Abstract
The mouse-human chimeric anti-epidermal growth factor receptor vIII (EGFRvIII) antibody C12 is a promising candidate for the diagnosis of hepatocellular carcinoma (HCC). In this study, 3 processes were successfully developed to produce C12 by cultivation of recombinant Chinese hamster ovary (CHO-DG44) cells in serum-free medium. The effect of inoculum density was evaluated in batch cultures of shaker flasks to obtain the optimal inoculum density of 5 × 105 cells/mL. Then, the basic metabolic characteristics of CHO-C12 cells were studied in stirred bioreactor batch cultures. The results showed that the limiting concentrations of glucose and glutamine were 6 and 1 mM, respectively. The culture process consumed significant amounts of aspartate, glutamate, asparagine, serine, isoleucine, leucine, and lysine. Aspartate, glutamate, asparagine, and serine were particularly exhausted in the early growth stage, thus limiting cell growth and antibody synthesis. Based on these findings, fed-batch and perfusion processes in the bioreactor were successfully developed with a balanced amino acid feed strategy. Fed-batch and especially perfusion culture effectively maintained high cell viability to prolong the culture process. Furthermore, perfusion cultures maximized the efficiency of nutrient utilization; the mean yield coefficient of antibody to consumed glucose was 44.72 mg/g and the mean yield coefficient of glutamine to antibody was 721.40 mg/g. Finally, in small-scale bioreactor culture, the highest total amount of C12 antibody (1,854 mg) was realized in perfusion cultures. Therefore, perfusion culture appears to be the optimal process for small-scale production of C12 antibody by rCHO-C12 cells.
Keywords: Mouse-human chimeric anti-EGFRvIII antibody, Recombinant CHO-C12 cells, Balanced amino acid feed, Perfusion culture
Introduction
Hepatocellular carcinoma (HCC), one of the most malignant diseases known to man, is the fifth most common solid tumor worldwide, and the fourth leading cause of cancer-related death (Thomas and Zhu 2005). It is especially prevalent in China, Korea, and sub-Saharan Africa, and increases by an estimated 0.5–1 million new cases per year (Parkin et al. 1984; Serag et al. 2001). Among the many methods to treat HCC, such as surgery, chemotherapy, intervention therapy, and liver transplantation, liver transplantation is considered the treatment with the best chances for a cure. Nonetheless, there is still a certain level of recurrence after transplantation (Yoo et al. 2003), and some research suggests that the survival rate of patients was not significantly improved (Zhu 2006). Therefore, innovative treatment approaches are urgently needed.
Recently, the epidermal growth factor receptor (EGFR) has emerged as a promising cancer therapy target (Gainet et al. 2003; Ciardiello and Tortora 2001). Abnormal, enhanced, or constitutive expression of EGFR, which was closely related to cell growth and survival, was found in a variety of tumors (Ciardiello and Tortora 2001). Generally, many naturally occurring EGFR gene mutations exist in tumor cells and normal tissues; EGFRvIII is the most commonly described mutation and produces a ligand-independent constitutively active variant. Surprisingly, this variant is not present in normal tissues but has been detected in multiple human malignancies such as glioma, non-small cell lung carcinoma, breast cancer, head and neck cancer, and ovarian carcinoma (Okamoto et al. 2003; Moscatello et al. 1995; Sok et al. 2006; Zeineldin et al. 2006). The level of EGFRvIII expression was closely correlated with increased tumorigenicity in mouse models (Nishikawa et al. 1994; Ramnarain et al. 2006) and poor prognosis of brain cancer in clinical settings (Diedrich et al. 1995; Liu et al. 2005). In addition, the EGFRvIII mutation has been detected in HCC tissue samples (Ou et al. 2005) and found to play a vital role in tumorigenicity and enhanced resistance to 5-Fluorouracil (5-FU) treatment of HCC (Wang et al. 2009). Therefore, in our previous work, we successfully developed and produced the chimeric anti-EGFRvIII antibody C12 from CHO-DG44 cells. This antibody binds to the same sites, possesses similar affinity to ligand as the monoclonal antibody 12H23, and effectively reduces the human anti-mouse antibody response (HAMA) (Wang 2009). Thus, there is an increasing demand for purified C12 antibody in large amounts for use in pre-clinical or clinical studies or in studies on its biological character. However, there is currently no ideal bioprocess for the production of C12 antibody here or abroad.
The dihydrofolate reductase-deficient CHO cell line DG44 is the major mammalian host for recombinant protein production, mainly because of its well-characterized genetic selection and amplification system (Derouazi et al. 2006). There are 18 therapeutic antibodies currently licensed for use, of which 10 are produced in CHO and the other 8 are produced in murine lymphoid cells (including NS0 and Sp2/0-Ag 14) (John et al. 2006). Three main culture processes, that is, batch culture, fed-batch culture, and perfusion culture have been developed for the production of cell-culture-based recombinant proteins or antibody production with CHO-DG44 cells. Each of these processes have their individual advantages. For example, the major advantage of fed-batch culture is its relatively high concentration of its final product. The final recombinant protein concentration in fed-batch processes is generally determined by the viable cell density, viable cell culture time, and specific rate of production of the recombinant protein. Therefore, fed-batch processes have been developed to maximize these parameters by optimizing different aspects such as culture medium and environmental parameters (Bibila and Robinson 1995). The viable cell density and time span of viable cell culture have been prolonged by adding limiting nutrients, e.g., glucose, amino acids, and minerals (Bibila et al. 1994; Xie and Wang 1996; DeZengotita 2000). The major advantages of perfusion culture are that it can avoid nutrient limitation, reduce growth inhibitory metabolites, and lower activities of protease and glycosidase in the culture media, which is expected to be beneficial for high-density culture (Yang et al. 2000; Konstantinov et al. 1996). In addition, perfusion culture is beneficial for the purity and quality of the product, especially those bio-products that are sensitive to protease and glycosidase (Rastilho et al. 2002; Ryll et al. 2000). However, the perfusion process has a limited scale-up capability when compared with the fed-batch process, which has scale-up potential to typical bioreactor sizes of 20 m3 or more (Meuwly et al. 2006; John et al. 2006). In this work, we evaluated batch, fed-batch, and perfusion operations to optimize the process for rapid production of anti-EGFRvIII C12 antibody from recombinant CHO cells cultivated in serum-free medium for pre-clinical and early stage clinical trials.
