Abstract
It has recently been reported that soluble epoxide hydrolase (sEH), the major enzyme that metabolizes epoxyeicosatrienoic acids (EETs), is expressed in axons of cortical neurons; however, the functional relevance of axonal sEH localization is unknown. Immunocytochemical analyses demonstrate predominant axonal localization of sEH in primary cultures of not only cortical but also sympathetic and sensory neurons. Morphometric analyses of cultured sensory neurons indicate that exposure to a regioisomeric mixture of EETs (0.01 to 1.0μM) cause a concentration-dependent increase in axon outgrowth. This axon promoting activity is not a generalized property of all regioisomers of EETs since axonal growth is enhanced in sensory neurons exposed to 14,15-EET but not 8,9 or 11,12-EET. 14,15-EET also promotes axon outgrowth in cultured cortical neurons. Co-exposure to EETs and either of two structurally diverse pharmacological inhibitors of sEH potentiate the axon-enhancing activity of EETs in sensory and cortical neurons. Mass spectrometry indicates that sEHi significantly increase EETs and significantly decrease dihydroxyeicosatrienoic acid metabolites in neuronal cell cultures. These data indicate that EETs enhance axon outgrowth and suggest that axonal sEH activity regulates EETs-induced axon outgrowth. These findings suggest a novel therapeutic use of sEH inhibitors in promoting nerve regeneration.
Keywords: axonal outgrowth, cortical neurons, epoxyeicosatrienoic acids, sensory neurons, soluble epoxide hydrolase
Introduction
Epoxyeicosatrienoic acids (EETs) are epoxide metabolites of arachidonic acid that critically influence neuronal and vascular function and disease, primarily via potent vasodilator, neuroprotective, anti-inflammatory and pro-angiogenic activities (Spector et al. 2004, Iliff et al. 2010, Inceoglu et al. 2007). While production of EETs in the brain was demonstrated soon after the initial discovery of these novel arachidonic acid metabolites (Snyder et al. 1983), much of the research on the biological activity of these novel eicosanoids has focused on regulatory roles of EETs in cardiovascular and renal physiology and pathology. Recently, however, EETs have emerged as key players in regulating central and peripheral nervous system function. EETs signaling has been linked to regulation of cerebral blood flow, modulation of neuronal pain processing in the brain stem, control of neurohormone release, and influence on synaptic transmission (Iliff et al. 2010, Inceoglu et al. 2007, Inceoglu et al. 2008).
The biological activity of EETs is rapidly terminated via their metabolism through multiple pathways (Iliff et al. 2010, Inceoglu et al. 2007), with the predominant metabolic pathway being hydration by soluble epoxide hydrolase (sEH, EC 3.3.2.10) to form the less active dihydroxyeicosatrienoic acids (DHETs) (Chacos et al. 1983, Spector et al. 2004). Our recent work has demonstrated that sEH is highly abundant and active in the central and peripheral nervous system. Immunohistochemical analyses of mouse brain revealed sEH immunoreactivity in the cerebral cortex that was predominantly localized to neurons (Zhang et al. 2007). Within cortical neurons, sEH immunoreactivity was observed in neuronal cell bodies and processes but the most striking finding was the localization of sEH immunoreactivity in axons in the neuropil and nerve fiber bundles within gray and white matter. Additional studies indicate that sensory neurons of the trigeminal ganglia and parasympathetic neurons of the sphenopalatine ganglia are also immunoreactive for sEH as are nerve fibers emanating from these ganglia (Iliff et al. 2009).
The functional relevance of sEH expression in axons is not known. We hypothesize that sEH regulates axonal growth via modulation of the local levels of epoxy fatty acids and particularly EETs. To test this novel hypothesis, we examined the subcellular localization of sEH in primary cultures of central (cortical) and peripheral (sympathetic and sensory) neurons and determined whether experimentally increasing levels of EETs by addition of exogenous EETs and/or pharmacologic inhibition of sEH influenced axonal growth in vitro. Our findings support this hypothesis and suggest pharmacologic inhibition of sEH as a novel therapeutic approach for preventing axon retraction or promoting axon regeneration following ischemic or traumatic insult.
Experimental Procedures
Materials
Recombinant human bone morphogenetic protein-7 (BMP-7) was generously provided by Curis (Cambridge, MA). All solvents used for liquid chromatography and mass spectroscopy were of HPLC grade or better and purchased from Fisher Scientific (Pittsburgh, PA). 4-Phenylchacone oxide (4-PCO) was synthesized and purified as previously described (Morisseau et al. 1999). 1-(1-Methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea (TUPS) was synthesized and purified according to recently published methods (Tsai et al. 2010). Unless otherwise noted, all other chemical reagents were purchased from Sigma Chemical Company (St. Louis, MO). A mixture of EET-methyl esters as well as individual EET regioisomers (free acid form) were synthesized and purified as described earlier (Campbell et al. 1991). To determine the ratio of individual EET-methyl ester regioisomers in the mixture, the methyl esters were base hydrolyzed as described (Newman et al. 2002) and the resulting free fatty acid EETs mixture was analyzed by LC-MS/MS (as described below under quantification of EETs). The regioisomeric EET-methyl ester mixture contained 14,15-, 11,12-, 8,9- and 5,6-EET at a ratio of 10:10:10:1. The relatively low level of 5,6-EET in this mixture likely reflects its susceptibility to conversion to the lactone derivative under the conditions used for LC-MS/MS. Aliquots of stock solutions of the individual 8,9-, 11,12- and 14,15-EET preparations used in axon outgrowth experiments were analyzed by LC-MS/MS to confirm purity and concentration.
Animals
All procedures involving animals were performed according to protocols approved by the Institutional Animal Care and Use Committees of Oregon Health & Science University and UC Davis. Timed-pregnant Sprague Dawley rats were purchased from Charles River Laboratories (Hollister, CA) and housed individually in standard plastic cages with Alpha-Dri bedding (Shepherd Specialty Papers, Watertown, TN) in a temperature (22±2°C) controlled room on a 12h reverse light-dark cycle. Food and water were provided ad libitum. Pain and discomfort were minimized by humane euthanasia of animals prior to any experimentation or surgery.
