Abstract
In this study, the effects of dimethyl sulfoxide (DMSO) on the spontaneous aging of ovulated murine oocyte were evaluated in vitro. When ovulated oocytes were cultured continuously in vitro without fertilization stimulation, they underwent several phenotypic changes, including non-activation, activation, fragmentation, and lysis. To investigate the effects of DMSO on these changes, I cultured ovulated oocytes with various concentrations of DMSO and evaluated the phenotypic changes for up to 3 days. After 3 days of culture, the frequency of oocyte fragmentation was significantly lower in oocytes treated with 2 and 4% DMSO (7 and 5%, respectively) than in control oocytes (80%). All control oocytes were activated or fragmented after 3 days of culture in vitro. However, more than 80% of the oocytes cultured with 4% DMSO for 3 days contained spindles and condensed chromosomes, although they displayed abnormal spindle structures. Next Cdk1 activity in DMSO-treated oocytes was examined. The results showed that DMSO treatment prevented the reduction in Cdk1 activity during prolonged culture. Moreover, DMSO inhibited the degradation of cyclin B. These results suggest that DMSO inhibits spontaneous oocyte fragmentation and maintains Cdk1 activity in ovulated murine oocytes during prolonged culture in vitro, possibly by inhibiting cyclin B degradation.
Keywords: Oocyte fragmentation, DMSO, Cdk1, cyclin B
Introduction
Ovulated mammalian oocytes that do not become fertilized ultimately die as a result of a process known as oocyte aging (Miao et al. 2009). Oocyte aging is influenced by donor age, with oocytes from older donors aging more rapidly (Thouas et al. 2005; Yamamoto et al. 2010; Wu et al. 2000). It is easily seen in ovulated oocytes cultured for prolonged periods of time in vitro. Such oocytes display phenotypic changes, including spontaneous activation, fragmentation, and lysis (Fissore et al. 2001). Molecular changes that occur during oocyte aging include decreases in the levels of maturation promoting factor (MPF) and of bcl2 and MDM2 transcripts, and disruption of calcium ion homeostasis (Kikuchi et al. 2000; Tatone et al. 2006; Steuerwald et al. 2005). However, the precise mechanisms of oocyte aging remain unknown.
Dimethyl sulfoxide (DMSO) is widely used as a solvent for water-insoluble chemicals and as a cryoprotectant to protect biological tissues that are frozen for storage purposes. DMSO also has various biological and pharmacological activities (Jacob and Torre 2009; Santos et al. 2003). Our group fortuitously observed DMSO’s effects on oocyte aging during the course of an experiment conducted to investigate the influence of various kinase inhibitors pre-dissolved in DMSO on oocyte aging.
In the present study, we investigated the effects of DMSO on ovulated oocyte aging in vitro. The results reveal that DMSO inhibits spontaneous oocyte fragmentation in a dose-dependent manner and maintains MPF activity. Moreover, the maintenance of MPF activity results from the inhibition of cyclin B degradation by DMSO in the oocyte culture medium.
Materials and methods
Collection and culture of ovulated oocytes
Ovulated oocytes were obtained from ICR female mice (4–5 weeks old). Mice were injected intraperitoneally with 5 IU of pregnant mare serum gonadotropin (Sigma, St. Louis, MO, USA) and, 48 h later, 5 IU of human chorionic gonadotropin (hCG; Sigma). Ovulated oocytes were collected from the ampullae of the oviducts 15–16 h post-hCG injection. Cumulus cells surrounding the oocytes were freed by incubation for 2–3 min in 0.1% hyaluronidase (type I–S; Sigma) in M16 culture medium (Sigma) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The cumulus cell–free oocytes were rinsed three times and used in subsequent experiments. Oocytes were activated through treatment for 7 min at room temperature (RT) in medium containing 7% (v/v) ethanol. They were then washed three times in medium and cultured for up to 6 h. Interphase samples were prepared and analyzed for Cdk1 activity and cyclin B levels. DMSO (cat # D4540) was purchased from Sigma Chemical Co.
