Abstract
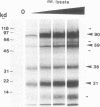
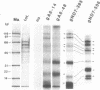
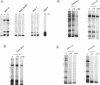
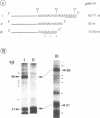
RNA editing is a mitochondrial transcript maturation process which evolved in kinetoplastid protozoa. It entails the insertion and deletion of exclusively uridine nucleotides directed by gRNAs into pre-mRNAs. Other participating components are not currently known. The aim of this study was to identify mitochondrial proteins that are in direct physical contact with gRNAs thereby possibly involved in the editing reaction. At low monovalent cation concentration (30 mM KCl) 8 polypeptides with apparent molecular weights ranging from 124 to 9 kDa specifically cross-linked to gRNAs. Three of the proteins, 90, 21, and 9 kDa in size, were able to bind at higher salt concentrations (> or = 100 mM) indicating an enhanced affinity to the gRNA molecules. No cross-links were identified at > or = 250 mM KCl. Four gRNAs, specific for different editing domains of the ATPase 6 and ND7 pre-mRNAs, were in contact with the same set of mitochondrial polypeptides suggesting the assembly of an identical RNP complex that does not include pre-mRNA molecules. The binding of the 90 kDa protein was sensitive to the presence of U-nucleotides at the 3'-end of the gRNAs and could specifically be blocked by modifying free sulfhydryl groups. The interaction with the 124 kDa polypeptide was inhibited by vanadyl ribonucleosides, implicating a role for 2', 3' hydroxyl groups in the gRNA-protein interaction.
Full text
PDF

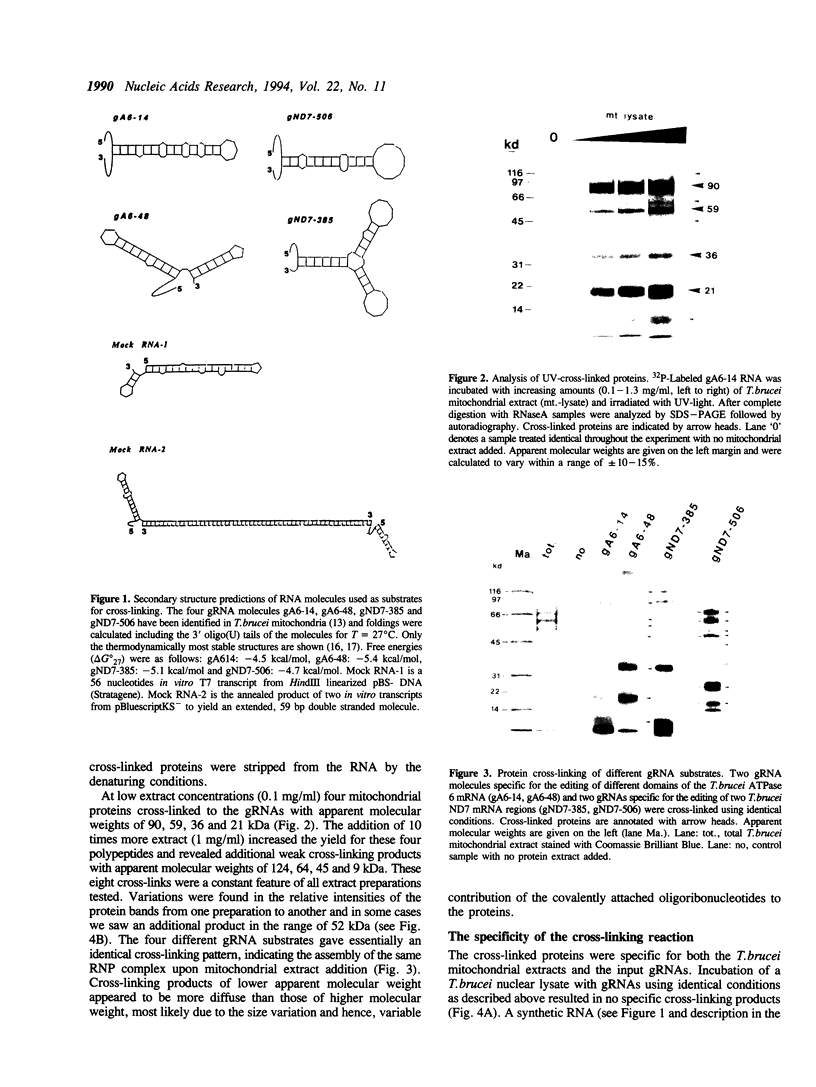
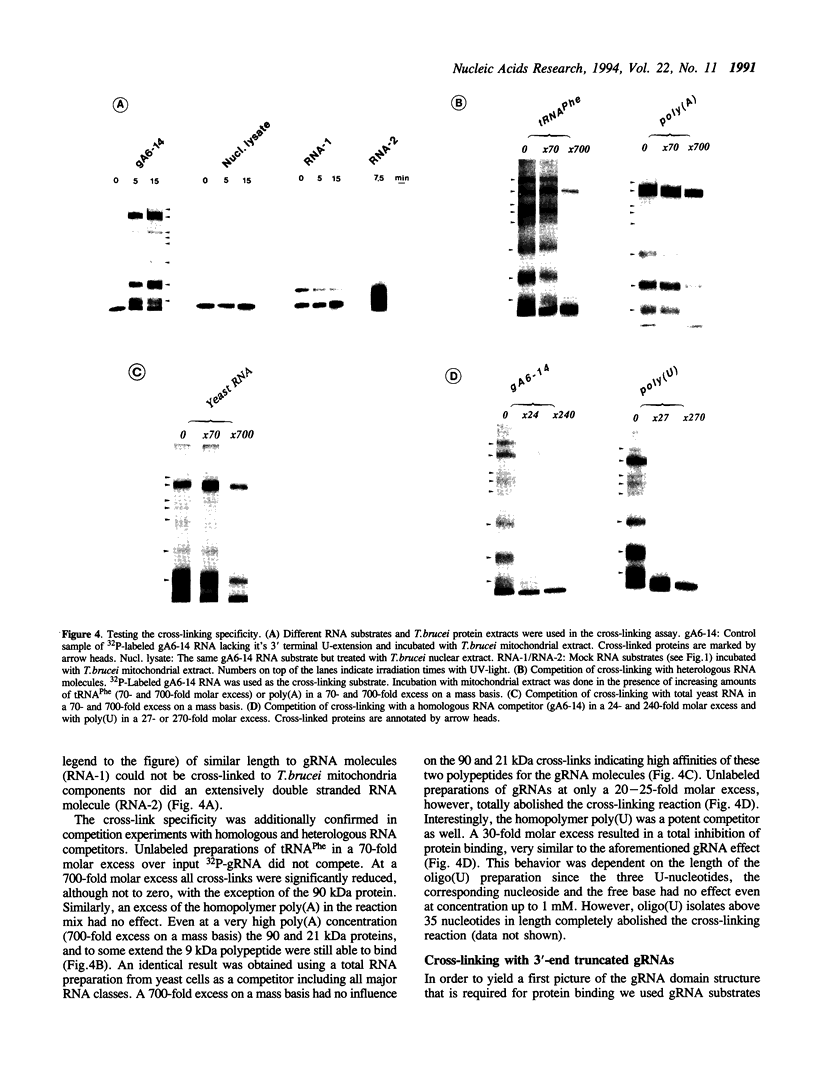
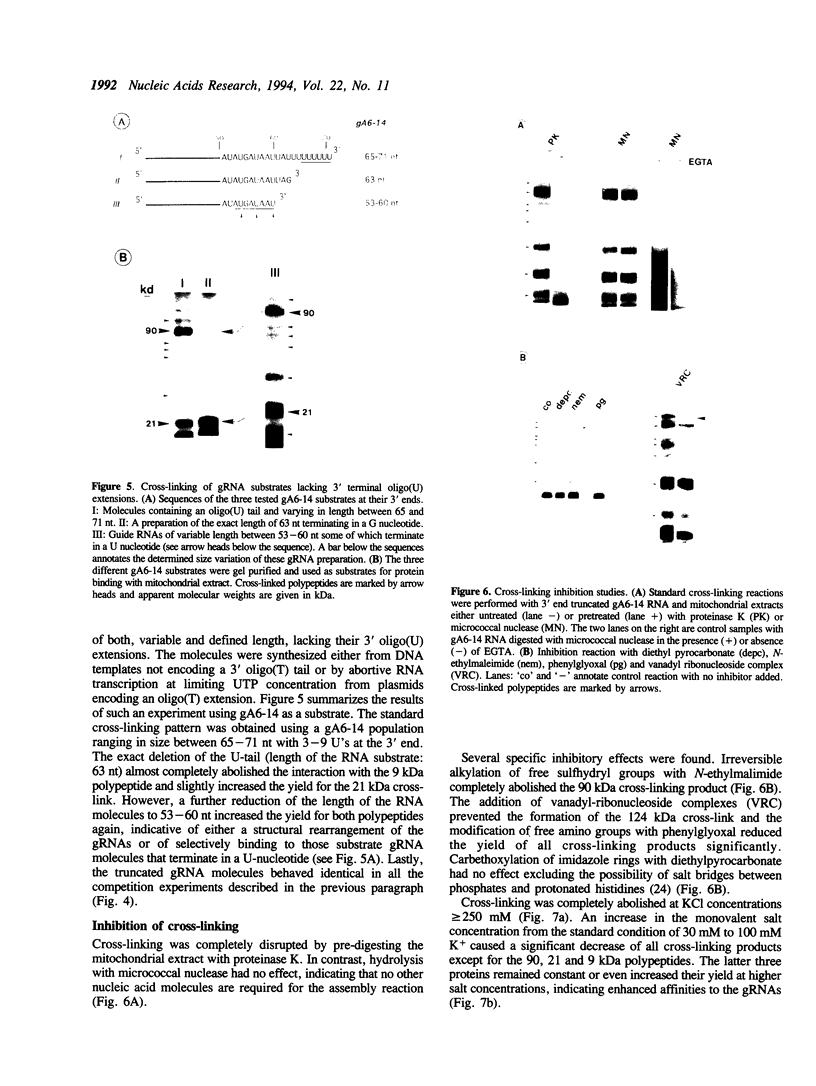


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalara N., Simpson A. M., Simpson L. The Leishmania kinetoplast-mitochondrion contains terminal uridylyltransferase and RNA ligase activities. J Biol Chem. 1989 Nov 5;264(31):18679–18686. [PubMed] [Google Scholar]
