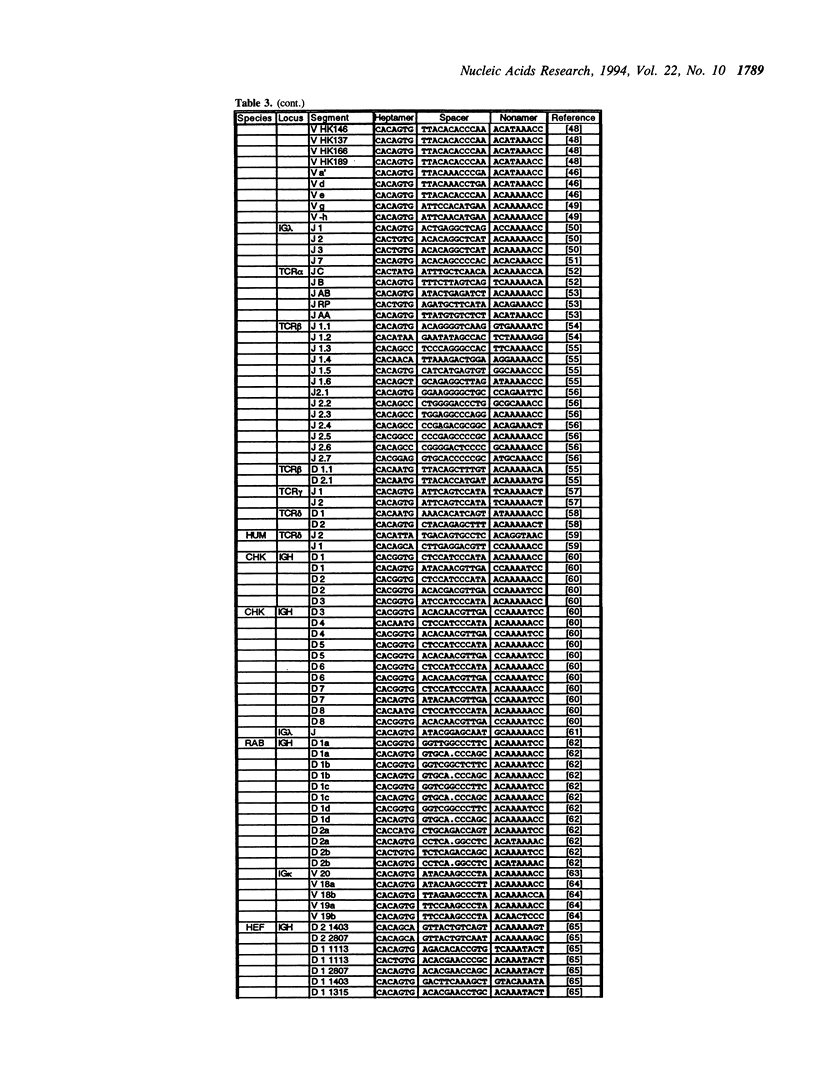
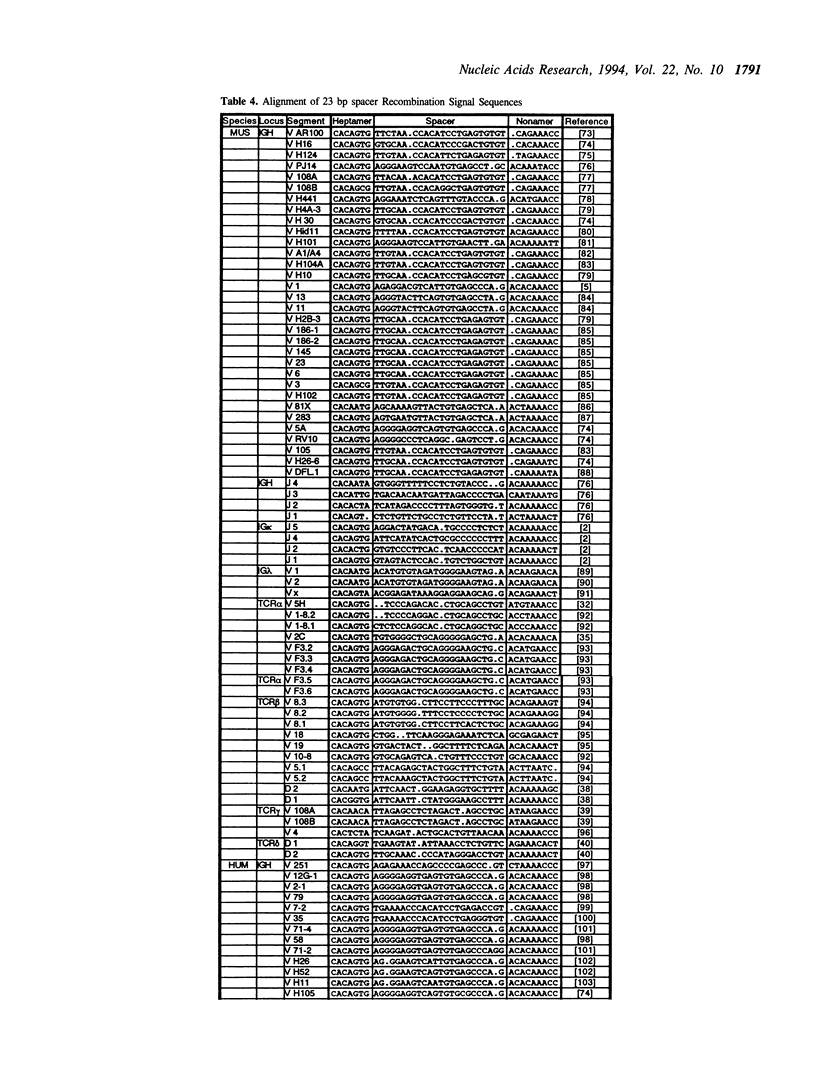
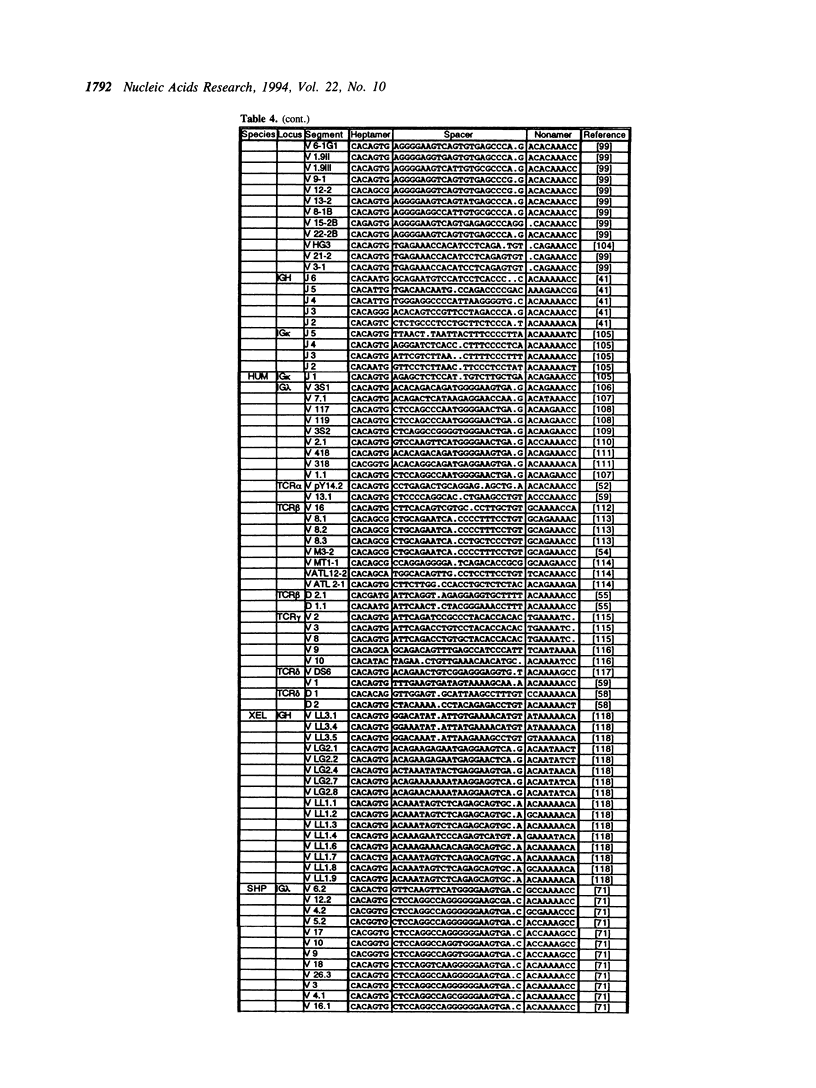
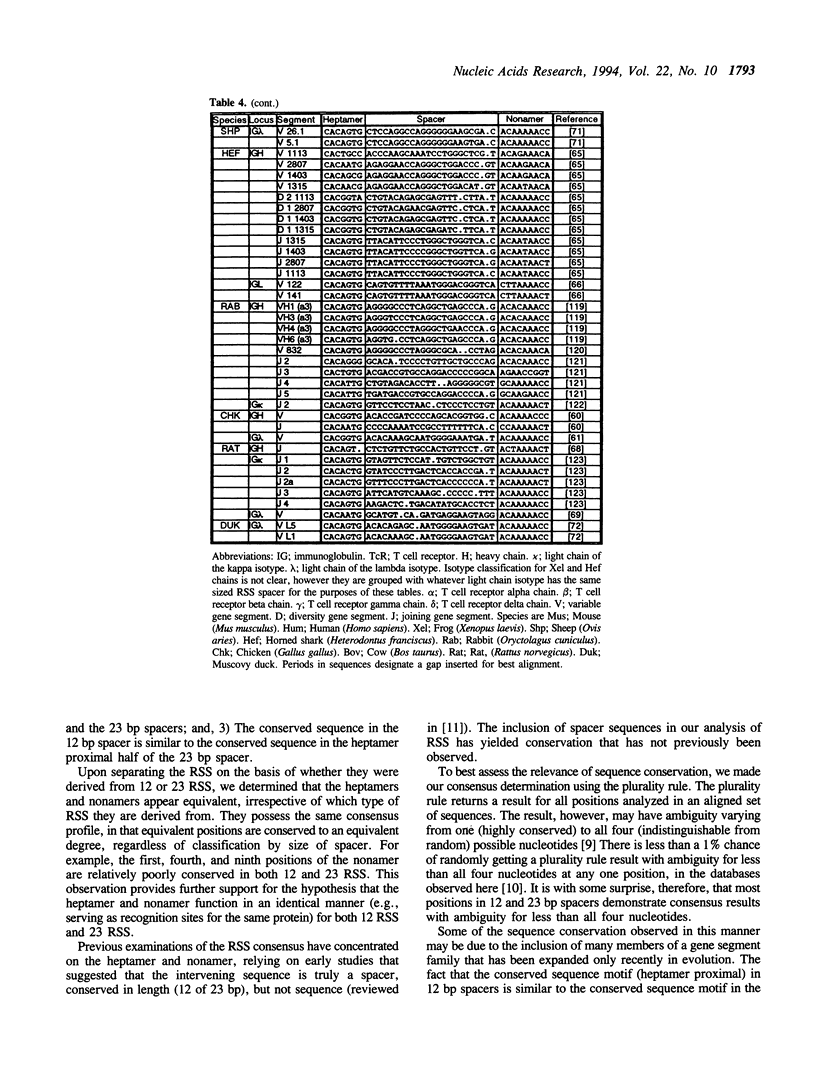
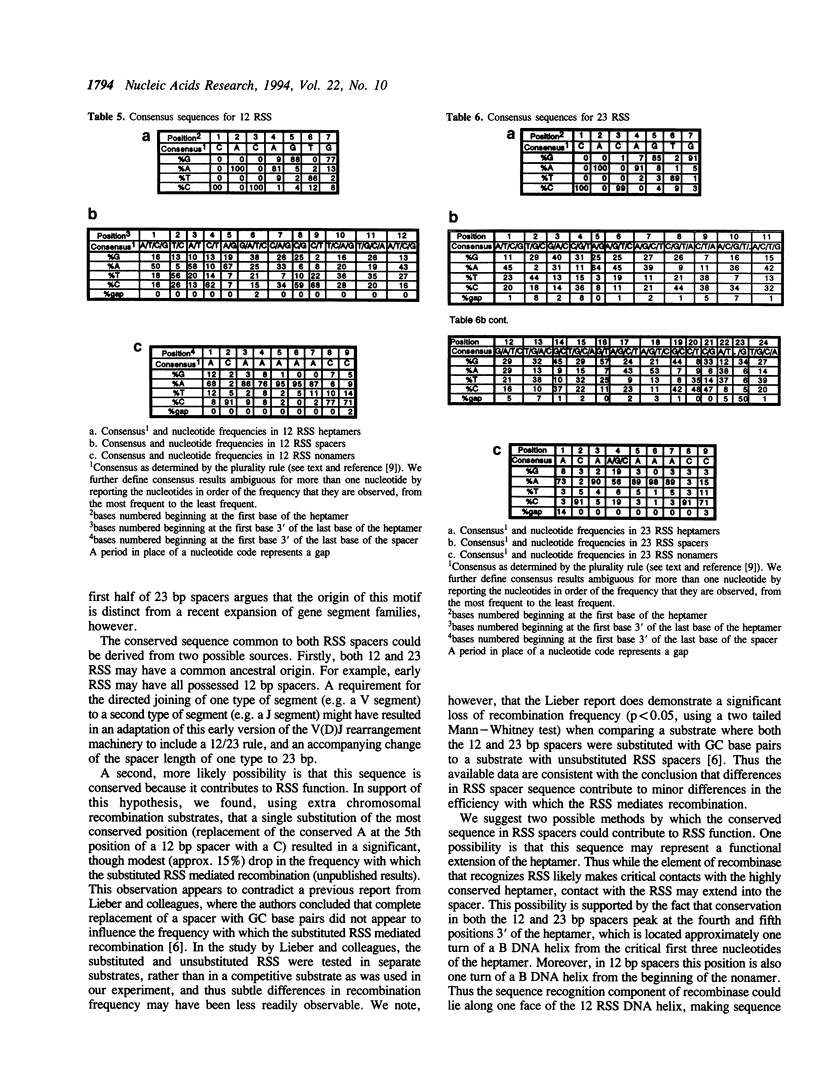
The variable domains of immunoglobulins and T cell receptors are assembled through the somatic, site specific recombination of multiple germline segments (V, D, and J segments) or V(D)J rearrangement. The recombination signal sequence (RSS) is necessary and sufficient for cell type specific targeting of the V(D)J rearrangement machinery to these germline segments. Previously, the RSS has been described as possessing both a conserved heptamer and a conserved nonamer motif. The heptamer and nonamer motifs are separated by a 'spacer' that was not thought to possess significant sequence conservation, however the length of the spacer could be either 12 +/- 1 bp or 23 +/- 1 bp long. In this report we have assembled and analyzed an extensive data base of published RSS. We have derived, through extensive consensus comparison, a more detailed description of the RSS than has previously been reported. Our analysis indicates that RSS spacers possess significant conservation of sequence, and that the conserved sequence in 12 bp spacers is similar to the conserved sequence in the first half of 23 bp spacers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Alexandre D., Chuchana P., Brockly F., Blancher A., Lefranc G., Lefranc M. P. First genomic sequence of a human Ig variable lambda gene belonging to subgroup I. Functional genes, pseudogenes and vestigial sequences are interspersed in the IGLV locus. Nucleic Acids Res. 1989 May 25;17(10):3975–3975. doi: 10.1093/nar/17.10.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden B., Klotz J. L., Siu G., Hood L. E. Diversity and structure of genes of the alpha family of mouse T-cell antigen receptor. 1985 Aug 29-Sep 4Nature. 316(6031):783–787. doi: 10.1038/316783a0. [DOI] [PubMed] [Google Scholar]
- Baer R., Boehm T., Yssel H., Spits H., Rabbitts T. H. Complex rearrangements within the human J delta-C delta/J alpha-C alpha locus and aberrant recombination between J alpha segments. EMBO J. 1988 Jun;7(6):1661–1668. doi: 10.1002/j.1460-2075.1988.tb02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A. M., Zhurkin V. B. CAP binding sites reveal pyrimidine-purine pattern characteristic of DNA bending. J Biomol Struct Dyn. 1990 Oct;8(2):213–232. doi: 10.1080/07391102.1990.10507803. [DOI] [PubMed] [Google Scholar]
- Becker R. S., Suter M., Knight K. L. Restricted utilization of VH and DH genes in leukemic rabbit B cells. Eur J Immunol. 1990 Feb;20(2):397–402. doi: 10.1002/eji.1830200224. [DOI] [PubMed] [Google Scholar]