Primary neuronal cell culture
Cortical neurons were dissociated from the neocortex of embryonic day 18 (E18) rat pups and maintained in serum-free Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with B27 (Invitrogen) as previously described (Howard et al. 2003). Under these culture conditions, immunocytochemical analyses indicated that ~95% of the cell population was immunoreactive for the neuronal marker, neurofilament, and ~5% of the cell population was immunoreactive for the astrocytic marker, glial fibrillary acidic protein. Sympathetic neurons were dissociated from the superior cervical ganglia (SCG) of embryonic day 21 (E21) rat pups and maintained in the absence of glial cells in serum-free medium supplemented with nerve growth factor (β-NGF, 100ng/ml, Harlan Bioproducts, Indianapolis, IN) as previously described (Higgins et al. 1991). BMP-7 (50 ng/ml) was added to the medium on day 5 in vitro to trigger dendritic growth (Lein et al. 1995). Sensory neurons were dissociated from the dorsal root ganglia (DRG) of embryonic day 15 (E15) rat pups and maintained in serum-free medium supplemented with β-NGF (100ng/ml) as previously described (Yang et al. 2008).
Immunocytochemical localization of sEH
To localize sEH immunoreactivity in primary neuronal cell cultures, cultures were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton-X in phosphate-buffered saline, blocked with 2% normal goat serum and 1% bovine serum albumin and reacted with rabbit polyclonal antibodies against sEH (1:10,000, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Antigen: antibody complexes were detected by indirect immunofluorescence as previously described (Lein et al., 1995). Fluorescent cell images were captured using a Spot digital camera. To identify dendrites in cortical and sympathetic neurons, cultures were immunostained with a monoclonal antibody to microtubule-associated protein-2 (MAP-2, Sternberger-Meyer Immunochemicals, Jarrettsville, MD) as previously described (Lein et al. 1995). To compare relative fluorescence intensity in dendrites versus axons of cultured sympathetic neurons, digital images were captured using identical exposure settings and signal intensity in the central shaft of individual dendrites and individual axons was quantified using MetaMorph image analysis software (Molecular Devices, Sunnyvale, CA). Twenty dendritic and twenty axonal images were captured per culture in 3 cultures derived from the same dissection for a total sample size of 60. The mean fluorescence intensity (in arbitrary units) in cytoplasm of central dendritic shafts was compared to that of axons.
Experimental manipulation of EETs levels in neuronal cell cultures
Levels of EETs were increased in cultures by addition of a mixture of EET-methyl ester regioisomers, individual EETs regioisomers, the sEHi 4-PCO or TUPS or a combination of EETs and one of the sEHi directly into the culture medium. Stock solutions of EETs and sEHi were prepared in 100% DMSO or ethanol, respectively. Stocks were diluted 1:1,000 in culture medium to give final concentrations of EETs and sEHi as indicated in the text. Control cultures were treated with culture medium supplemented with DMSO and ethanol at 0.1% (v/v) final. Previous studies indicated that this amount of DMSO and ethanol does not alter axonal growth relative to medium alone (Howard et al. 2003, Yang et al. 2008). Experiments were initiated 1h after plating by adding EETs and/or sEHi directly to the medium of individual cultures. Each culture was treated again 12h after plating by exchanging the existing medium in the culture for freshly prepared medium containing EETs ± sEHi at the same final concentration as added previously. During this process, less than 5% of the cells were lost due to poor adherence to the culture dish.
1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) Assay of Cell Viability
Primary cultures of sensory and cortical neurons (2×104 cells per well in 24-well plates) were exposed to the regioisomeric EET-methyl ester mixture in the absence or presence of sEHi TUPS or 4-PCO under the same conditions used for morphometric analyses. Cultures were then incubated at 37°C for 4h in HBSS supplemented with MTT at a final concentration of 500μg/ml then solubilized in 5% Triton X-100. Absorbance was measured at 562nm using a Spectraflour Plus spectrophotometer (Tecan, Research Triangle Park, NC).
Morphometric analyses
To confirm individual cells as neuronal and to visualize their axons, cultured DRG neurons were fixed with 4% paraformaldehyde and immunostained for Protein Gene Product 9.5 (PGP 9.5; Biogenesis Inc. Brentwood, NH) while cultured cortical neurons were reacted with a tau-specific antibody (Chemicon-Millipore, Billerica, MA). Antigen-antibody complexes were detected by indirect immunofluorescence as previously described (Lein et al., 1995), and fluorescent cell images were captured using a Spot digital camera. An observer blinded to the experimental conditions quantified axonal length using Metamorph image analysis software (Molecular Devices, Sunnyvale, CA). Processes were identified as axons if they were immunopositive for either PGP9.5 or tau, exhibited a distinct growth cone and their length was equal to or greater than the diameter of the cell body. Axonal growth was evaluated in a minimum of 50 randomly chosen neurons from three different cultures (≥15 neurons per culture) of each experimental condition. Each experimental result was confirmed in cultures obtained from three independent dissections; all data presented in the text are from a single representative culture series.