- Benne R. RNA editing in trypanosomes. The us(e) of guide RNAs. Mol Biol Rep. 1992 Sep;16(4):217–227. doi: 10.1007/BF00419661. [DOI] [PubMed] [Google Scholar]
- Benne R. RNA-editing in trypanosome mitochondria. Biochim Biophys Acta. 1989 Mar 1;1007(2):131–139. doi: 10.1016/0167-4781(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Blum B., Simpson L. Formation of guide RNA/messenger RNA chimeric molecules in vitro, the initial step of RNA editing, is dependent on an anchor sequence. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11944–11948. doi: 10.1073/pnas.89.24.11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B., Simpson L. Guide RNAs in kinetoplastid mitochondria have a nonencoded 3' oligo(U) tail involved in recognition of the preedited region. Cell. 1990 Jul 27;62(2):391–397. doi: 10.1016/0092-8674(90)90375-o. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göringer H. U., Koslowsky D. J., Morales T. H., Stuart K. The formation of mitochondrial ribonucleoprotein complexes involving guide RNA molecules in Trypanosoma brucei. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1776–1780. doi: 10.1073/pnas.91.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduk S. L., Harris M. E., Pollard V. W. RNA editing in kinetoplastid mitochondria. FASEB J. 1993 Jan;7(1):54–63. doi: 10.1096/fasebj.7.1.8422975. [DOI] [PubMed] [Google Scholar]
- Harris M. E., Hajduk S. L. Kinetoplastid RNA editing: in vitro formation of cytochrome b gRNA-mRNA chimeras from synthetic substrate RNAs. Cell. 1992 Mar 20;68(6):1091–1099. doi: 10.1016/0092-8674(92)90080-v. [DOI] [PubMed] [Google Scholar]
- Harris M. E., Moore D. R., Hajduk S. L. Addition of uridines to edited RNAs in trypanosome mitochondria occurs independently of transcription. J Biol Chem. 1990 Jul 5;265(19):11368–11376. [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Koontz S. W., Schimmel P. R. Aminoacyl-tRNA synthetase-catalyzed cleavage of the glycosidic bond of 5-halogenated uridines. J Biol Chem. 1979 Dec 25;254(24):12277–12280. [PubMed] [Google Scholar]