- Becker R. S., Zhai S. K., Currier S. J., Knight K. L. Ig VH, DH, and JH germ-line gene segments linked by overlapping cosmid clones of rabbit DNA. J Immunol. 1989 Feb 15;142(4):1351–1355. [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Human immunoglobulin variable region genes--DNA sequences of two V kappa genes and a pseudogene. Nature. 1980 Dec 25;288(5792):730–733. doi: 10.1038/288730a0. [DOI] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard F., Chuchana P., Frippiat J. P., Buluwela L., Lefranc M. P. Genomic sequence of IGLV1S2, a human immunoglobulin variable lambda gene belonging to subgroup I. Nucleic Acids Res. 1990 Dec 11;18(23):7139–7139. doi: 10.1093/nar/18.23.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Blankenstein T., Bonhomme F., Krawinkel U. Evolution of pseudogenes in the immunoglobulin VH-gene family of the mouse. Immunogenetics. 1987;26(4-5):237–248. doi: 10.1007/BF00346518. [DOI] [PubMed] [Google Scholar]
- Bolshoy A., McNamara P., Harrington R. E., Trifonov E. N. Curved DNA without A-A: experimental estimation of all 16 DNA wedge angles. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2312–2316. doi: 10.1073/pnas.88.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Boyd R. T., Goldrick M. M., Gottlieb P. D. Structural differences in a single gene encoding the V kappa Ser group of light chains explain the existence of two mouse light-chain genetic markers. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9134–9138. doi: 10.1073/pnas.83.23.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockly F., Alexandre D., Chuchana P., Huck S., Lefranc G., Lefranc M. P. First nucleotide sequence of a human immunoglobulin variable lambda gene belonging to subgroup II. Nucleic Acids Res. 1989 May 25;17(10):3976–3976. doi: 10.1093/nar/17.10.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann M., Free J., Diamond A., Howard J., Cobbold S., Waldmann H. Immunoglobulin heavy chain locus of the rat: striking homology to mouse antibody genes. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6075–6079. doi: 10.1073/pnas.83.16.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P., Albrandt K., Orida N. K., Radoux V., Chen E. Y., Schrantz R., Liu F. T., Carson D. A. Genetic basis for the cross-reactive idiotypes on the light chains of human IgM anti-IgG autoantibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8318–8322. doi: 10.1073/pnas.83.21.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chou H. S., Anderson S. J., Louie M. C., Godambe S. A., Pozzi M. R., Behlke M. A., Huppi K., Loh D. Y. Tandem linkage and unusual RNA splicing of the T-cell receptor beta-chain variable-region genes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1992–1996. doi: 10.1073/pnas.84.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou H. S., Behlke M. A., Godambe S. A., Russell J. H., Brooks C. G., Loh D. Y. T cell receptor genes in an alloreactive CTL clone: implications for rearrangement and germline diversity of variable gene segments. EMBO J. 1986 Sep;5(9):2149–2155. doi: 10.1002/j.1460-2075.1986.tb04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Effron K., Rechavi G., Ben-Neriah Y., Zakut R., Givol D. Simple DNA sequences in homologous flanking regions near immunoglobulin VH genes: a role in gene interaction? Nucleic Acids Res. 1982 Jun 11;10(11):3353–3370. doi: 10.1093/nar/10.11.