Evaluation of isolated oocytes
At the end of the culture period, the oocytes were fixed through incubation with 1.8% paraformaldehyde in PBS for 40 min and then permeabilized through incubation with 1% Triton X-100 in PBS for 20 min. They were then washed with 0.1% Tween 20 in PBS for 20 min and blocked with PBS containing 3% bovine serum albumin (blocking solution) for 1 h at RT. The oocytes were next incubated for 1 h at RT with an anti-tubulin antibody (YL1/2; dilution 1:100; Accurate Chemical) and then for 1 h at RT with a fluorescein isothiocyanate-conjugated secondary antibody (Sigma; 1:50). Oocytes were co-stained with 4,6-diamidinophenylindole (DAPI; Sigma) to allow DNA to be visualized.
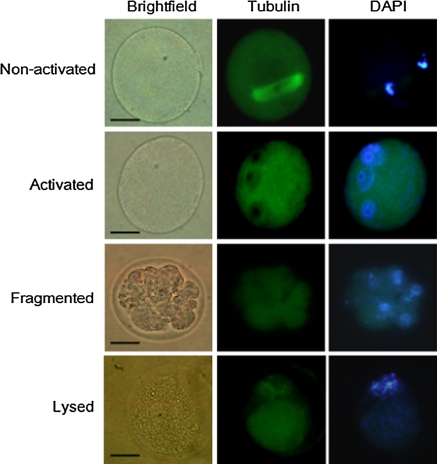
Immunostained oocytes were examined using an Olympus IX71 fluorescence microscope and classified as non-activated, activated, fragmented, or lysed. Pronuclear oocytes were deemed to be activated oocytes. Oocytes containing meiotic spindles and condensed chromosomes were considered to be non-activated, even though their spindles were abnormal and their chromosomes dispersed. Oocytes with more than three asymmetric cells were considered to be fragmented, whereas those that appeared brown and whose plasma membranes appeared indistinct on microscopic analysis were considered to be lysed (Lim and Choi 2004).
Measurement of Cdk1 assay
A Cdk1 assay was conducted according to the method described by Ito et al. (2005) with certain modifications. Briefly, 10 denuded oocytes were lysed in 5 μl ice-cold RIPA buffer containing protease and phosphatase inhibitors (Sigma) and then frozen at −70 °C prior to use. The kinase assay of each oocyte lysate was measured using a MESACUP Cdk1 assay kit (MBL, Nagoya, Japan). Cdk1 activity was calculated as a percentage of maximal activity (activity in ovulated oocytes was defined as 100%, and that in oocytes that were ethanol activated for 6 h was defined as 0%).
Immunoblot analysis of cyclin B
A total of 100 oocytes in each treatment group were analyzed by immunoblotting. Oocytes were suspended in 10 μl of 2× Laemmli sample buffer, boiled for 5 min, and then subjected to standard SDS–PAGE (10% gel). Separated proteins were transferred to polyvinylidene fluoride membranes and analyzed using an anti-cyclin B antibody (Millipore).
Statistical analysis
Each experiment was repeated at least three times. Statistically significant differences between groups were identified by one-way analysis of variance. P < 0.05 was considered to be statistically significant. Data are presented as the mean ± SEM.