Materials and methods
Cell line and culture medium
The recombinant CHO-C12 cell lines were established by transfecting the chimeric anti-EGFRvIII C12 antibody expression vector into CHO-DG44 cells, as described in our previous work (Wang 2009). Stable recombinant cells were selected in Dulbecco’s Modified Eagle Medium (DMEM), containing 7% fetal bovine serum (FBS) and 400 nM methotrexate (MTX) and adapted to suspension culture in shaker flasks. Cloned cells were cultivated in basic medium (serum-free EX-CELL 302 (Sigma, USA) with 4 mM glutamine (Gln), feeding medium A (basic medium with 2 g/L glucose), or feeding medium B (basic medium with 2 g/L glucose, 2 mM Asp, 6 mM Glu, 10 mM Asn, 8 mM Ser, 5 mM Ile, 6 mM Leu, and 8 mM Lys).
Cell cultivation in shake flasks
Parallel shake flask cultures (in triplicate) were performed using 120-mL shake flasks with a 30-mL working volume to determine the influence of the inoculum density on cell growth and metabolism. The shake flask cultures were carried out at 37 °C in a humidified incubator containing 5% CO2 with an agitation speed of 120 r/min.
Parallel batch cultures in bioreactor
Parallel batch cultures (in triplicate) were performed using 5-L stirred bioreactors (Model: FUS-5 L (A), NCBIO, China) with a 2-L working volume. The temperature was maintained at 37 °C, and the stirring speed was set to 50–70 r/min. The dissolved oxygen (DO) tension was maintained at 40% of air saturation using a 4-way-gas control system with a gas mixture of nitrogen, air, CO2, and pure oxygen.
Fed-batch bioreactor operation
Fed-batch cultures were carried out in 5-L bioreactors with a 4-L working volume. The initial culture volume was 2 L, unless otherwise noted. The culture set points were DO of 40% air saturation, pH of 6.8–7.0, temperature of 37 °C, and stirred speed of 50 r/min, which was adjusted to 70 r/min when the cell density reached 2 × 106 cells/mL. Feeding medium B was gradually fed into the serum-free basal medium. Samples of 5 mL were taken with a syringe for off-line analysis once or twice daily.
Perfusion wave-bioreactor operation
Perfusion cultures were conducted in a 2-L cell bag of a wave-bioreactor (SYSTEM20/50, GE) with a 1-L working volume. The initial inoculum density was 3 × 105 cells/mL, and the feed medium was continuously fed into the basal medium. The CO2 content was controlled at 5%, DO was maintained at 40% of air saturation, the rotation speed and angle were initially set to 15–25 r/min and 6°, respectively. The shot was set to 50 mL at the start of the perfusion culture. In the continuous process, the initial dilution rate was imposed at 0.083 h−1. To maintain the substrate concentrations well above non-limiting levels, the dilution rate was increased up to 0.042 h−1, depending on the substrate consumption observed (see below for substrate quantification).
Analytical methods
Viable cells were determined by observation using a hemocytometer with trypan blue exclusion dye. Concentrations of glucose, lactate and ammonia were analyzed using commercially available kits (glucose and lactate; Nan Jing Jiancheng Bio-engineering Institute, China, and ammonia; Shanghai Jiemen Biotechnology Corporation, China). Osmolality was measured with the Fully Automated Freezing Point Osmolality Measuring Instrument (FM-8P, Shanghai Medical Instrument Factory, China).
The concentrations of free amino acids were analyzed with an automatic reverse phase HPLC system (HP1110, Aligent) with pre-column derivatization by the OPA (o-phthaldialdehyde) method. Quantification of proline was performed through double derivatization of the sample with OPA and FMOC (9-fluorenylmethyl chloroformate).
The chimeric anti-EGFRvIII C12 antibody concentrations in the supernatants were estimated by sandwich ELISA. Briefly, polyvinyl chloride 96-well microtiter plates were coated with 1 μg/mL goat anti-human F(ab′)2 (Chemicon, USA). After washing with 0.05% Tween 20 and 3% BSA Tris buffer, a standard curve was generated using serial dilutions of purified chimeric anti-EGFRvIII C12 antibody and a dilution series of culture supernatants was loaded into the microtiter plates. After incubation and a subsequent wash, the bound chimeric anti-EGFRvIII C12 antibody was recognized by HRP-conjugated goat anti-human IgG Fc (Chemicon, USA) and detected by ABTS (Sigma). After the substrate reaction was completed, the absorbance was read at a wavelength of 405 nm.
Parameter calculation
The following equations were used to calculate the specific growth rate:
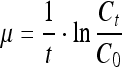 |
(where t is culture time, Ct is the viable cell density (cells/mL) at time t, C0 is the viable cell density (cells/mL) of the initial inoculum)
The specific glucose or Gln consumption rate:
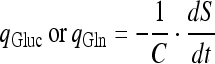 |
The specific lactate or ammonia production rate:
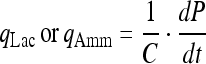 |
Yield coefficient of lactate production to glucose consumption:
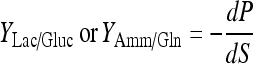 |
Yield coefficient of CHO cells to substrate consumption:
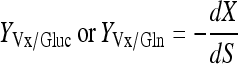 |
(where C is the viable cell density (×105 cells/mL), S is the glucose or Gln concentration (mM), P is the lactate or ammonia concentration (mM), t is time).