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Givol D. Allelic immunoglobulin VH genes in two mouse strains: possible germline gene recombination. EMBO J. 1983;2(11):2013–2018. doi: 10.1002/j.1460-2075.1983.tb01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet S., Milili M., Fougereau M., Schiff C. Two V kappa germ-line genes related to the GAT idiotypic network (Ab1 and Ab3/Ab1') account for the major subfamilies of the mouse V kappa-1 variability subgroup. J Immunol. 1987 Feb 1;138(3):932–939. [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Daley M. D., Peng H. Q., Misener V., Liu X. Y., Chen P. P., Siminovitch K. A. Molecular analysis of human immunoglobulin V lambda germline genes: subgroups V lambda III and V lambda IV. Mol Immunol. 1992 Dec;29(12):1515–1518. doi: 10.1016/0161-5890(92)90226-n. [DOI] [PubMed] [Google Scholar]
- Day W. H., McMorris F. R. Critical comparison of consensus methods for molecular sequences. Nucleic Acids Res. 1992 Mar 11;20(5):1093–1099. doi: 10.1093/nar/20.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day W. H., McMorris F. R. Interpreting consensus sequences based on plurality rule. Math Biosci. 1992 Oct;111(2):231–247. doi: 10.1016/0025-5564(92)90072-5. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Rock E. P., Patten P. A., Davis M. M., Chien Y. H. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988 Feb 18;331(6157):627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- Frippiat J. P., Chuchana P., Bernard F., Buluwela L., Lefranc G., Lefranc M. P. First genomic sequence of a human Ig variable lambda gene belonging to subgroup III. Nucleic Acids Res. 1990 Dec 11;18(23):7134–7134. doi: 10.1093/nar/18.23.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman R. D., Doherty P. J., Raulet D. H. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986 Jun 6;45(5):733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Gellert M. Molecular analysis of V(D)J recombination. Annu Rev Genet. 1992;26:425–446. doi: 10.1146/annurev.ge.26.120192.002233. [DOI] [PubMed] [Google Scholar]
- Givol D., Zakut R., Effron K., Rechavi G., Ram D., Cohen J. B. Diversity of germ-line immunoglobulin VH genes. Nature. 1981 Jul 30;292(5822):426–430. doi: 10.1038/292426a0. [DOI] [PubMed] [Google Scholar]
- Guglielmi P., Davi F., d'Auriol L., Bories J. C., Dausset J., Bensussan A. Use of a variable alpha region to create a functional T-cell receptor delta chain. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5634–5638. doi: 10.1073/pnas.85.15.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman G. A., Besta R. M., Frank M. B., Baverstock P. R. Duplication of J kappa genes within genus Rattus. Immunogenetics. 1987;26(1-2):14–20. doi: 10.1007/BF00345449. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Diamond D. J., Tanigawa G., Heilig J. S., Folsom V., Saito H., Tonegawa S. Unusual organization and diversity of T-cell receptor alpha-chain genes. 1985 Aug 29-Sep 4Nature. 316(6031):828–832. doi: 10.1038/316828a0. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Heidmann O., Rougeon F. Diversity in the rabbit immunoglobulin kappa chain variable regions is amplified by nucleotide deletions and insertions at the V-J junction. Cell. 1983 Oct;34(3):767–777. doi: 10.1016/0092-8674(83)90533-0. [DOI] [PubMed] [Google Scholar]