Results
Effect of DMSO on spontaneous oocyte fragmentation and activation during prolonged culture of ovulated oocytes
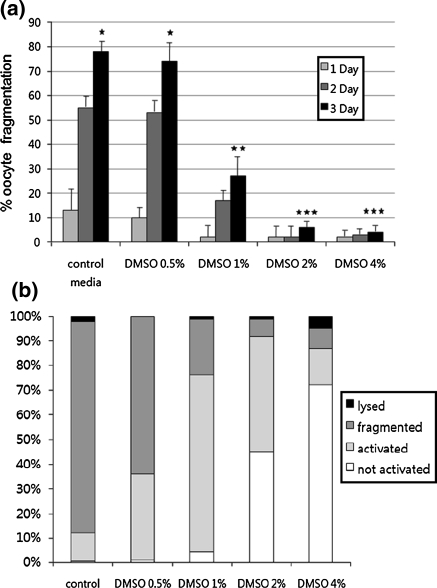
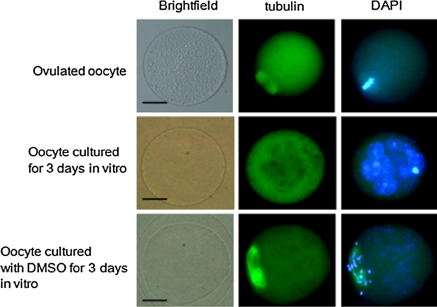
Culturing ovulated oocytes in control medium for 3 days increased the frequency of oocyte fragmentation to 80 ± 5%. Treatment with 2 or 4% DMSO reduced the frequency of oocyte fragmentation (to 7 ± 2% and 5 ± 2%, respectively; n = 90; Fig. 1a). The inhibitory effects of DMSO were concentration dependent. Low concentrations of DMSO had no significant effect: the fragmentation frequency following treatment with 0.5% DMSO was 78 ± 5%. After culture in medium containing 2 or 4% DMSO for 3 days, most oocytes appeared as single cells. To determine whether they were activated, we stained oocytes with the combination of an anti-tubulin antibody and DAPI. The frequency of each phenotype (non-activated, activated, fragmented, and lysed) was calculated for oocytes cultured in the presence of various concentrations of DMSO for 3 days (Fig. 1b). DMSO inhibited oocyte activation in a concentration-dependent manner. After culture for 3 days, most activated oocytes had multiple nuclei, whereas inactivated oocytes contained dispersed chromosomes and abnormal or week meiotic spindles (Fig. 2).
Fig. 1.
Oocytes were classified into four groups: non-activated, activated, fragmented and lysed oocyte. Scale bar: 50 μm
Fig. 2.
Effect of DMSO on spontaneous oocyte aging. a The rate of fragmentation increased in a time-dependent manner in oocytes cultured in M16 control medium. Spontaneous oocyte fragmentation was inhibited in a concentration-dependent manner by the addition of DMSO to the M16 medium. Data represent the mean ± SEM (n = 3), and values with different superscripts are significantly different (P < 0.05). b The frequencies of four different oocyte phenotypes were evaluated after culture for 3 days in the presence or absence of DMSO. DMSO increased the frequency of non-activated oocytes, including those with condensed chromosomes and meiotic spindles, in a concentration-dependent manner
Cdk1 activity in DMSO-treated oocytes
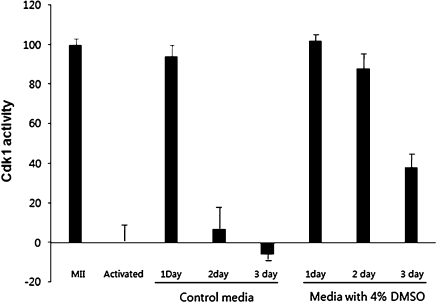
To determine whether oocytes cultured with DMSO displayed MPF activity, we measured Cdk1 activity as described in Materials and Methods. As is shown in Fig. 4, kinase activity in oocytes cultured in control medium for 1, 2, and 3 days decreased by 94, 7, and 6%, respectively. The kinase activity of oocytes cultured with 4% DMSO was 102, 88, and 47%, respectively.
Fig. 3.
Fluorescence photomicrographs of oocytes stained with anti-tubulin antibody and DAPI. Ovulated oocytes were fixed within 2 h of ovulation. Most single-cell oocytes cultured for 3 days in vitro were activated and contained multiple nuclei. Most oocytes cultured with DMSO for 3 days contained abnormal spindles and condensed chromosomes. Scale bar: 50 μm
Fig. 4.