Quantification of EETs
Cultured DRG neurons were exposed to 14, 15- EET (free acid) in the absence or presence of the sEHi TUPS for 24h. Following this exposure, conditioned medium was collected from cultures and snap frozen on dry ice. Cells were rinsed once with PBS, removed with a cell scraper, spun down to remove PBS and the resulting cell pellet snap frozen. Both media and cell pellets were stored at −80°C until analyzed. Immediately prior to analysis, cell pellets were resuspended in 1mL cold methanol and refrozen at −80°C for at least 1h. Upon thawing on ice, an antioxidant solution (10 μL) containing 0.2mg/mL butylated hydroxytoluene (BHT, Sigma Chemical Company, St. Louis, MO) and 0.2mg/mL triphenyl phosphate (TPP, Sigma) was added to each sample. Samples were centrifuged for 2 min at 16,000 × g at 4°C, the supernatant recovered and the remaining pellet re-extracted two additional times each in 0.5 ml of cold methanol followed by another two rounds of extraction each using 0.5 ml of cold ethyl acetate. All extracts for each sample were then combined into one tube. A trap solution (30% glycerol in methanol) of 6 μL volume was added to the pooled extracts to prevent complete dryness of the samples. These samples were then evaporated under vacuum and reconstituted using a solution containing 200 nM 1-cyclohexyl ureido, 3-dodecanoic acid (CUDA), which was synthesized as previously described (Watanabe et al. 2003). Conditioned culture medium (3 mL per sample) was extracted by solid phase extraction using 60 mg Waters Oasis-HLB cartridges (Milford, MA) as described (Yang et al. 2009). The lipid fraction eluted from the SPE cartridge was evaporated and reconstituted in methanol containing an internal standard solution.
An Agilent 1200 SL liquid chromatography (LC) instrument (Agilent Corporation, Palo Alto, CA) equipped with an auto sampler (set to 4°C) and an Eclipse Plus C18 2.1 × 150 mm, 1.8 μm LC column was used for the separation of the analytes. For separation, a gradient of solvent was formed using water with 0.1% glacial acetic acid (solvent A) and acetonitrile/methanol (84:16) with 0.1% glacial acetic acid (solvent B). Gradient elution was performed at a flow rate of 250 μL/min. Chromatography was optimized to separate target analytes in 21.5 min as described (Yang et al. 2009). The output from the LC column was directed to a 4000 QTrap tandem mass spectrometer (Applied Biosystems Instrument Corporation, Foster City, CA) equipped with an electrospray source (Turbo V). The instrument was operated in negative multiple reaction monitoring (MRM) mode. The MRM transitions used were 14(15)-EET (m/z 319/219) and 14,15-DHET (m/z 337/207). The Analyst software 1.4.2 from Applied Biosystems was used to quantify the analytes based on the standard curves as described (Yang et al. 2009).
Statistical Analyses
Data are presented as the mean ± SEM. Statistical significance for in vitro experiments was assessed by a one-way ANOVA with p < 0.05 considered significant, followed by post hoc comparison of means using Student Newman-Keuls analysis.
Results
To confirm and extend previous in vivo observations of sEH immunoreactivity in central and peripheral neurons (Iliff et al. 2009, Zhang et al. 2007), we examined the expression pattern of sEH immunoreactivity in primary cultures of cortical neurons dissociated from the embryonic rat neocortex, sympathetic neurons dissociated from embryonic rat SCG and sensory neurons derived from embryonic rat DRG. Neurons cultured from the neocortex were immunostained for sEH after 4 to 5d in vitro, at which time cortical neurons are polarized and extend morphologically, biochemically and functionally distinct axons and dendrites (Dotti & Banker 1987). To determine whether sEH immunoreactivity was differentially localized to axons versus dendrites, cortical cultures were co-labeled for MAP-2 immunoreactivity, which is selectively expressed in the somatodendritic compartment (Lein et al. 1995). As demonstrated in Figure 1(A and B), a subset of cortical neurons in the culture (~ 30–50%) are immunopositive for sEH. Within sEH immunopositive cortical neurons, the most intense sEH immunofluorescence is observed in the somata and the axons, the latter distinguishable as the thin non-tapering processes that do not react with MAP-2 antibody. In contrast, dendrites, recognizable as tapered MAP-2 immunopositive processes that end proximal to the cell body, exhibit noticeably less intense sEH immunofluorescence.
Figure 1. Soluble epoxide hydrolase (sEH) is selectively localized to axons in primary neuronal cell cultures.
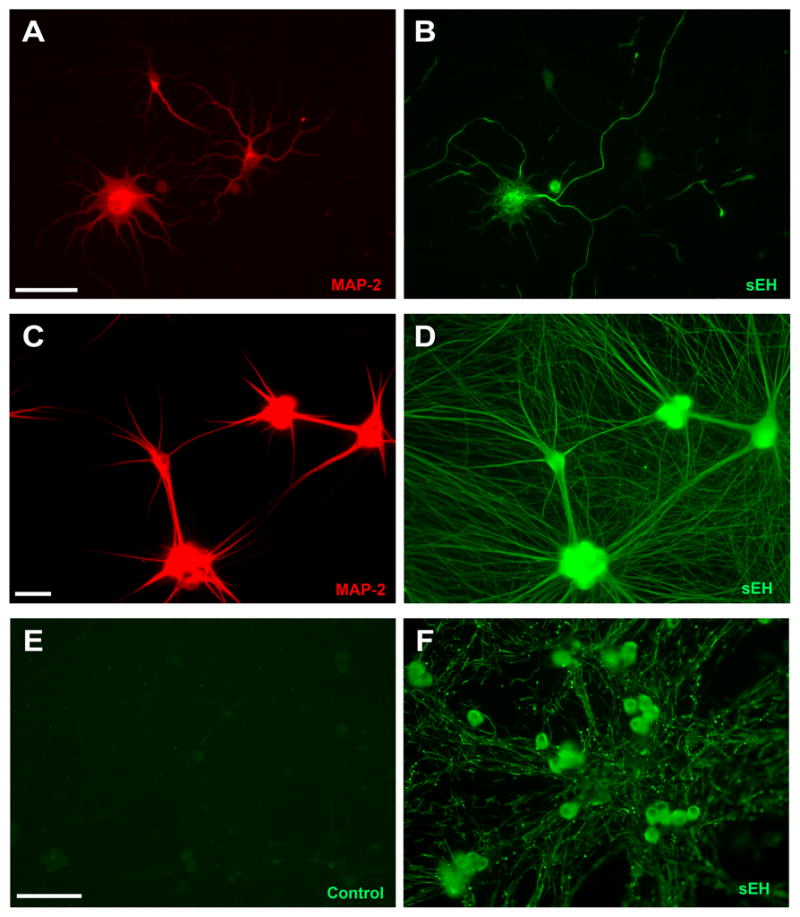
Neuronal cell cultures derived from the neocortex of embryonic rat pups (A, B) or the superior cervical ganglia (C, D) were co-labeled with antibodies specific for the somatodendritic cytoskeletal protein MAP-2 (A, C) and sEH (B, D). Comparison of MAP-2 (A) versus sEH (B) patterns in cultured cortical neurons suggests that only a subset of cortical neurons express sEH. In those neurons that are immunopositive for sEH, fluorescence is significantly more intense in axons relative to dendrites. Comparison of MAP-2 (C) versus sEH (D) immunostaining in cultured sympathetic neurons suggests that all neurons are immunopositive for sEH. Consistent with results in cultured cortical neurons, sEH immunofluorescence is more intense in axonal than in dendritic processes. Intense sEH immunofluorescence was also observed in the axons of sensory neurons cultured from dorsal root ganglia of embryonic rat pups (F) relative to control cultures reacted with secondary antibody only (E). Bar = 50 μm.