- Heidmann O., Rougeon F. Immunoglobulin kappa light-chain diversity in rabbit is based on the 3' length heterogeneity of germ-line variable genes. Nature. 1984 Sep 6;311(5981):74–76. doi: 10.1038/311074a0. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Traunecker A., Tonegawa S. Somatic mutation creates diversity in the major group of mouse immunoglobulin kappa light chains. J Exp Med. 1984 Feb 1;159(2):417–435. doi: 10.1084/jem.159.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Maizel J. V., Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [PubMed] [Google Scholar]
- Hirama T., Takeshita S., Matsubayashi Y., Iwashiro M., Masuda T., Kuribayashi K., Yoshida Y., Yamagishi H. Conserved V(D)J junctional sequence of cross-reactive cytotoxic T cell receptor idiotype and the effect of a single amino acid substitution. Eur J Immunol. 1991 Feb;21(2):483–488. doi: 10.1002/eji.1830210234. [DOI] [PubMed] [Google Scholar]
- Hockett R. D., de Villartay J. P., Pollock K., Poplack D. G., Cohen D. I., Korsmeyer S. J. Human T-cell antigen receptor (TCR) delta-chain locus and elements responsible for its deletion are within the TCR alpha-chain locus. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9694–9698. doi: 10.1073/pnas.85.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck S., Dariavach P., Lefranc M. P. Variable region genes in the human T-cell rearranging gamma (TRG) locus: V-J junction and homology with the mouse genes. EMBO J. 1988 Mar;7(3):719–726. doi: 10.1002/j.1460-2075.1988.tb02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara Y., Matsuoka H., Kurosawa Y. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 1988 Dec 20;7(13):4141–4150. doi: 10.1002/j.1460-2075.1988.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Ogura T., Shimizu A., Honjo T. Low frequency of somatic mutation in beta-chain variable region genes of human T-cell receptors. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7701–7705. doi: 10.1073/pnas.82.22.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R., Gershenfeld H., Weissman I. L. Evolution of a multigene family of V kappa germ line genes. EMBO J. 1984 Jan;3(1):185–191. doi: 10.1002/j.1460-2075.1984.tb01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvin-Marche E., Rudikoff S. Evolution of a V kappa gene family. Immunogenetics. 1986;24(3):191–201. doi: 10.1007/BF00364748. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Nikaido T., Miyata T., Moriwaki K., Honjo T. The nucleotide sequences of rearranged and germline immunoglobulin VH genes of a mouse myeloma MC101 and evolution of VH genes in mouse. J Biol Chem. 1982 Jan 10;257(1):277–285. [PubMed] [Google Scholar]
- Knight K. L., Becker R. S. Molecular basis of the allelic inheritance of rabbit immunoglobulin VH allotypes: implications for the generation of antibody diversity. Cell. 1990 Mar 23;60(6):963–970. doi: 10.1016/0092-8674(90)90344-e. [DOI] [PubMed] [Google Scholar]
- Kodaira M., Kinashi T., Umemura I., Matsuda F., Noma T., Ono Y., Honjo T. Organization and evolution of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1986 Aug 20;190(4):529–541. doi: 10.1016/0022-2836(86)90239-1. [DOI] [PubMed] [Google Scholar]