Considering the natural degradation of Gln, the concentration of its consumption, dS = dSapparent − Kt, (where dSapparent is the apparent metabolic concentration, K is the natural degradation of the constant value for the 0.0048 mmol L−1 h−1, t is culture time.)
Mass balance in continuous perfusion culture:
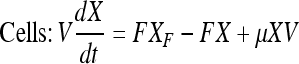 |
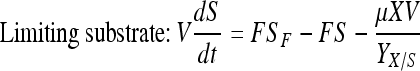 |
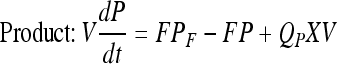 |
Here, X, S, and P are concentrations of cells (×105 per mL), substrate (mM) and product (mg/L) concentrations in the bioreactor, respectively. V is the culture volume (L). F is the flow rate (L/d). XF, SF, and PF are the concentrations of cells (×105 per mL), substrate (mM) and product (mg/L) added to the medium, respectively. There are no cells or product in the feed medium, so XF = 0, PF = 0. Qp is the specific rate of C12 production.
Statistical analysis
Data are presented as mean ( ) and standard deviation, and SPSS 10.0 software was employed for ANOVA test, the data was accepted when p ≤ 0.05.
) and standard deviation, and SPSS 10.0 software was employed for ANOVA test, the data was accepted when p ≤ 0.05.
Results
Influences of inoculum cell density on CHO-C12 cell growth
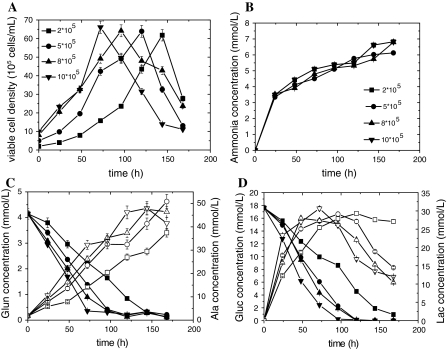
To determine an optimal inoculum cell concentration, parallel batch cultures (in triplicate, p < 0.05) were performed in shake flasks at the following cell inoculum concentrations 2 × 105 cells/mL, 5 × 105 cells/mL, 8 × 105 cells/mL, and 10 × 105 cells/mL. The maximum viable cell concentration in each of the original inocula was approximately 6 × 106 cells/mL, but the lag period was much longer when the cells were inoculated at 2 × 105 cells/mL (Fig. 1a). It was observed that glucose and Gln were exhausted quickly (Fig. 1c, d) when the inoculum concentration was higher than 8 × 105 cells/mL, while metabolites such as Ala and lactate that accumulated in a short period of time, were harmful to the maintenance of the cell cultures. However, there was no significant difference in ammonium amounts when the cell density of the inoculum increased (Fig. 1b). Cells grew relatively faster, but nutrients were not quickly exhausted at inoculum concentrations of 5 × 105 cells/mL. Based on these observations, an inoculum cell density of 5 × 105 cells/mL was employed for subsequent investigations.
Fig. 1.
Time course of viable CHO cell density, ammonia, glutamine, alanine, glucose, and lactate concentration with different inoculation cell densities in shake flask cultures. a: viable cell density; b: Ammonia concentration; c: Glutamine and alanine concentrations; d: Glucose and lactate concentrations. Symbols: 2 × 105 cells/mL (filled square), 5 × 105 cells/mL (filled circle), 8 × 105 cells/mL (filled triangle), 10 × 105 cells/mL (filled inverted triangle)
Development of the batch culture process in a 5-L stirred bioreactor
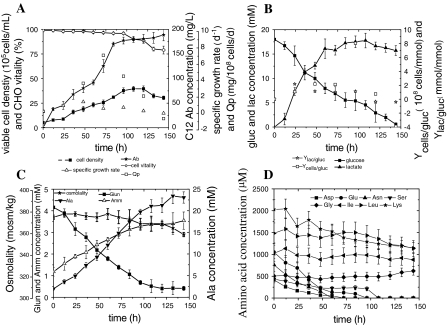
Based on the shaker-flask batch process results, parallel batch cultures in bioreactors were inoculated at 5 × 105 cells/mL (in triplicate, p < 0.05). The number of viable cells increased gradually and reached the maximal value of 4 × 106 cells/mL at 108 h, but then immediately declined, and viability dropped to approximately 80% after 120 h (Fig. 2a). The maximal concentration of C12 antibody was 190 mg/L in a 5-L bioreactor. The concentration of glucose declined from 18 to 6 mM and the Gln concentration declined from 4 to 1 mM within the first 84 h (Fig. 2b, c). The cell viability began to decrease after 84 h, mainly due to the deficiency of glucose and Gln in the medium. At the same time, lactate was produced, and it reached a maximal value of 18 mM at 96 h. Then, the specific lactate production rate (qlac) values fell below zero, indicating that lactate started to be consumed. This was accompanied by a slow rise in pH from 6.78 to 7.1. Furthermore, it was observed that Ala was also consumed at 132 h during the late cultivation stage (Fig. 2c). This phenomenon also occurred when a high inoculum of cells was cultured in shake flasks (Fig. 1c). Figure 2c also shows that the osmolality for cells varied between 358 and 374 mOsm/kg when they were cultured in EX302 medium. Ammonia concentration increased during cell culture, reaching 3.6 mM at the end of the cultivation period.
Fig. 2.