Sympathetic neurons similarly extend both multiple dendrites and a single axon in vivo and this morphology can be recapitulated in vitro by exposing neurons to BMP-7 (Lein et al. 1995). MAP-2 and sEH were colocalized in sympathetic neurons after a 5d exposure to BMP-7. In contrast to cortical neuronal cell cultures, all the sympathetic neurons in the culture were immunopositive for sEH (Figure 1, C and D). However, similar to cortical neurons, intense sEH immunofluorescence was evident throughout the MAP-2-negative axons. While it appears that dendrites may also exhibit sEH immunoreactivity, close the signal intensity appears weaker in the center of the dendritic shaft relative to the edge of the process, and the bright sEH immunofluorescence evident along the edge of dendritic processes continues well beyond the tip of the of the MAP-2-positive process. These observations suggest the possibility that the sEH immunofluorescence observed in MAP-2 positive processes may be largely associated with axons running along the length of the dendrite, which is a common occurrence in these cultures (Lein et al. 1996). In support of this possibility, a semi-quantitative analysis of sEH fluorescence indicated that the mean signal intensity in the central shaft of axons (54 ± 3.2) was approximately twice that observed in the central shaft of dendrites (17 ± 5.2).
DRG sensory neurons extend only axons both in vivo and in vitro. All neurons within cultures derived from DRG are immunoreactive for sEH (Figure 1F). Intense sEH immunofluorescence is observed throughout the extranuclear space of the somata. As with cortical and sympathetic neurons, the axons of DRG neurons are immunopositive for sEH; however, the pattern of axonal sEH immunoreactivity varies from that observed in sympathetic neurons. Particularly, the axons of DRG neurons exhibit discontinuous axonal sEH immunoreactivity. Most striking is the bright punctuate staining superimposed on a pattern of less intense sEH immunoreactivity that extends in an interrupted pattern throughout the shaft of the axon.
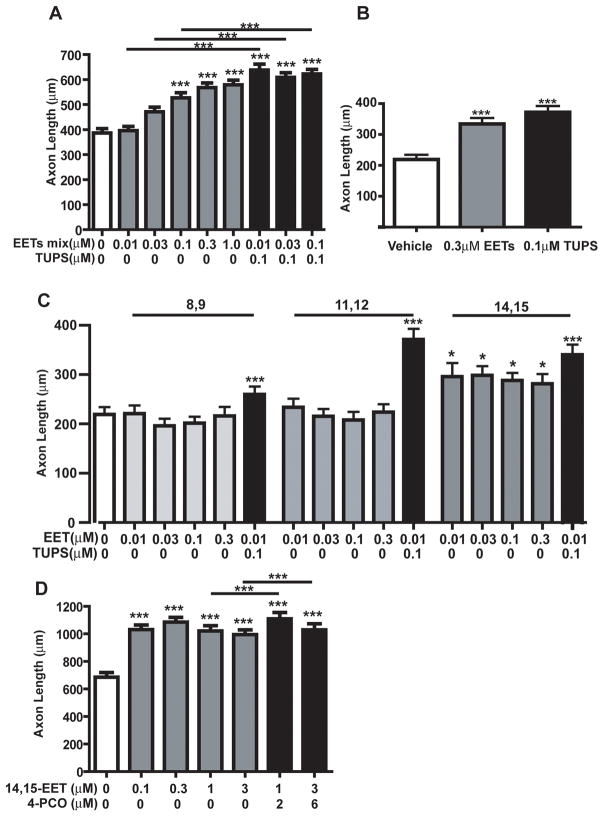
To test the hypothesis that EETs regulate axonal growth, we determined whether experimentally increasing EETs altered axon outgrowth during the first 24h after plating. A regioisomeric EET-methyl ester mixture was added to DRG cultures at concentrations ranging from 0.01 to 1.0 μM. Because EETs are rapidly degraded by sEH, the regioisomeric EET-methyl ester mixture was added 1h and again at 12h after plating and a subset of cultures were also treated with the sEHi TUPS (see Table 1). DRG cultures assessed in the MTT assay revealed no effect of this exposure paradigm on mitochondrial activity (data not shown). Morphometric analyses of DRG cultures fixed and immunostained for an axon-selective antigen indicated that the addition of the regioisomeric EET-methyl ester mixture did not alter the number of axons extended by DRG neurons (Figure 2). However, the regioisomeric EET-methyl ester mixture did significantly increase total axonal length per neuron in a concentration-dependent manner starting at a concentration of 0.1μM (Figure 2 and Figure 3A). Simultaneous exposure of DRG cultures to the EET-methyl ester mixture and the sEHi TUPS at 0.1 μM significantly enhanced axonal outgrowth relative to cultures exposed to the same concentration of the EET-methyl ester mixture in the absence of TUPS (Figure 3A). Notably, inhibition of sEH with TUPS significantly increased axon length even at concentrations of the regioisomeric EET-methyl ester mixture (0.01 and 0.03 μM) that had no significant effect on axonal growth in the absence of sEHi. Addition of TUPS alone also significantly increased axonal growth relative to control cultures (Figure 3B).