- Kokubu F., Litman R., Shamblott M. J., Hinds K., Litman G. W. Diverse organization of immunoglobulin VH gene loci in a primitive vertebrate. EMBO J. 1988 Nov;7(11):3413–3422. doi: 10.1002/j.1460-2075.1988.tb03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- LeFranc M. P., Forster A., Baer R., Stinson M. A., Rabbitts T. H. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986 Apr 25;45(2):237–246. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Matsuda F., Kinashi T., Kodaira M., Honjo T. A novel family of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1987 Jun 20;195(4):761–768. doi: 10.1016/0022-2836(87)90482-7. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Forster A., Rabbitts T. H. Rearrangement of two distinct T-cell gamma-chain variable-region genes in human DNA. 1986 Jan 30-Feb 5Nature. 319(6052):420–422. doi: 10.1038/319420a0. [DOI] [PubMed] [Google Scholar]
- Lieberman R., Emorine L., Max E. E. Structure of a germline rabbit immunoglobulin V kappa-region gene: implications for rabbit V kappa-J kappa recombination. J Immunol. 1984 Nov;133(5):2753–2756. [PubMed] [Google Scholar]
- Liu M. F., Robbins D. L., Crowley J. J., Sinha S., Kozin F., Kipps T. J., Carson D. A., Chen P. P. Characterization of four homologous L chain variable region genes that are related to 6B6.6 idiotype positive human rheumatoid factor L chains. J Immunol. 1989 Jan 15;142(2):688–694. [PubMed] [Google Scholar]
- Louie M. C., Nelson C. A., Loh D. Y. Identification and characterization of new murine T cell receptor beta chain variable region (V beta) genes. J Exp Med. 1989 Dec 1;170(6):1987–1998. doi: 10.1084/jem.170.6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F., Lee K. H., Nakai S., Sato T., Kodaira M., Zong S. Q., Ohno H., Fukuhara S., Honjo T. Dispersed localization of D segments in the human immunoglobulin heavy-chain locus. EMBO J. 1988 Apr;7(4):1047–1051. doi: 10.1002/j.1460-2075.1988.tb02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthyssens G., Rabbitts T. H. Structure and multiplicity of genes for the human immunoglobulin heavy chain variable region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6561–6565. doi: 10.1073/pnas.77.11.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Miller H., Leder P. Variation in the crossover point of kappa immunoglobulin gene V-J recombination: evidence from a cryptic gene. Cell. 1980 Oct;21(3):793–799. doi: 10.1016/0092-8674(80)90442-0. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Carlson L. M., Tjoelker L. W., Thompson C. B. Evolutionary comparison of the avian IgL locus: combinatorial diversity plays a role in the generation of the antibody repertoire in some avian species. Int Immunol. 1989;1(4):332–341. doi: 10.1093/intimm/1.4.332. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Laster S. M., Marzluff W. F., Roux K. H. Dynamic gene interactions in the evolution of rabbit VH genes: a four codon duplication and block homologies provide evidence for intergenic exchange. Nucleic Acids Res. 1985 Oct 11;13(19):7041–7054. doi: 10.1093/nar/13.19.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Selsing E., Storb U. Structural alterations in J regions of mouse immunoglobulin lambda genes are associated with differential gene expression. Nature. 1982 Feb 4;295(5848):428–430. doi: 10.1038/295428a0. [DOI] [PubMed] [Google Scholar]
