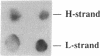Abstract
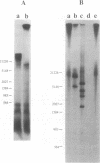
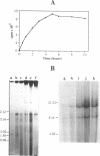
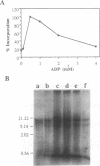
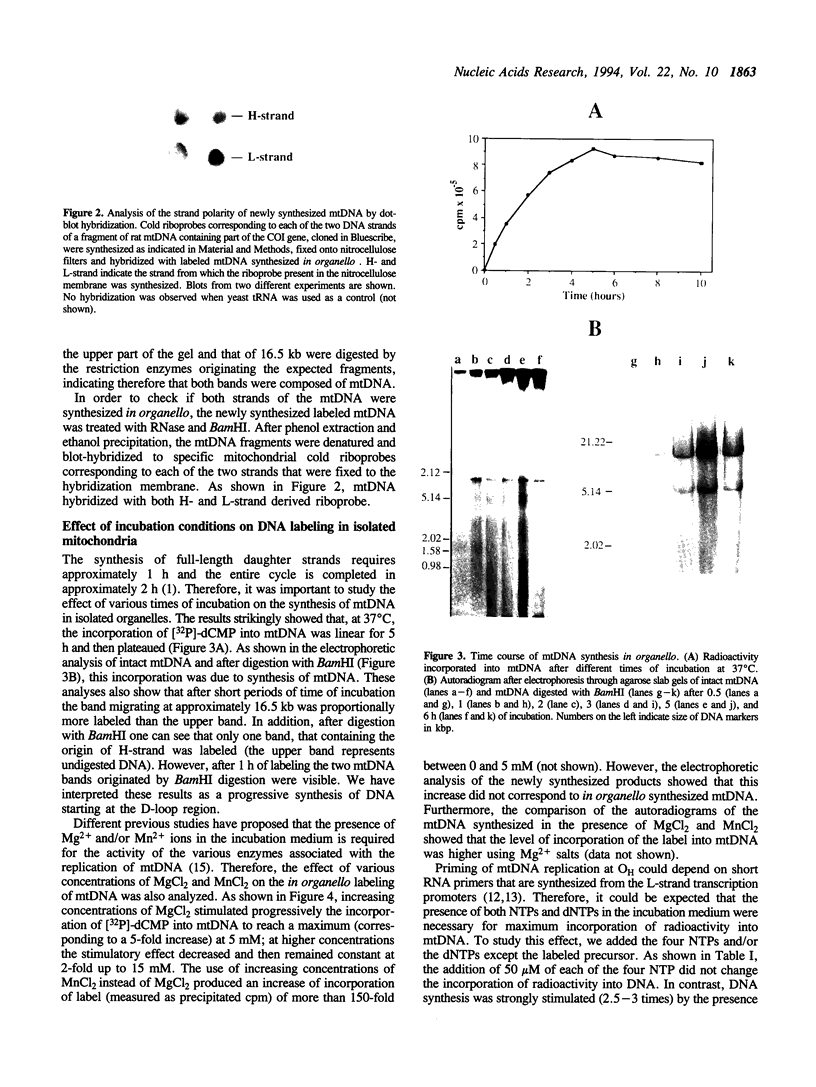
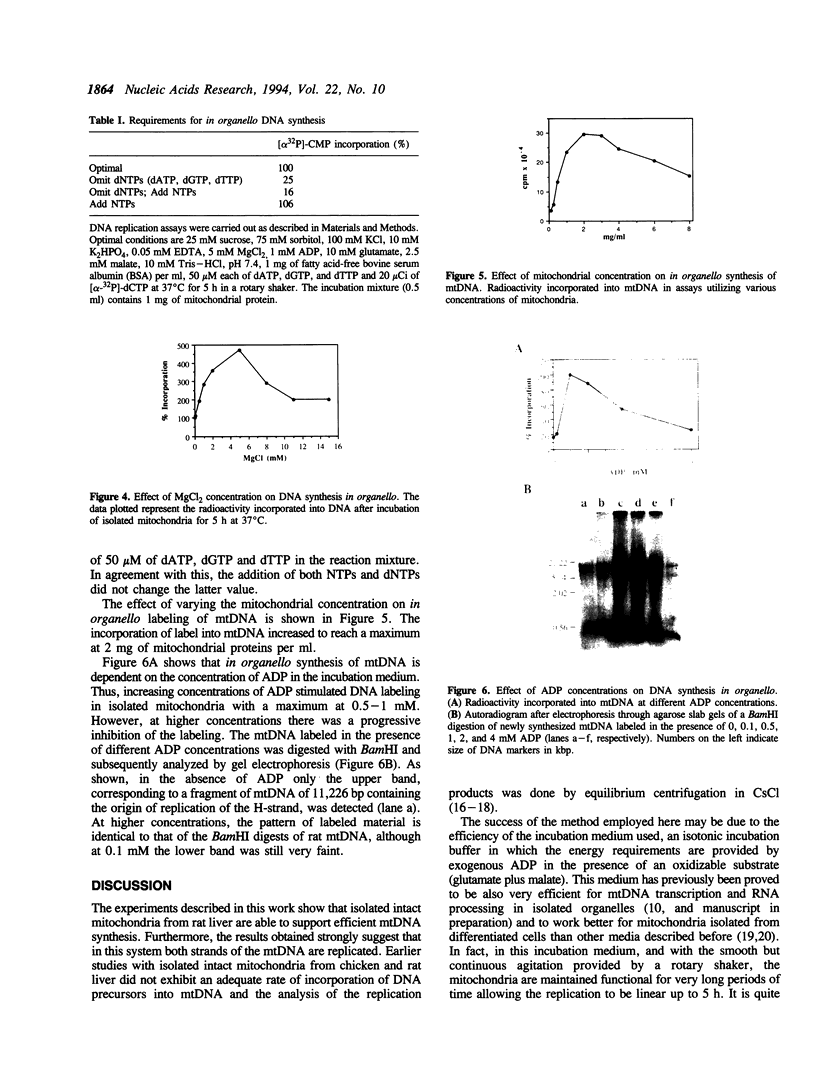
We have developed a highly efficient DNA-synthesizing system with isolated intact rat liver mitochondria. The ATP requirements for this in organello DNA synthesis are provided by endogenous synthesis in the presence of exogenous ADP and an oxidizable substrate. In this system, mitochondrial DNA synthesis strikingly proceeds at a constant rate for about 5 h at 37 degrees C. Gel electrophoresis, hybridization and restriction enzyme analyses show that intact mitochondria synthesize nucleic acids with a size of 16.5 kb, that correspond to mitochondrial DNA, and that both DNA strands are replicated. This in organello DNA synthesis requires the supply of dNTPs and decreases at high ADP concentration in the incubation medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Montoya J. Analysis of human mitochondrial RNA. Methods Enzymol. 1983;97:435–469. doi: 10.1016/0076-6879(83)97154-9. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Loguercio Polosa P., Mustich A., Petruzzella V., Gadaleta M. N. Faithful and highly efficient RNA synthesis in isolated mitochondria from rat liver. Curr Genet. 1988 Nov;14(5):477–482. doi: 10.1007/BF00521272. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Morisco P., Rainaldi G., Gadaleta M. N., Saccone C. A novel gene order in the Paracentrotus lividus mitochondrial genome. Gene. 1987;53(1):41–54. doi: 10.1016/0378-1119(87)90091-6. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc Natl Acad Sci U S A. 1985 Jan;82(2):351–355. doi: 10.1073/pnas.82.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Hauswirth W. W., Clayton D. A. Replication priming and transcription initiate from precisely the same site in mouse mitochondrial DNA. EMBO J. 1985 Jun;4(6):1559–1567. doi: 10.1002/j.1460-2075.1985.tb03817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Dunon-Bluteau D., Cordonnier A., Brun G. DNA synthesis in a mitochondrial lysate of Xenopus laevis oocytes. H strand replication in vitro. J Mol Biol. 1987 Sep 20;197(2):175–185. doi: 10.1016/0022-2836(87)90116-1. [DOI] [PubMed] [Google Scholar]