Table 1.
Structure and pharmacologic properties of soluble epoxide hydrolase inhibitors
| Inhibitor Name | Structure | Melting point, °C | IC50 (nM)a |
Mass (Da) | ||
|---|---|---|---|---|---|---|
| Human | Mouse | Rat | ||||
| 4-PCOb | 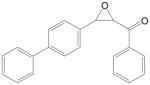 |
103.5 | 200 | 140 | d | 300.3 |
| TUPSc | 237 | 3 | 5 | 9 | 381.4 | |
Values represent the in vitro inhibitory potency on affinity purified recombinant sEH proteins from human, mouse and rat expressed using a baculovirus expression system. The potency of 4-PCO was tested using 4-nitrophenyl-trans-2,3-epoxy-3-phenyl-propyl carbonate as substrate; the potency of TUPS was tested using the fluorescent substrate, cyano-(2-methoxynaphthalen-6-yl)methyl trans-(3-phenyl-oxyran-2-yl) methyl carbonate.
4-Phenyl chalcone oxide
1-(1-Methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea).
Not determined
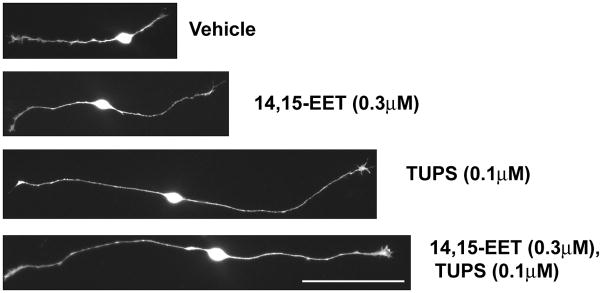
Figure 2. Epoxyeicosatrienoic acids (EETs) enhance axon outgrowth in cultured sensory neurons.
Representative fluorescence micrographs of sensory neurons derived from embryonic rat dorsal root ganglia exposed to vehicle (ethanol and DMSO) or 14, 15-EET in the absence or presence of the sEH inhibitor TUPS. 14, 15-EET (0.3 μM) and TUPS (0.1 μM) were added 1h and 12h after plating. At 20 to 24h in vitro, cultures were fixed and immunostained for the peripheral neuronal cell marker PGP9.5. Bar = 100 μm.
Figure 3. Epoxyeicosatrienoic acids (EETs) enhance axon outgrowth in cultured sensory neurons.
Sensory neurons derived from embryonic rat dorsal root ganglia were exposed to vehicle (ethanol and DMSO) or EETs in the absence or presence of sEH inhibitors (sEHi). EETs ± sEHi were added 1h and 12h after plating. At 20 to 24h in vitro, cultures were fixed and immunostained for the peripheral neuronal cell marker PGP9.5. Exposure to a mixture of EET isomers caused a dose-dependent enhancement of axon outgrowth (A). Co-exposure to the sEHi TUPS further enhanced the effects of EETs on axon outgrowth (A). Addition of TUPS enhanced axon outgrowth even in the absence of exogenous EETs (B). Morphometric analyses of sensory neurons exposed to purified EET isomers indicated that relative to vehicle control cultures, EET 14,15-EET, but not 8,9- or 11,12-EET significantly enhanced axon outgrowth at concentrations ranging from 0.01 to 0.3 μM (C). Addition of TUPS further enhanced axonal outgrowth in cultures co-exposed to 11,12- and 14,15-EET at 0.01 μM, but not 8,9-EET at 0.01 μM (C). Exposure to 14,15-EET at even higher concentrations (1 to 3 μM) also enhanced axon outgrowth and these effects were further enhanced by co-exposure to 4-PCO at 2 or 6 μM (D). Data presented as the mean ± S.E.M. (n = 100 neurons per experimental condition); ***p < 0.001 relative to vehicle control (One-way ANOVA with post-hoc Student Newman-Keuls analysis).
There are 4 regioisomers of EETs, 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET, which are distinguished by the location of the epoxide along the carbon chain of arachidonic acid. The closer the epoxide ring to the methyl-terminal or omega carbon of any given EET isomer, the more efficiently it is metabolized by sEH. Thus, the order of substrate selectivity for sEH is as follows: 14,15-EET > 11,12-EET > 8,9-EET > 5,6-EET. To determine whether the axon-promoting activity of the regioisomeric EET-methyl ester mixture is a general property of all EETs, we tested the ability of the three EET regioisomers with the greatest affinity for sEH to promote axonal growth in DRG cultures. At concentrations ranging from 0.01 to 0.3 μM, 8,9-EET and 11,12 EET had no significant effect on axon outgrowth, whereas the same concentration range of 14,15-EET significantly increased axon outgrowth (Figure 3C). The addition of the TUPS (0.1 μM) significantly enhanced axonal growth observed in cultures exposed to 8,9-EET, 11,12-EET or 14,15-EET at 0.01 μM (Figure 3C).
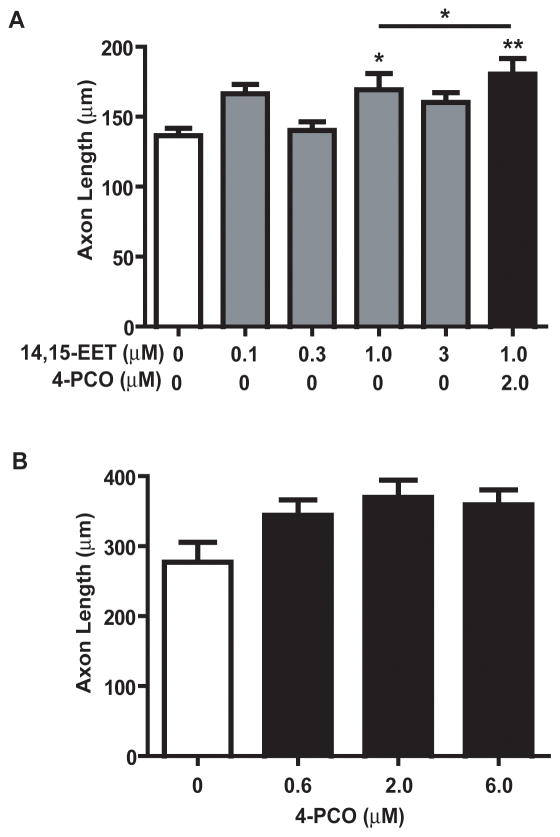
To confirm that the enhancement axon outgrowth by TUPS was due to inhibition of sEH, we tested the effects on axonal growth of a structurally diverse sEHi, 4-PCO (see Table 1). Because 4-PCO is less potent than TUPS, higher concentrations were tested. As observed with TUPS, treatment of DRG cultures with 4-PCO (at 2 and 6 μM) significantly enhanced the axon-promoting activity of 14,15-EET (Figure 3D).