- Milstein C., Even J., Jarvis J. M., Gonzalez-Fernandez A., Gherardi E. Non-random features of the repertoire expressed by the members of one V kappa gene family and of the V-J recombination. Eur J Immunol. 1992 Jun;22(6):1627–1634. doi: 10.1002/eji.1830220642. [DOI] [PubMed] [Google Scholar]
- Ng K. H., Lavigueur A., Ricard L., Boivrette M., Maclean S., Cloutier D., Gibson D. M. Characterization of allelic V kappa-1 region genes in inbred strains of mice. J Immunol. 1989 Jul 15;143(2):638–648. [PubMed] [Google Scholar]
- Ollo R., Auffray C., Sikorav J. L., Rougeon F. Mouse heavy chain variable regions: nucleotide sequence of a germ-line VH gene segment. Nucleic Acids Res. 1981 Aug 25;9(16):4099–4109. doi: 10.1093/nar/9.16.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Sikorav J. L., Rougeon F. Structural relationships among mouse and human immunoglobulin VH genes in the subgroup III. Nucleic Acids Res. 1983 Nov 25;11(22):7887–7897. doi: 10.1093/nar/11.22.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech M., Jaenichen H. R., Pohlenz H. D., Neumaier P. S., Klobeck H. G., Zachau H. G. Organization and evolution of a gene cluster for human immunoglobulin variable regions of the kappa type. J Mol Biol. 1984 Jun 25;176(2):189–204. doi: 10.1016/0022-2836(84)90420-0. [DOI] [PubMed] [Google Scholar]
- Pech M., Zachau H. G. Immunoglobulin genes of different subgroups are interdigitated within the VK locus. Nucleic Acids Res. 1984 Dec 21;12(24):9229–9236. doi: 10.1093/nar/12.24.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden D. A., Wu G. E. Mouse kappa light-chain recombination signal sequences mediate recombination more frequently than do those of lambda light chain. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10721–10725. doi: 10.1073/pnas.88.23.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Bienz B., Ram D., Ben-Neriah Y., Cohen J. B., Zakut R., Givol D. Organization and evolution of immunoglobulin VH gene subgroups. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4405–4409. doi: 10.1073/pnas.79.14.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Ram D., Glazer L., Zakut R., Givol D. Evolutionary aspects of immunoglobulin heavy chain variable region (VH) gene subgroups. Proc Natl Acad Sci U S A. 1983 Feb;80(3):855–859. doi: 10.1073/pnas.80.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Anquez V., Weill J. C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989 Oct 6;59(1):171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Mackay C. R., Müller R. G., Weill J. C. Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyer's patches. Cell. 1991 Mar 8;64(5):995–1005. doi: 10.1016/0092-8674(91)90323-q. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Kurosawa Y., Weigert M., Tonegawa S. Identification and nucleotide sequence of a diversity DNA segment (D) of immunoglobulin heavy-chain genes. Nature. 1981 Apr 16;290(5807):562–565. doi: 10.1038/290562a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sanchez P., Marche P. N., Rueff-Juy D., Cazenave P. A. Mouse V lambda x gene sequence generates no junctional diversity and is conserved in mammalian species. J Immunol. 1990 Apr 1;144(7):2816–2820. [PubMed] [Google Scholar]