- Koslowsky D. J., Göringer H. U., Morales T. H., Stuart K. In vitro guide RNA/mRNA chimaera formation in Trypanosoma brucei RNA editing. Nature. 1992 Apr 30;356(6372):807–809. doi: 10.1038/356807a0. [DOI] [PubMed] [Google Scholar]
- Koslowsky D. J., Riley G. R., Feagin J. E., Stuart K. Guide RNAs for transcripts with developmentally regulated RNA editing are present in both life cycle stages of Trypanosoma brucei. Mol Cell Biol. 1992 May;12(5):2043–2049. doi: 10.1128/mcb.12.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I., Koehler K. A., Lindquist R. N. Enzymatic catalysis and the transition state theory of reaction rates: transition state analogs. Cold Spring Harb Symp Quant Biol. 1972;36:45–51. doi: 10.1101/sqb.1972.036.01.009. [DOI] [PubMed] [Google Scholar]
- Navaratnam N., Shah R., Patel D., Fay V., Scott J. Apolipoprotein B mRNA editing is associated with UV crosslinking of proteins to the editing site. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):222–226. doi: 10.1073/pnas.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. C., Souza A. E., Parsons M. Characterization of a Trypanosoma brucei nuclear gene encoding a protein homologous to a subunit of bovine NADH:ubiquinone oxidoreductase (complex I). Mol Biochem Parasitol. 1993 Mar;58(1):63–70. doi: 10.1016/0166-6851(93)90091-b. [DOI] [PubMed] [Google Scholar]
- Piñol-Roma S., Adam S. A., Choi Y. D., Dreyfuss G. Ultraviolet-induced cross-linking of RNA to proteins in vivo. Methods Enzymol. 1989;180:410–418. doi: 10.1016/0076-6879(89)80114-4. [DOI] [PubMed] [Google Scholar]
- Pollard V. W., Harris M. E., Hajduk S. L. Native mRNA editing complexes from Trypanosoma brucei mitochondria. EMBO J. 1992 Dec;11(12):4429–4438. doi: 10.1002/j.1460-2075.1992.tb05543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. M., Abelson J. N. In vitro synthesis of end-mature, intron-containing transfer RNAs. Methods Enzymol. 1989;180:63–69. doi: 10.1016/0076-6879(89)80092-8. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Uhlenbeck O. C. Nucleoside and nucleotide inactivation of R17 coat protein: evidence for a transient covalent RNA-protein bond. Biochemistry. 