To determine whether the axon-promoting activity of EETs could be generalized across diverse neuronal cell types, we quantified the effects of 14,15-EET and/or sEHi 4-PCO on axonal growth in cortical neurons. At a concentration of 1.0 μM, 14-15-EET significantly increased axon outgrowth in 4-day-old cortical cultures and this effect was significantly enhanced by simultaneous exposure to sEHi at 2.0 μM (Figure 4A). Addition of 4-PCO in the absence of exogenous EETs at concentrations ranging from 0.6 to 6.0 μM increased axonal length but this effect did not reach statistical significance (Figure 4B). MTT assays of parallel cultures indicated that these exposure paradigms did not affect mitochondrial activity in cortical neurons.
Figure 4. Epoxyeicosatrienoic acids (EETs) enhance axon outgrowth in cultured cortical neurons.
Cortical neurons derived from embryonic rat neocortex were exposed to vehicle (ethanol and DMSO) or 14,15-EET at concentrations ranging from 0.1 to 3 μM in the absence or presence of the sEH inhibitor (sEHi) 4-PCO. EETs ± sEHi were added 1h and 12h after plating. On day 4 in vitro, cultures were fixed and immunostained for neurofilaments. 14,15-EET significantly enhanced axon outgrowth at 1.0 μM and this effect was further enhanced by co-exposure to 4-PCO (A). Exposure to 4-PCO alone did not cause a significant increase in axon outgrowth relative to neurons exposed only to vehicle (B). Data presented as the mean ± S.E.M. (n = 50 neurons per experimental condition); *p < 0.05 and **p< 0.01 (One-way ANOVA with post-hoc Student Newman-Keuls analysis). An asterisk above bar of a treated group indicates a significant difference from vehicle control; whereas an asterisk above a horizontal line indicates a significant difference between cultures grown in the absence or presence of sEHi at the same EETs concentration.
Liquid chromatography-mass spectrometry (LC-MS) demonstrated that cultured DRG neurons produce endogenous 14,15-EET and 14,15-DHET (Figure 5); however, endogenous levels of 8,9-EET and 11,12-EET and their dihyroxy metabolites were below the level of detection. In these cells, the ratio of 14,15-EET to 14,15-DHET is approximately 10 and the endogenous levels of these eicosanoids are approximately 30-fold higher in cells relative to culture medium. The addition of the free acid 14,15-EET to DRG cultures increased the concentration of 14,15-EET and 14,15-DHET in the culture medium and in cells by several orders of magnitude relative to vehicle control cultures (Figure 5). Addition of the regioisomeric EET-methyl ester mixture similarly increased 14,15-EET and 14-15-DHET (data not shown). Simultaneous exposure to TUPS had a pronounced effect on levels of these compounds in the cell medium, further elevating the concentration of 14,15-EET coincident with a significant decrease in the concentration of 14,15-DHET relative to cultures exposed to EETs in the absence of TUPS (Figure 5).
Figure 5. Pharmacologic inhibition of soluble epoxide hydrolase (sEH) significantly decreases hydration of 14,15-epoxyeicosatrienoic acid (EET) in neuronal cell cultures.
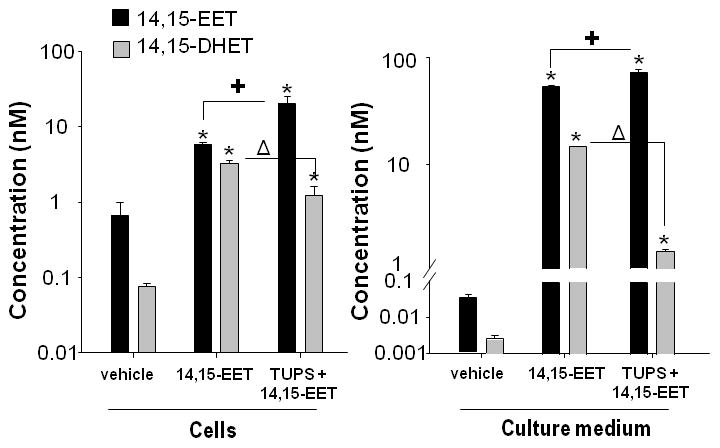
Sensory neurons derived from embryonic rat DRG were exposed to vehicle (ethanol and DMSO) or 14,15-EET (free fatty acid, 0.3 μM) in the absence or presence of the sEH inhibitor TUPS (0.1μM) added 1h and 12h after plating. At 24h in vitro, conditioned medium and cell pellets were collected. The concentration of 14,15-EET and its metabolite, 14,15-dihydroxyeicosatrienoic acid (DHET), were determined in both culture medium and cells using liquid chromatography-mass spectroscopy. Addition of 14,15-EET significantly increased the concentration of 14,15-EET in the cells and media (* p<0.05 vehicle versus 14,15-EET ) and the concentration of 14,15-DHET in the cells and media (*p<0.001 vehicle versus 14,15-EET). Simultaneous treatment with TUPS further increased the levels of 14,15-EET in cells and media (+ p<0.05 14,15-EET versus 14,15-EET+TUPS) and decreased the levels of 14,15-DHET in cells and media (Δ p<0.002 14,15-EET versus 14,15-EET+TUPS). Data are presented as the mean ± S.E.M. (n = 3 per experimental condition); statistical significance was determined by One-way ANOVA followed by Student-Newman-Keuls post hoc analysis.