- Satyanarayana K., Hata S., Devlin P., Roncarolo M. G., De Vries J. E., Spits H., Strominger J. L., Krangel M. S. Genomic organization of the human T-cell antigen-receptor alpha/delta locus. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8166–8170. doi: 10.1073/pnas.85.21.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff C., Milili M., Hue I., Rudikoff S., Fougereau M. Genetic basis for expression of the idiotypic network. One unique Ig VH germline gene accounts for the major family of Ab1 and Ab3 (Ab1') antibodies of the GAT system. J Exp Med. 1986 Mar 1;163(3):573–587. doi: 10.1084/jem.163.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Structure and evolution of mammalian VH families. Int Immunol. 1990;2(1):41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- Schwager J., Bürckert N., Courtet M., Du Pasquier L. Genetic basis of the antibody repertoire in Xenopus: analysis of the Vh diversity. EMBO J. 1989 Oct;8(10):2989–3001. doi: 10.1002/j.1460-2075.1989.tb08449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Leder A., Edgell M. H., Polsky F., Tilghman S. M., Tiemeier D. C., Leder P. Multiple related immunoglobulin variable-region genes identified by cloning and sequence analysis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3881–3885. doi: 10.1073/pnas.75.8.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Shamblott M. J., Litman G. W. Genomic organization and sequences of immunoglobulin light chain genes in a primitive vertebrate suggest coevolution of immunoglobulin gene organization. EMBO J. 1989 Dec 1;8(12):3733–3739. doi: 10.1002/j.1460-2075.1989.tb08549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A., Humphries C., Tucker P., Blattner F. Human heavy-chain variable region gene family nonrandomly rearranged in familial chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8563–8567. doi: 10.1073/pnas.84.23.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Ravetch J. V., Korsmeyer S., Waldmann T., Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981 Dec 17;294(5842):631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Siminovitch K. A., Misener V., Kwong P. C., Song Q. L., Chen P. P. A natural autoantibody is encoded by germline heavy and lambda light chain variable region genes without somatic mutation. J Clin Invest. 1989 Nov;84(5):1675–1678. doi: 10.1172/JCI114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu G., Clark S. P., Yoshikai Y., Malissen M., Yanagi Y., Strauss E., Mak T. W., Hood L. The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell. 1984 Jun;37(2):393–401. doi: 10.1016/0092-8674(84)90369-6. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Siu G., Strauss E. C., Lai E., Hood L. E. Analysis of a human V beta gene subfamily. J Exp Med. 1986 Nov 1;164(5):1600–1614. doi: 10.1084/jem.164.