Time course of of recombinant CHO cells grown in batch culture in a 5 L bioreactor. a: viable cell density and C12 antibody concentration (Ab); viability; specific growth rate; specific C12 antibody production rate (Qp); b: residual glucose, lactate concentration, Ycells/gluc and Ylac/gluc; c: osmolality, glutamine, ammonia and alanine concentration; d: amino acid concentration
An amino acid analysis of the culture supernatants was carried out. As shown in Fig. 2 c and d, Glu, Asp, Asn, Ser, Ile, Leu and Lys were significantly consumed throughout the 144-h culture period. Glu, Asp, Asn, and Ser were exhausted at early stages of culture, while Ile, Leu, and Lys were consumed to 85, 76, and 56% of their initial values, respectively, by the end of culture. In contrast, Gly and Ala were produced during the culture period. The concentrations of the other amino acids had no apparent tendency in variation; their values fluctuated significantly during the culture period, but maintained an almost balanced state (data not shown). Therefore, Glu, Asp, Asn, and Ser were postulated to be limiting factors for cell growth.
Performance of the balanced amino acid fed-batch process
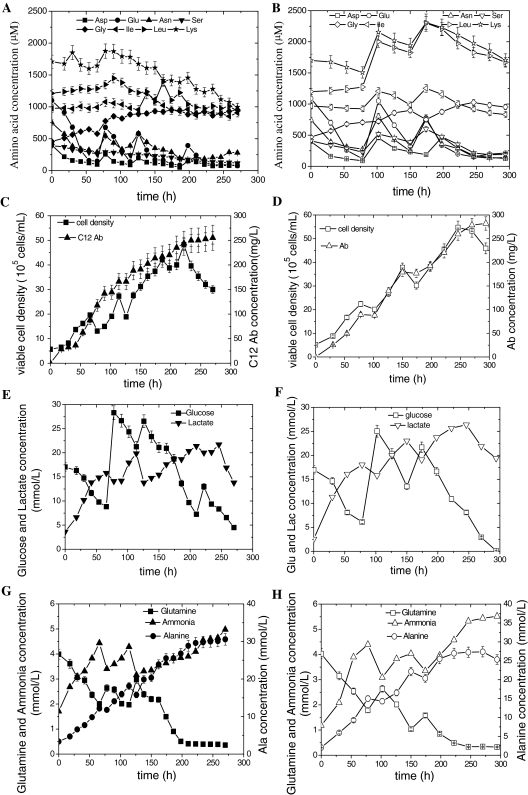
Based on the conclusion achieved from parallel batch cultures, the concentrations of residual glucose and Gln should be maintained above 6 and 1 mM, respectively. To test the hypotheses that these 7 amino acids Asp, Glu, Asn, Ser, Ile, Leu, Lys are limiting factors for cell growth, balanced amino acid feed experiments were employed. Replicate parallel fed-batch cultures were carried out using both the existing feed medium with an imbalanced amino acid content (strategy A) and a new feed medium with a balanced amino acid component that was reformulated on the basis of cell-specific amino acid requirements (strategy B). In strategy B, Asp, Glu, Asn, Ser were increased by 4–10 times, and Ile, Leu, and Lys were increased by 2–4 times the original concentration in the feeding medium to maintain adequate amino acids throughout the duration of the culture according to their specific rate of consumption. The analysis of amino acids indicated that Gly and Ala were both produced in strategy A and B. The concentrations of the 7 consumed amino acids were greater than 50–100% of that in strategy A at 270 h (Fig. 3a, b), while the concentrations of other amino acids such as Thr, Tyr, Val, Met, and Arg etc. fluctuated around initial values (data not shown). Thus, the balanced amino acid feeding used in strategy B enhanced the maximum viable cell number and C12 antibody concentration by 14.6 and 10.6%, respectively, compared with the imbalanced amino acid feeding strategy A throughout the culture (Fig. 3c, d). Up to 250 h, the concentrations of glucose and Gln were maintained above 6 mM and around 0.2 mM, respectively, and accompanied by the continuous accumulation of lactate in both fed-batch strategies. The use of lactate started when glucose was consumed to a limiting concentration of 6 mM (Fig. 3e, f).
Fig. 3.
Effect of balanced amino acid feeding strategy in fed-batch culture of CHO-C12 cells. Parallel fed-batch cultures were carried out using the feed medium with imbalanced (closed icons) and balanced amino acid concentrations (open icons). a,b: amino acid concentrations; c, d: viable cell densities and Ab concentrations; e,f: glucose and lactate concentrations; g, h: glutamine, ammonia and alanine concentrations
Simultaneously, the concentration of net Ala declined slightly in the late culture stage in strategy B but not A (Fig. 3g, h). However, the balanced amino acid feeding had no effect on the average specific glucose consumption rate (qGluc) or lactate accumulation (qLac), but improved the specific Ab production rate (QP) (Table 1). These results imply that the balanced amino acid feeding strategy can enhance the level of C12 by increasing the viable cell number and average specific C12 production rate. The concentration of ammonia accumulated during the whole culture process reaching 5 mM in both strategies.
Table 1.