Discussion
Our data support the novel hypothesis that sEH regulates axonal growth via modulation of EETs levels. Specifically we demonstrate that: 1) sEH immunoreactivity is localized to axons in primary cultures of cortical, sympathetic and sensory neurons; 2) addition of exogenous EETs significantly increases axonal length in sensory and cortical neurons; 3) pharmacologic inhibition of sEH increases axonal length when added alone and enhances the axon promoting activity of exogenous EETs; and 4) primary neuronal cell cultures produce endogenous EETs and pharmacologic inhibition of sEH decreases conversion of 14,15-EET to its less active dihydroxy metabolite.
Immunocytochemical localization of sEH in cultured cortical, sympathetic and sensory neurons confirms and extends our previous work demonstrating that sEH is abundantly expressed in the central and peripheral nervous systems (Iliff et al. 2009, Zhang et al. 2007). This previous work suggested that sEH immunoreactivity is expressed in neuronal cell bodies, dendrites and axons of the cerebral cortex (Zhang et al. 2007). Interestingly, we observed sEH immunoreactivity only in a subset of neurons in cortical cultures. This is consistent with a recent report that sEH immunoreactivity is detected in neurons in layers II and V but not other layers of the mouse neocortex (Bianco et al. 2009) and the known neuronal heterogeneity of neurons of primary cortical cultures (Banker & Goslin 1998). In those cortical neurons that were immunopositive for sEH, sEH immunofluorescence was significantly more intense in axons relative to dendrites. Although the differential between axons and dendrites was not as marked as in cortical neurons, a similar pattern of subcellular distribution was observed in sympathetic neurons immunostained for sEH, suggesting that selective enrichment of sEH in axons versus dendrites may be a generalized property of neurons. In neurons that extend only axons, such as DRG neurons, axons similarly exhibited intense sEH immunoreactivity. The pattern of axonal staining varied somewhat from that observed in cortical and sympathetic neurons in that DRG axons exhibited intense punctuate sEH immunofluorescence superimposed on less intense but above background sEH immunostaining within the axon shaft. Cell-type specific differences in the subcellular immunolocalization of sEH have been previously reported (Enayetallah et al. 2006). These earlier studies demonstrated that sEH may be cytosolic or peroxisomal and the relative distribution between these subcellular sites varied according to cell type such that it was predominantly cytosolic in some cell types but both cytosolic and peroxisomal in other cell types (Enayetallah et al. 2006). These observations suggest the possibility that sEH is localized to peroxisomes in the axons of DRG neurons but not sympathetic or cortical neurons. While this hypothesis has yet to be tested, the observation of different patterns of sEH immunoreactivity between different neuronal cell types suggests the intriguing possibility that the profile of sEH cellular function(s) varies according to neuronal cell type.
While arachidonic acid and its prostanoid metabolites have been previously implicated in regulating neurite outgrowth (Campello-Costa et al. 2006, Hiruma et al. 2000, Leu & Schmidt 2008, Williams et al. 1994), our data are the first to describe a role for EETs in the regulation of neuronal morphogenesis. Specifically, we observed that a 24h exposure to exogenous EETs added as a mixture of regioisomers significantly increased axonal length in DRG neurons in a concentration-dependent manner. The axon-promoting activity of the regioisomeric EET-methyl ester mixture appears to be mediated primarily by 14,15-EET since this regioisomer but not 8,9-EET or 11,12-EET significantly enhanced axonal growth when added as purified preparations to DRG neurons. However, since we did not test the 5,6-EET, we cannot rule out the possibility that it may also exhibit axon-promoting activity. These findings are consistent with previous reports that different EET regioisomers exhibit different activity profiles. For example, 5,6-EET is a potent dilator of cerebral blood vessels in vivo and in vitro, whereas 14,15-EET is a weak vasodilator in the cerebral circulation (Alkayed et al. 1996, Ellis et al. 1990).
Cortical neurons also responded to exogenous 14,15-EET with significantly increased axonal growth. This finding suggests increased axonal growth may be a generalized neuronal response to EETs across neuronal cell types. The axonal effects of 14,15-EET in cortical neurons did not exhibit a clear dose-response relationship nor were they as robust in cortical neurons relative to DRG neurons. The former suggests the possibility that similar to the expression of sEH in a subpopulation of cortical neurons, only some cortical neurons express the putative EET receptor, leading to potentially increased variability in cortical versus DRG neuronal responses. Increased variability could also account for the less robust response to the axon-promoting activity of EETs. Alternatively or in addition, it may also reflect differences in culture medium and time in culture between EETs exposure and morphometric analyses. With respect to the latter, axonal length was analyzed in DRG neurons fixed for morphometric analyses immediately after the 24h EETs exposure, whereas axonal length was analyzed in cortical neurons fixed 72h after the 24h EETs exposure to allow development of cortical neurites into fully differentiated axons and dendrites.
Since EETs are rapidly metabolized by sEH, pharmacologic inhibition of sEH would be expected to enhance the axon-promoting activity of EETs. Our data support this prediction: simultaneous exposure to sEHi enhanced axonal length in cultured DRG neurons exposed to either a mixture of EET-methyl esters or purified 14,15-EET. Interestingly, simultaneous exposure to sEHi also enhanced axon outgrowth in DRG cultures exposed to 8,9-EET or 11,12-EET, neither of which increased axonal growth when administered in the absence of sEHi. This effect is likely not explained by increased metabolic degradation of 8,9 or 11,12-EET relative to 14,15-EET in the absence of sEHi since the substrate selectivity of sEH is significantly greater for 14,15-EET than either 8,9-EET or 11,12-EET, Two alternative explanations include: 1) decreased affinity of 8,9-EET and 11,12-EET relative to 14,15-EET for the putative receptor(s) mediating the axon-promoting activity of EETs; or 2) the possibility that sEHi increases the bioavailability of endogenous 14,15-EET in neuronal cell cultures which mediates the increased axonal growth observed in cultures exposed to 8,9 EET or 11-12 EETs in the presence of sEHi. Since a specific receptor that mediates the biological activity of EETs has yet to be described, we cannot address the former possibility; however, our data support the latter possibility (see below). Moreover, our observations suggest that 8,9-EET and 11,12-EET do not inhibit the axon-promoting activity of 14,15-EET.