- Enriquez J. A., López-Pérez M. J., Montoya J. Saturation of the processing of newly synthesized rRNA in isolated brain mitochondria. FEBS Lett. 1991 Mar 11;280(1):32–36. doi: 10.1016/0014-5793(91)80197-b. [DOI] [PubMed] [Google Scholar]
- Enríquez J. A., Pérez-Martos A., Fernández-Silva P., López-Pérez M. J., Montoya J. Specific increase of a mitochondrial RNA transcript in chronic ethanol-fed rats. FEBS Lett. 1992 Jun 15;304(2-3):285–288. doi: 10.1016/0014-5793(92)80639-x. [DOI] [PubMed] [Google Scholar]
- Gadaleta M. N., Petruzzella V., Renis M., Fracasso F., Cantatore P. Reduced transcription of mitochondrial DNA in the senescent rat. Tissue dependence and effect of L-carnitine. Eur J Biochem. 1990 Feb 14;187(3):501–506. doi: 10.1111/j.1432-1033.1990.tb15331.x. [DOI] [PubMed] [Google Scholar]
- Gaines G., Attardi G. Highly efficient RNA-synthesizing system that uses isolated human mitochondria: new initiation events and in vivo-like processing patterns. Mol Cell Biol. 1984 Aug;4(8):1605–1617. doi: 10.1128/mcb.4.8.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson J. E., Wong T. W., Clayton D. A. Both the conserved stem-loop and divergent 5'-flanking sequences are required for initiation at the human mitochondrial origin of light-strand DNA replication. J Biol Chem. 1986 Feb 15;261(5):2384–2390. [PubMed] [Google Scholar]
- Jui H. Y., Wong T. W. In vitro replication of heavy strand DNA in permeabilized human mitochondria. Nucleic Acids Res. 1991 Feb 25;19(4):905–911. doi: 10.1093/nar/19.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Kobayashi M., Fujisawa T. Novel properties of two classes of nascent mitochondrial DNA formed in vitro. Biochim Biophys Acta. 1974 Aug 29;361(2):144–154. doi: 10.1016/0005-2787(74)90342-6. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. A detailed physical map of HeLa cell mitochondria DNA and its alignment with the positions of known genetic markers. Plasmid. 1977 Nov;1(1):78–105. doi: 10.1016/0147-619x(77)90010-5. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Wong T. W., Clayton D. A. In vitro replication of human mitochondrial DNA: accurate initiation at the origin of light-strand synthesis. Cell. 1985 Oct;42(3):951–958. doi: 10.1016/0092-8674(85)90291-0. [DOI] [PubMed] [Google Scholar]
- ter Schegget J., Borst P. DNA synthesis by isolated mitochondria. I. Effect of inhibitors and characterization of the product. Biochim Biophys Acta. 1971 Aug 26;246(2):239–248. [PubMed] [Google Scholar]