Average qGluc, qLac, QP of rCHO-C12 cultures using different fed-batch strategies from start of medium supplementation (78 h) to the point of reaching maximum viable cell density
| Fed-batch strategy |
qGluc mmol/108cells/h |
qLac mmol/108cells/h |
Qp mg/108cells/h |
|---|---|---|---|
| A | 0.0022 | 0.0014 | 0.0182 |
| B | 0.0021 | 0.0013 | 0.0218 |
qGluc specific glucose consumption rate, qLac specific lactate production rate, QP specific C12 production rate
Development of a perfusion process in a wave-bioreactor
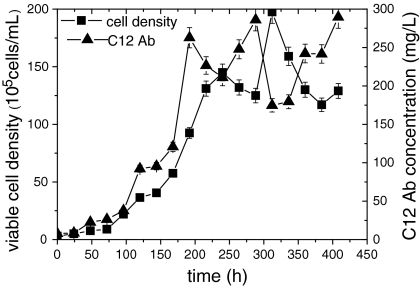
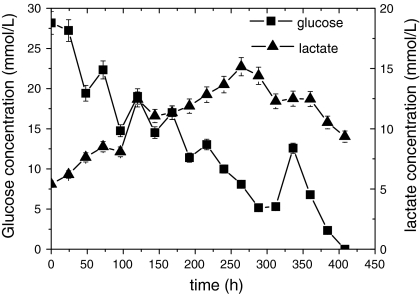
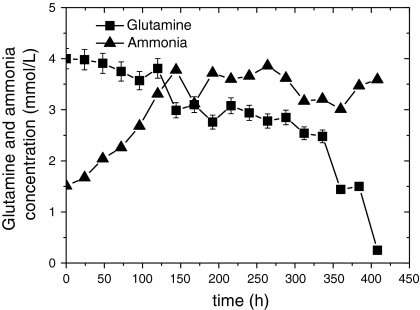
CHO-C12 cells were cultured in a 2-L wave bioreactor with a working volume of 1 L. The culture was transferred to continuous mode after a cell density of 1 × 106 cells/mL was reached and concentrations of glucose and Gln were maintained above limiting concentrations (6 mM for glucose and 1 mM for Gln). The maximum cell density of 1.98 × 107 cells/mL was reached with this strategy (Fig. 4). As shown in Figs. 5 and 6, glucose and Gln were maintained above limiting concentrations until the late cultivation stage (>384 h) so as to meet the energy demand of cell growth in the culture. The cell viability remained above 85% throughout the entire culture process, and the maximum concentration of metabolic by-products (lactate and ammonia) were about 15 and 3.5 mM, respectively, which were less than those in the fed-batch culture processes. The maximum concentration of C12 antibody was 289 mg/L (Fig. 4) in the wave bioreactor. The harvested cultivation supernatant was 12 times the volume of the initial culture volume, but the average concentration of C12 antibody in the supernatant was only 130 mg/L in the perfusion culture. This may be due to the continuous supplementation and outflow of medium, which was much lower than that in the batch culture (190 mg/L) and balanced amino acid fed-batch culture (282 mg/L).
Fig. 4.
Cell growth and C12 antibody concentration produced by rCHO-C12 cells in perfusion culture in a wave bioreactor
Fig. 5.
Time course of glucose and lactate concentrations of rCHO-C12 cells in perfusion culture of rCHO-C12 cells in a wave bioreactor
Fig. 6.
Time course of glutamine and ammonia concentrations of rCHO-C12 cells in perfusion culture of rCHO-C12 cells in a wave bioreactor
Discussion
Three culture processes were evaluated for C12 antibody production by CHO-C12 cells in this work to gain understanding of suspension culture performance of these cells and the roles of the main nutrients in this complex system. In batch cultures of shaker flasks, we found that there was no significant difference in ammonium production when the cell density of the inoculum increased. That may have been caused by Gln and glutamate metabolism, as higher inocula will result in a corresponding increased rate of Gln consumption. However, glutamine, an essential amino acid, can be synthesized by glutamine synthetase (GS) from glutamate combined with ammonia (Sun and Zhang 2001).
In stirred bioreactor batch cultures, we observed that the concentration of the residual glucose and glutamine should be maintained above 6 and 1 mM, respectively. More importantly, 7 amino acids including glutamate, aspartate, asparagine, serine, isoleucine, leucine and lysine were effectively restricted nutrients, and the maximum levels of accumulated metabolic by-products like ammonia and lactate were 3.5 and 18 mmol/L, respectively. Generally, cells suffer from toxicity only when the concentration of ammonia reaches 4.5–5.5 mM in the medium (Dai and Liu 2002). Other researchers have reported that the addition of 5 mM ammonium to the medium prior to inoculation did not significantly inhibit the CHO cell growth rate (Chen and Harcum 2005). Ozturk found that lactate itself inhibited cell growth only when the concentration of lactate exceeded 40 mM, however cell growth inhibition arised when the concentration fell below 40 mM, largely due to fluctuations of osmolality in the environment (Ozturk and Palsson 1992). In the culture period, the osmolality for cells varied between 358 and 374 mOsm/kg when they were cultured in EX302 medium. Other researchers have also reported that the majority of mammalian cells show a great tolerance to changes in osmolality, and that only osmolality in excess of 400 mOsm/kg would significantly impact cell growth (Ljunggren and Haggstrom 1990; Banik 1996), suggesting that the osmolality seen in these cultures would not influence cell growth.