1985 Jul 16;24(15):4239–4244. doi: 10.1021/bi00336a064. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Doxsey S. J. Purification of nuclei from a flagellate protozoan, Trypanosoma brucei. Anal Biochem. 1982 Nov 15;127(1):112–115. doi: 10.1016/0003-2697(82)90152-x. [DOI] [PubMed] [Google Scholar]
- Simpson L. RNA editing--a novel genetic phenomenon? Science. 1990 Oct 26;250(4980):512–513. doi: 10.1126/science.1700474. [DOI] [PubMed] [Google Scholar]
- Smith H. C., Kuo S. R., Backus J. W., Harris S. G., Sparks C. E., Sparks J. D. In vitro apolipoprotein B mRNA editing: identification of a 27S editing complex. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1489–1493. doi: 10.1073/pnas.88.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Sanders J. Z., Kaiser R. J., Hughes P., Dodd C., Connell C. R., Heiner C., Kent S. B., Hood L. E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986 Jun 12;321(6071):674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- Stuart K., Gobright E., Jenni L., Milhausen M., Thomashow L., Agabian N. The IsTaR 1 serodeme of Trypanosoma brucei: development of a new serodeme. J Parasitol. 1984 Oct;70(5):747–754. [PubMed] [Google Scholar]
- Stuart K. RNA editing: new insights into the storage and expression of genetic information. Parasitol Today. 1989 Jan;5(1):5–8. doi: 10.1016/0169-4758(89)90211-1. [DOI] [PubMed] [Google Scholar]
- Vakalopoulou E., Schaack J., Shenk T. A 32-kilodalton protein binds to AU-rich domains in the 3' untranslated regions of rapidly degraded mRNAs. Mol Cell Biol. 1991 Jun;11(6):3355–3364. doi: 10.1128/mcb.11.6.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Jaeger J. A., Turner D. H. A comparison of optimal and suboptimal RNA secondary structures predicted by free energy minimization with structures determined by phylogenetic comparison. Nucleic Acids Res. 1991 May 25;19(10):2707–2714. doi: 10.1093/nar/19.10.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]