Kinetics of Cdk1 activity in oocytes during in vitro culture. Cdk1 activity was calculated as a percentage of maximal activity (activity in ovulated oocytes was defined as 100%, and that in oocytes that were ethanol activated for 6 h was defined as 0%). The decrease in Cdk1 activity caused by the prolonged culture of ovulated oocytes was delayed by the addition of 4% DMSO to the culture medium
DMSO inhibits cyclin B degradation during the prolonged culture of ovulated oocytes
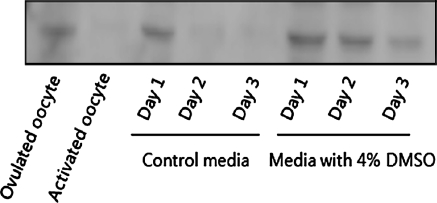
It is well known that the inactivation of Cdk1 during the transition from meiosis to interphase results from the degradation of cyclin B. Therefore, we measured (by immunoblotting) cyclin B protein levels in oocytes cultured in the presence and absence of DMSO. The results showed that cyclin B was degraded during the prolonged culture of oocytes in vitro. The addition of DMSO to the culture medium inhibited the degradation of cyclin B (Fig. 5).
Fig. 5.
Changes in cyclin B levels during the culture of oocytes in vitro. Lysates from oocytes cultured under various conditions and for different lengths of time were analyzed by immunoblotting using an anti-cyclin B antibody. Each sample was prepared from 100 oocytes
Discussion
The results of the present study show that DMSO (at a concentration of 2 or 4%) effectively inhibits spontaneous oocyte fragmentation and activation during the prolonged culture of ovulated murine oocytes. What is significant is that the inhibitory effects of DMSO were accompanied by delayed inactivation of Cdk1 as a result of cyclin B degradation. Although the direct targets of DMSO were not identified, this is the first report describing the inhibitory effects of DMSO on oocyte aging.
Oocyte fragmentation occurs during prolonged culture in vitro (spontaneous fragmentation) and as a result of treatment with chemotherapeutic agents such as doxorubicin (induced fragmentation; Fujino et al. 1996; Perez et al. 1999). In induced fragmentation, oocyte activation occurs within several hours, with key features of apoptotic pathway activation, including DNA cleavage, caspase activation, and the translocation of phosphatidyl serine, also being observed (Jurisicova et al. 2006). The results of the present study also reveal that spontaneous fragmentation of oocytes during prolonged culture in vitro is correlated with an increased rate of activation. Oocyte activation may be a prerequisite for induced and spontaneous oocyte fragmentation. Culture in DMSO (2 or 4%) efficiently blocked oocyte activation and fragmentation. However, treatment with DMSO at concentrations above 6% negatively affected oocytes, increasing fragmentation and lysis (data not shown).
Cellular fragmentation during apoptosis is inhibited by treatment with inhibitors of microtubules (nocodazole, vinblastine, demecolcine) and microfilaments (cytochalasins; Moss et al. 2006; Cotter et al. 1992). In this study, treatment with nocodazole or cytochalasin inhibited spontaneous oocyte fragmentation (data not shown). Figure 2 shows that the inhibitory effects of DMSO did not result from microtubule disassembly, because oocytes treated with DMSO still had spindle structures. To determine whether DMSO targets microfilaments, oocytes were activated with ethanol and then cultured with DMSO for 24 h. Following treatment, the number of two-cell oocytes in the control and DMSO treatment groups was similar. These findings show that DMSO does not affect cytokinesis in activated oocytes (data not shown).
DMSO is involved in a number of biological actions, influencing cell differentiation, apoptosis, ion channel expression, and lipid metabolism and producing neuroprotective effects (Santos et al. 2003). In addition, it was recently shown that it inhibits histone deacetylase (HDAC; Marks and Breslow 2007). Moreover, Jeseta et al. (2008) showed that HDAC inhibition delays spontaneous aging in pig oocytes. In the present study, mouse oocytes were treated with HDAC inhibitors such as trichostatin A and sodium butyrate to determine whether the effects of DMSO are linked to HDAC inhibition. In mouse oocytes, HDAC inhibition had no effect on spontaneous aging, including fragmentation (data not shown). Huang et al. (2007) previously reported similar results in mouse oocytes.