Altogether, these results suggest that factors that may influence cell growth and C12 production are pH, osmolality, DO, and the metabolic by-products (lactate and ammonium). All factors varied in the range of cell tolerance levels during the culture as reported, but cellular energy substrates (including glucose and Gln) and the 7 amino acids consumed to low levels, may be major contributors to the restriction in cell growth and C12 productivity observed in the late culture period. The reduced availability of cellular energy caused by the decrease in qGluc, led to the decrease in Qp. Thus, the decline in viable cell density and Qp might be partially prevented by increasing residual glucose and Gln concentrations to above 6 and 1 mM, respectively, and by increasing the 7 easily consumed amino acids in the basic medium. This conclusion plays a fundamental role for the balanced fed-batch and continuous perfusion bioprocesses performed in the following experiments. An improved understanding of CHO-C12 cell metabolism through analysis of the role of the main nutrients in this complex system may contribute to optimization of C12 production. Also in the batch culture, the specific lactate production rate (qlac) values fell below zero, indicating that lactate started to be consumed after 96 h of culture, while alanine began to be consumed after 132 h of culture. This suggests that the decrease in glucose limited the availability of pyruvate from the glycolytic pathway that in turn, led to the simultaneous consumption of lactate and alanine to produce pyruvate. In addition, a decrease in the specific C12 production rate (Qp) occurred after a decrease of the yield coefficient of lactate production to glucose consumption (Ylac/Gluc). The Qp was greatest at 60–84 h, but when the substrates continued to decrease after reaching limiting levels, the Qp would also begin to decrease. Therefore, glucose and Gln should be maintained at low concentrations but carefully monitored to ensure that they are not consumed below limitation levels. Kuwae and Ohda (2005) also reported a similar phenomenon, that is, cell metabolism shifted from net lactate production to lactate consumption in late cultivation phases.
In fed-batch culture mode, the glucose and Gln in the culture medium were both consumed to limiting concentrations at later time points in culture in both strategies A and B. The restriction imposed by the volume of the bioreactor was the limiting factor for fed-batch culture because the cells were ultimately restricted by energy availability. Gln and glucose can complement each other as an important energy source for cells. Gln cannot be completely digested but is maintained at around 0.2 mM. The reason is that Gln is one of the essential amino acids for cell growth; as Gln is metabolized, it can be synthesized by the reaction of glutamate and ammonia under the catalysis of glutamine synthetase to maintain cell basic metabolism. When there is sufficient Gln in the medium, Gln metabolism proceeds mainly through the α-ketoglutarate and alanine pathway. When Gln is restricted, Gln metabolism begins to switch to generate α-ketoglutaric acid and the ammonia pathway, since the ammonia pathway produces more ATP, thereby alleviating the decline in energy substrates (Kuwae and Ohda 2005; Paredes et al. 1998). The co-consumption of lactate and Ala after 250 h of cultivation suggests that the decrease in residual glucose concentration caused a limited availability of pyruvate from the glycolytic pathway and then led to the simultaneous consumption of lactate and Ala. Lactate and Ala can be metabolized via pyruvate as a source of energy to yield ATP. Hence, the metabolic shift from net lactate production to its consumption was presumably caused by the decrease in residual glucose concentration.
This phenomenon that net Ala declined at the late culture stage was not observed in strategy A because the viable cell density in strategy B was higher than that in strategy A, therefore the cells in strategy B required more energy at the late culture stage, resulting in Ala consumption after 250 h. As seen in Fig. 1c, this conclusion could be verified, because at inoculum densities ≥8 × 105 cells/mL, Ala was consumed at the late culture stage, while at inoculum densities ≤5 × 105 cells/mL, Ala was not consumed. Furthermore, the slow accumulation of Ala after 250 h implied that Ala production and consumption had attained a balanced state.
Continuous perfusion culture has the potential to become increasingly important for industrial cell culture technology (Kuwae and Ohda 2005; Chu and Robinson 2001). Perfusion culture using a feeding strategy can avoid nutrient limitations and maintain a very high cell concentration. In our culture, a cell density of 1.98 × 107 cells/mL was achieved, greater than threefold increase compared to the fed-batch culture, with a cell viability >85% through the whole culture process. As shown in Figs. 4, 5, and 6, we also found that viable cell density, C12 Ab, glucose, Gln, lactate, and ammonia concentrations fluctuated around some constant value from 192 to 360 h, indicating that the perfusion culture had adapted to a steady state optimal for cell and product stability.
Tables 2 and 3 summarize the results achieved throughout this work. Fed-batch and particularly, perfusion culture bioprocesses effectively maintain high cell viability, thereby extending the duration of cell culture. In addition, the maximum mean yield coefficients YVx/Gluc of 13.87 × 108 cells/g, YVx/Gln of 291.94 × 108 cells/g, YAb/gluc of 44.72 mg/g, and YAb/gln of 721.40 mg/g were achieved in perfusion culture. The highest total amount of antibody C12 (1,854 mg) was produced in perfusion culture; however, the maximum C12 Ab concentration of 282 mg/L was reached in the balanced amino acid fed-batch bioprocess. In general, as shown in Table 3, lactate and ammonia were not strongly produced by the cells, except in the fed-batch mode. The low levels of lactate and ammonia could contribute to the extension of culture duration. Moreover, it was observed that the concentrations of the metabolic by-product lactic acid was the lowest in the perfusion culture among the culture processes assayed.
Table 2.
Comparision of culture duration, total consumed glucose and glutamine, produced lactatemax, ammoniamax and C12 antibody, and cell density (Xmax) in batch, fed-batch, and perfusion culture processes
| Culture process |
Culture duration h |
Total Gluc g |
Total Glun g |
Lacmax mmol/L |
Ammmax mmol/L |
C12 Ab amount mg |
Xmax 105cells/mL |
Broth volume L |
|---|---|---|---|---|---|---|---|---|
| Batch | 144 | 5.97 | 1.095 | 17.76 | 3.6 | 377.64 | 39.6 | 2.0 |
| Fed-batch A | 270 | 29.80 | 2.399 | 24 | 5.2 | 1,020.94 | 48.2 | 4.0 |
| Fed-batch B | 294 | 25.67 | 2.093 | 26.4 | 5.5 | 1,128.40 | 54.7 | 4.0 |
| Perfusion | 408 | 41.46 | 2.57 | 15.15 | 3.8 | 1,854.00 | 197.5 | 12.0 |
Table 3.