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. J., Tunnacliffe A., Rabbitts T. H. Germline sequence of two human T-cell receptor V beta genes: V beta 8.1 is transcribed from a TATA-box promoter. Nucleic Acids Res. 1987 Jun 25;15(12):4991–4991. doi: 10.1093/nar/15.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen M. L., Hellman L., Pettersson U. The immunoglobulin lambda locus in rat consists of two C lambda genes and a single V lambda gene. Gene. 1987;55(1):75–84. doi: 10.1016/0378-1119(87)90250-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi N., Ishiguro N., Shinagawa M. Molecular cloning and sequence analysis of bovine T-cell receptor gamma and delta chain genes. Immunogenetics. 1992;35(2):89–96. doi: 10.1007/BF00189517. [DOI] [PubMed] [Google Scholar]
- Timsit Y., Vilbois E., Moras D. Base-pairing shift in the major groove of (CA)n tracts by B-DNA crystal structures. Nature. 1991 Nov 14;354(6349):167–170. doi: 10.1038/354167a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyonaga B., Yoshikai Y., Vadasz V., Chin B., Mak T. W. Organization and sequences of the diversity, joining, and constant region genes of the human T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8624–8628. doi: 10.1073/pnas.82.24.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnacliffe A., Rabbitts T. H. Sequence of the D beta 2-J beta 2 region of the human T-cell receptor beta-chain locus. Nucleic Acids Res. 1985 Sep 25;13(18):6651–6661. doi: 10.1093/nar/13.18.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udey J. A., Blomberg B. Human lambda light chain locus: organization and DNA sequences of three genomic J regions. Immunogenetics. 1987;25(1):63–70. doi: 10.1007/BF00768834. [DOI] [PubMed] [Google Scholar]
- Vasicek T. J., Leder P. Structure and expression of the human immunoglobulin lambda genes. J Exp Med. 1990 Aug 1;172(2):609–620. doi: 10.1084/jem.172.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Lieber M. R. Lymphoid V(D)J recombination. Functional analysis of the spacer sequence within the recombination signal. J Biol Chem. 1993 Feb 15;268(5):3180–3183. [PubMed] [Google Scholar]
- Wilson R. K., Koop B. F., Chen C., Halloran N., Sciammis R., Hood L. Nucleotide sequence analysis of 95 kb near the 3' end of the murine T-cell receptor alpha/delta chain locus: strategy and methodology. Genomics. 1992 Aug;13(4):1198–1208. doi: 10.1016/0888-7543(92)90038-t. [DOI] [PubMed] [Google Scholar]
- Winoto A., Mjolsness S., Hood L. Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):832–836. doi: 10.1038/316832a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Clark S. P., Taylor S., Sohn U., Wilson B. I., Minden M. D., Mak T. W. Organization and sequences of the variable, joining and constant region genes of the human T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):837–840. doi: 10.1038/316837a0. [DOI] [PubMed] [Google Scholar]
- Zezza D. J., Stewart S. E., Steiner L. A. Genes encoding Xenopus laevis Ig L chains. Implications for the evolution of kappa and lambda chains. J Immunol. 1992 Dec 15;149(12):3968–3977. [PubMed] [Google Scholar]