This study shows that DMSO maintains oocyte Cdk1 kinase activity by inhibiting cyclin B degradation. Cyclin B degradation results from its ubiquitination by anaphase-promoting complex/cyclosome (APC/C), an E3 ligase complex, and its subsequent destruction by the proteasome (Madgwick and Jones 2007; Wu et al. 2007; Wu and Kornbluth 2008). Emi2, a negative regulator of APC/C (Shoji et al. 2006), and calcium/calmodulin kinase II, a negative regulator of Emi2, are known upstream regulators of APC/C (Rauh et al. 2005; Hansen et al. 2006). An increase in intracellular calcium ions is required for normal oocyte activation after fertilization. At the molecular level, calcium ion oscillations are a prerequisite for cyclin B degradation (Alberio et al. 2001). Takahashi et al. (2000) reported that cytosolic calcium ion oscillations are abnormal in aged oocytes. Spontaneous oocyte activation linked to oocyte aging may result from a gradual increase in oocyte calcium ions. Accordingly, it is interesting to note that a previous study found that DMSO acts as a calcium desensitizer in rabbit detrusor muscle cells (Shiga et al. 2007). Nitric oxide is one of the most effective molecules for delaying murine oocyte aging. Goud et al. (2005) showed that oocyte aging was delayed in young and old murine oocytes treated with the NO donor S-nitroso-N-acetylpenicillamine. What is interesting is that DMSO induces NO synthesis in bladder afferent neurons (Birder et al. 1997). The possibility of increased nitric oxide production in oocytes treated with DMSO cannot be ruled out.
Acknowledgments
This study was supported by research funds provided by Dankook University in 2008.
References
- Alberio R, Zakhartchenko V, Motlik J, Wolf E. Mammalian oocyte activation: lessons from the sperm and implications for nuclear transfer. Int J Dev Biol. 2001;45:797–809. [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, Groat WC. DMSO: effect on bladder afferent neurons and nitric oxide release. J Urol. 1997;158:1989–1995. doi: 10.1016/S0022-5347(01)64199-5. [DOI] [PubMed] [Google Scholar]
- Cotter TG, Lennon SV, Glynn JM, Green DR. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res. 1992;52:997–1005. [PubMed] [Google Scholar]
- Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2001;124:745–754. doi: 10.1530/rep.0.1240745. [DOI] [PubMed] [Google Scholar]
- Fujino Y, Ozaki K, Yamamasu S, Ito F, Matsuoka I, Hayashi E, Nakamura H, Ogita S, Sato E, Inoue M. DNA fragmentation of oocytes in aged mice. Hum Reprod. 1996;11:1480–1483. doi: 10.1093/oxfordjournals.humrep.a019421. [DOI] [PubMed] [Google Scholar]
- Goud AP, Goud PT, Diamond MP, Abu-Soud HM. Nitric oxide delays oocyte aging. Biochemistry. 2005;44:11361–11368. doi: 10.1021/bi050711f. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Tung JJ, Jackson PK. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc Natl Acad Sci USA. 2006;103:608–6132. doi: 10.1073/pnas.0509549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, Wang Q, Ouyang YC, Sun QY, Chen DY. Changes in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod. 2007;77:666–670. doi: 10.1095/biolreprod.107.062703. [DOI] [PubMed] [Google Scholar]
- Ito J, Hirabayashi M, Kato M, Takeuchi A, Ito M, Shimada M, Hochi S. Contribution of high p34cdc2 kinase activity to premature chromosome condensation of injected somatic cell nuclei in rat oocytes. Reproduction. 2005;129:171–180. doi: 10.1530/rep.1.00431. [DOI] [PubMed] [Google Scholar]
- Jacob SW, Torre JCDL. Pharmacology of dimethyl sulfoxide in cardiac and CNS damage. Pharmacol Rep. 2009;61:225–235. doi: 10.1124/pr.109.001875. [DOI] [PubMed] [Google Scholar]
- Jeseta M, Petr J, Krejcová T, Chmelíková E, Jílek F. In vitro ageing of pig oocytes: effects of the histone deacetylase inhibitor trichostatin A. Zygote. 2008;16:145–152. doi: 10.1017/S0967199408004668. [DOI] [PubMed] [Google Scholar]