Comparision of YVx/Gluc, YVx/Gln,YLac/Gluc,YAmm/Gln,YAb/Vx,YAb/Gluc, and YAb/Gln in batch, fed-batch, perfusion culture processes
| Culture process |
YVx/Gluc 108cells/g |
YVx/Glun 108cells/g |
Ylac/Gluc g/g |
YAmm/Glun mg/g |
YAb/Vx mg/105cells |
YAb/gluc mg/g |
YAb/glun mg/g |
|---|---|---|---|---|---|---|---|
| Batch | 13.25 | 72.31 | 0.84 | 77.73 | 4.77 | 63.17 | 344.9 |
| Fed-batch A | 6.48 | 80.42 | 0.42 | 115.24 | 5.29 | 34.26 | 425.57 |
| Fed-batch B | 8.53 | 104.54 | 0.38 | 154.32 | 5.16 | 43.97 | 539.13 |
| Perfusion | 13.87 | 291.94 | 0.30 | 258.93 | 4.69 | 44.72 | 721.40 |
YVx/Gluc yield coefficient of rCHO-C12 cells to glucose consumption, YVx/Gln yield coefficient of rCHO-C12 cells to glutamine consumption, YLac/Gluc yield coefficient of lactate production to glucose consumption, YAmm/Gln yield coefficient of ammonia production to glutamine consumption, YAb/Vx yield coefficient of rCHO-C12 cells to antibody, YAb/Gluc mean yield coefficient of antibody to consumed glucose, YAb/Gln mean yield coefficient of antibody to consumed Gln
Altogether, we conclude that the perfusion culture bioprocess is beneficial to high-density cell culture, maintains the duration of viable culture, is effective in avoiding nutrient limitations and in reducing the concentration of inhibitory metabolites, but the concentration of harvested C12 antibody is lower than that in batch and fed-batch cultures. Besides, perfusion culture had the maximum utilization of nutrients and the highest mean yield coefficients of antibody produced per unit of glucose or Gln. In addition, the potential profit for mini-scale culture was the ability to harvest more total C12 Ab from this system as compared with other processes, indicating that perfusion culture bioprocess was the optimum bioprocess for small-scale antibody C12 production by rCHO-C12 cells.
Acknowledgments
This study was supported by the National High Technology Research & Development Program (863 Program) of China (No. 2007AA02Z216), the National Special Fund for State Key Laboratory of Bioreactor Engineering (No.2060204),the National Basic Research Program of China (No.2007CB714303) and the Qianjiang Scholarship grant from Hangzhou Municipal Government, China.
Contributor Information
Yingping Zhuang, Phone: +86-21-64251131, FAX: +86-21-64253702, Email: ypzhuang@ecust.edu.cn.
Meijin Guo, Email: guo_mj@ecust.edu.cn.
References
- Banik GG. High-density hybridoma perfusion culture. Appl Biochem Biotechnol. 1996;61:211–229. doi: 10.1007/BF02787797. [DOI] [PubMed] [Google Scholar]
- Bibila TA, Robinson DK. In pursuit of the optimal fed-batch process for monoclonal antibody production. Biotechnol Prog. 1995;11:1–13. doi: 10.1021/bp00031a001. [DOI] [PubMed] [Google Scholar]
- Bibila TA, Ranucci CS, Glazomitsky K. Monocloal antibody process development using medium concentrates. Biotechnol Prog. 1994;10:87–96. doi: 10.1021/bp00025a011. [DOI] [PubMed] [Google Scholar]
- Birch JR, Racher AJ. Antibody production. Adv Drug Deliv Rev. 2006;58:671–685. doi: 10.1016/j.addr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Chen PF, Harcum SW. Effects of amino acid additions on ammonium stressed CHO cells. J Biotechnol. 2005;117:277–286. doi: 10.1016/j.jbiotec.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Chu L, Robinson DK. Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol. 2001;2:180–187. doi: 10.1016/S0958-1669(00)00197-X. [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- Dai MS, Liu TQ. A study of the metabolism parameters of NSCs. Tissue Eng. 2002;12:101–110. [Google Scholar]
- Derouazi M, Martinet D, Besuchet SN, Flaction R, Wicht M, Bertschinger M, Hacker DL, Beckmann JS, Wurm FM. Genetic characterization of CHO production host DG44 and derivative recombinant cell lines. Biochem Biophys Res Commun. 2006;340:1069–1077. doi: 10.1016/j.bbrc.2005.12.111. [DOI] [PubMed] [Google Scholar]
- DeZengotita VM. Phosphate feeding improves high-cell-concentration NS0 myeloma culture performance for monoclonal antibody production. Biotechnol Bioeng. 2000;69:566–576. doi: 10.1002/1097-0290(20000905)69:5<566::AID-BIT11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Diedrich U, Lucius J, Baron E, Behnke J, Pabst B, Zoll B. Distribution of epidermal growth factor receptor gene amplifecation in brain tumors and correlation to prognosis. J Neurol. 1995;242:683–688. doi: 10.1007/BF00866920. [DOI] [PubMed] [Google Scholar]
- Gainet M, Guardiola E, Dufresne A, Pivot X. Epidermal growth factor receptors (EGFR): a new target for anticancer therapy. Cancer Radiother. 2003;7:195–199. doi: 10.1016/s1278-3218(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Konstantinov KB, Tsai Y, Moles D, Matanguihan R. Control of long-term perfusion Chinese hamster ovary cell culture by glucose auxostat. Biotechnol Prog. 1996;12:100–109. doi: 10.1021/bp950044p. [DOI] [PubMed] [Google Scholar]
- Kuwae S, Ohda T. Development of a fed-Batch culture process for enhanced production of recombinant human antithrombin by Chinese hamster ovary cells. J Biosci Bioeng. 2005;100:502–510. doi: 10.1263/jbb.100.502. [DOI] [PubMed] [Google Scholar]
- Liu L, Backlund LM, Nilsson BR, Grander D, Ichimura K, Goike HM, Collins VP. Clinical significance of EGFR amplification and the aberrant EGFRvIII transcript in conventionally treated astrocytic gliomas. J Mol Med. 2005;83:917–926. doi: 10.1007/s00109-005-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren J, Haggstrom L. Glutamine limited fed-batch culture reduces ammonium ion production in animal cells. Biotechnol Lett. 1990;12:705–710. doi: 10.1007/BF01024725. [DOI] [Google Scholar]
- Meuwly F, Weber U, Ziegler T, et al. Conversion of a CHO cell culture process from perfusion to fed-batch technology without altering product quality. J Biotechnol. 2006;123:106–116. doi: 10.1016/j.jbiotec.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Moscatello DK, Holgado-Madruga M, Godwin AK, Ramirenz G, Gunn G, Zoltick PW, Biegel JA, Hayes RL, Wong AJ. Frequent expression of a utant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. P Natl Aacd Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto I, Kenyon LC, Emlet DR, Moi T, Sasaki J, Hirosako S, Ichikawa Y, Kishi H, Godwin AK, Yoshioka M, Suga M, Matsumoto M, Wong AJ. Expression of constitutively activated EGFRvIII in non-small cell lung cancer. Cancer Sci. 2003;94:50–56. doi: 10.1111/j.1349-7006.2003.tb01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C, Wu FX, Luo Y, Cao J, Zhao YN, Yuan WP, Li Y, Su JJ. Expression and significance of epidermal growth factor receptor variant type III in hepatocellular carcinoma. Chin J Cancer. 2005;24:166–169. [PubMed] [Google Scholar]
- Ozturk SS, Palsson BO. Effects of ammonia and L-lactate on hybridoma growth, metabolism, and antibody production. Biotechnol Bioeng. 1992;39:234–239. doi: 10.1002/bit.260390408. [DOI] [PubMed] [Google Scholar]
- Paredes C, Sanfeliu A, Gardenas F, et al. Estimation of the intracellular fluxes for a hybridoma cell line by material ballances. Enzym Microb Tech. 1998;22:187–198. doi: 10.1016/S0141-0229(98)00023-4. [DOI] [Google Scholar]
- Parkin DM, Stjernsward T, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ. 1984;62:163–182. [PMC free article] [PubMed] [Google Scholar]
- Ramnarain DB, Park S, Lee DY, Hatanpaa KJ, Scoggin SO, Otu H, Libermann TA, Raisanen JM, Ashfaq R, Wong ET, Wu J, Elliott R, Habib AA. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res. 2006;66:867–874. doi: 10.1158/0008-5472.CAN-05-2753. [DOI] [PubMed] [Google Scholar]
- Rastilho CL, Anspach FB, Deckwer WD. Comparison of affinity membranes for the purification of immunoglobulins. Biotechnol. 2002;18:776–781. doi: 10.1021/bp0255154. [DOI] [PubMed] [Google Scholar]
- Ryll T, Dutina G, Reyes A, Gunson J, Krummen L, Etcheverry T. Performance of small-scale CHO perfusion cultures using an acoustic cell filtration device for cell retention: characterization of separation efficiency and impact of perfusion on product quality. Biotechnol Bioeng. 2000;69:440–449. doi: 10.1002/1097-0290(20000820)69:4<440::AID-BIT10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Serag EHB, Andrew C, Mason MD. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. doi: 10.1016/S1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, Freilino ML, Graner MW, Wikstrand CJ, Bigner DD, Gooding WE, Furnari FB, Grandis JR. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targenting. Clin Cancer Res. 2006;12:5064–5973. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- Sun XM, Zhang YX. Effects of ammonia on cell metabolismin in the culture of recombinant CHO cells. Chin J Biotechnol. 2001;17:304–309. [PubMed] [Google Scholar]
- Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. Clin Oncol. 2005;23:2892–2899. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- Wang HM (2009) Epidermal growth factor receptor vIII antibody development and application in hepatocellular carcinoma therapy. Dissertation, Fudan University
- Wang HM, Jiang H, Zhou M, Xu ZB, Liu SG, Li ZH. Epidermal growth factor receptor vIII enhances tumorigenicity and resistance to 5-fluorouracil in human hepatocellular carcinoma. Cancer Lett. 2009;279:30–38. doi: 10.1016/j.canlet.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Xie L, Wang DIC. High cell density and high monoclonal antibody production through medium desigh and rational control in a bioreactor. Biotechnol Bioeng. 1996;151:725–729. doi: 10.1002/(SICI)1097-0290(19960920)51:6<725::AID-BIT12>3.3.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Yang JD, Angelillo Y, Chaudhry M, Goldenberg C, Goldenberg DM. Achievement of high cell density and high antibody productivity by a controlled-fed perfusion bioreactor process. Biotechnol Bioeng. 2000;69:74–82. doi: 10.1002/(SICI)1097-0290(20000705)69:1<74::AID-BIT9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with Hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. doi: 10.1200/JCO.2003.11.137. [DOI] [PubMed] [Google Scholar]
- Zeineldin R, Rosenberg M, Ortega D, Buhr C, Chavez MG, Stack MS, Kusewitt DF, Hudson LG. Mesenchymal transformation in epithelial ovarian tumor cells expressing epidermal growth factor receptor variant III. Mol Carcinog. 2006;45:851–860. doi: 10.1002/mc.20237. [DOI] [PubMed] [Google Scholar]
- Zhu AX. Systemic therapy of advanced Hepatocellular carcinoma: how hopeful should we be. Oncol. 2006;11:790–800. doi: 10.1634/theoncologist.11-7-790. [DOI] [PubMed] [Google Scholar]