- Jurisicova A, Lee HJ, D’Eataing SG, Tilly J, Perez GI. Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ. 2006;13:1466–1474. doi: 10.1038/sj.cdd.4401819. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Aoki F, Toyoda Y. Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biol Reprod. 2000;63:715–722. doi: 10.1095/biolreprod63.3.715. [DOI] [PubMed] [Google Scholar]
- Lim E, Choi T. A phenotypic study of murine oocyte death in vivo. J Reprod Develop. 2004;50:179–183. doi: 10.1262/jrd.50.179. [DOI] [PubMed] [Google Scholar]
- Madgwick S, Jones KT. How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div. 2007;2:4. doi: 10.1186/1747-1028-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod. 2009;15:573–585. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- Moss DK, Betin VM, Malesinski SD, Lane JD. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci. 2006;119:2362–2374. doi: 10.1242/jcs.02959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GI, Tao XJ, Tilly JL. Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol Hum Reprod. 1999;5:414–420. doi: 10.1093/molehr/5.5.414. [DOI] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035–1041. doi: 10.1016/S0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Shiga KI, Hirano K, Nishimura J, Niiro N, Naito S, Kanaide H. Dimethyl sulphoxide relaxes rabbit detrusor muscle by decreasing the Ca2+ sensitivity of the contractile apparatus. Br J Pharmacol. 2007;151:1014–1024. doi: 10.1038/sj.bjp.0707317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry ACF. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 2006;25:834–845. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuerwald NM, Steuerwald MD, Maihes JB. Post-ovulatory aging of mouse oocytes leads to decreased MAD2 transcripts and increased frequencies of premature centromere separation and anaphase. Mol Hum Reprod. 2005;11:623–630. doi: 10.1093/molehr/gah231. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Saito H, Hiroi M, Doi K, Takahashi E. Effects of aging on inositol 1, 4, 5-triphosphate-induced Ca(2+) release in unfertilized mouse oocytes. Mol Reprod Dev. 2000;55:299–306. doi: 10.1002/(SICI)1098-2795(200003)55:3<299::AID-MRD8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tatone C, Carbone MC, Gallo R, Monache SD, Cola MD, Alesse E, Amicarelli F. Age-associated changes in mouse oocytes during postovulatory in vitro culture: possible role for meiotic kinases and survival factor BCL2. Biol Reprod. 2006;74:395–402. doi: 10.1095/biolreprod.105.046169. [DOI] [PubMed] [Google Scholar]
- Thouas GA, Trounson AO, Jones GM. Effect of female age on mouse oocyte developmental competence following mitochondrial injury. Biol Reprod. 2005;73:366–373. doi: 10.1095/biolreprod.105.040956. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Kornbluth S. Across the meiotic divide-CSF activity in the post-Emi2/XErp1 era. J Cell Sci. 2008;121:3509–3514. doi: 10.1242/jcs.036855. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang L, Wang X. Maturation and apoptosis of human oocytes in vitro are age-related. Fertil Steril. 2000;74:1137–1141. doi: 10.1016/S0015-0282(00)01597-1. [DOI] [PubMed] [Google Scholar]
- Wu Q, Guo Y, Yamada A, Perry JA, Wang MZ, Araki M, Freel CD, Tung JJ, Tang W, Margolis SS, Jackson PK, Yamano H, Asano M, Kornbluth S. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr Biol. 2007;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Iwata H, Goto H, Shiratuki S, Tanaka H, Monji Y, Kuwayama T. Effect of maternal age on the developmental competence and progression of nuclear maturation in bovine oocytes. Mol Reprod Dev. 2010;77:595–604. doi: 10.1002/mrd.21188. [DOI] [PubMed] [Google Scholar]