Two observations support the conclusion that sEHi enhanced EETs-induced axonal growth in DRG cultures by increasing the bioavailability of EETs: 1) enhanced EETs-induced axonal growth was observed using either of two structurally diverse sEHi; and 2) quantification of eicosanoids by LC-MS confirmed increased levels of 14,15-EET and decreased levels of the dihydroxy metabolite of 14,15-EET in cultures treated with sEHi. Alternative explanations that cannot be ruled out with the available data include: 1) if 14,15-DHET inhibits axonal growth, the axon promoting activity of sEHi could be due in part to its effect of decreasing 14,15-DHET levels; and 2) sEHi increases the levels of a number of other epoxy fatty acids from not only arachidonic acid (e.g., 8,9- and 11,12-EET) but also linoleic acid, eicosapentaenoic acid and docosahexaenoic acid, and these other epoxy fatty acids may contribute to the enhanced axon outgrowth observed in cultures treated with TUPS or 4-PCO. While additional studies are required to address these possibilities, the data in this study identify a novel function for sEH in regulating axonal growth.
That EETs are specifically targeting axonal growth and not increasing axonal growth via non-specific effects on general cell viability or enhanced survival of neuronal subpopulations is suggested by the finding that addition of exogenous EETs and/or pharmacologic inhibition of sEH had no effect on mitochondrial activity as determined using the MTT assay. However, the mechanism by which 14,15-EET promotes axonal growth remains to be determined. Accumulating evidence suggests that 14,15-EET exerts its actions through a membrane-associated protein receptor, although a specific receptor has yet to be identified (Iliff et al. 2010). 14,15-EET binding is reported to be sensitive to cholera toxin, a cAMP analog, and the protein kinase A (PKA) selective inhibitor H-89 but not a PKC selective inhibitor, suggesting that the putative 14,15-EET receptor is linked to the adenylyl cyclase-cAMP-PKA signaling pathway (Wong et al. 2000). This is in agreement with earlier observations demonstrating that 14,15-EET dose-dependently increases intracellular concentrations of cAMP in pituitary somatotrophic cells (Snyder et al. 1989). The adenylyl cyclase-cAMP-PKA has been implicated in axonal growth both via transcription-independent and CREB-mediated transcriptional mechanisms (Aglah et al. 2008, Chen et al. 2007, Cox et al. 2008, Tam et al. 2006, Teng & Tang 2006). However, our preliminary studies suggest that this signaling pathway does not mediate the effects of EETs on axonal growth: (a) addition of a mixture of EET-methyl ester regioisomers at concentrations that enhance axonal growth does not activate PKA or induce phosphorylation of CREB in cultured DRG neurons; and (b) EETs-induced axonal growth is not consistently inhibited by the PKA inhibitor H-89 or by expression of a dominant-negative CREB construct (Abdu and Lein, unpublished observations).
The relevance of our in vitro observations to regulation of axonal growth by sEH modulation of EETs levels under normal physiologic and pathologic conditions is suggested by several observations. First, LC-MS analyses confirmed that primary cultures of neurons produce endogenous EETs. Second, exposure to sEHi in the absence of exogenous EETs was sufficient to increase axonal length in both cortical and sensory neurons. This effect was more robust in DRG neurons than cortical neurons, which may reflect our observation that only a subpopulation of neurons in cortical cultures is immunoreactive for sEH. Third, previous in vivo studies indicate that 14,15-EET, the regioisomer that exhibits the most potent axon-promoting activity in vitro, is the predominant regioisomer produced in brain (Iliff et al. 2010). Collectively, these data suggest a model in which axonal sEH constrains the limits of axonal growth by inactivating local 14,15-EET. This model would suggest that axonal sEH expression may be low or excluded from the axonal growth cone during periods of active axonal growth and that upregulation of axonal sEH expression prevents extraneous axonal branching and/or functions in the differentiation of the growth cone into a presynaptic structure.
Our findings also suggest a novel therapeutic action of sEHi. Pharmacologic inhibition of sEH has been shown to decrease infarct size and promote functional recovery following ischemic injury in the brain (Zhang et al. 2007, Zhang et al. 2008, Dorrance et al. 2005) and to reduce non-neuropathic pain (Inceoglu et al. 2007). These therapeutic effects are mediated in part by the vasodilator, anti-apoptotic and anti-inflammatory activity of EETs (Iliff et al. 2010, Inceoglu et al. 2007). Our findings suggest that enhanced axonal growth may contribute to the therapeutic effects of sEHi in these pathologic settings. Although the axon-promoting effect of EETs and sEHi were observed in uninjured embryonic neurons, the fact that these neurons were regenerating axons sheared off during the dissociation process suggests the novel therapeutic use of EETs with concomitant inhibition of sEHi to prevent axonal degeneration or promote axonal regeneration following traumatic injury. Testing this possibility in in vivo models of peripheral nerve injury is the goal of future experimentation.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS044313 to NJA; ES002710 and P42ES004699 to BDH; NS046649 and ES014901 to PJL) and by the Cystic Fibrosis Foundation Inc (Elizabeth Nash Memorial Fellowship to JY). The authors have no competing interests to declare.
Abbreviations used in text
- BMP-7
bone morphogenetic protein-7
- DHET
dihydroxyeicosatrienoic acid
- DRG
dorsal root ganglia
- EET
epoxyeicosatrienoic acid
- MAP-2
microtubule-associated protein-2
- 4-PCO
4-phenyl chalcone oxide
- PGP9.5
protein gene product 9.5
- SCG
superior cervical ganglia
- sEH
soluble epoxide hydrolase
- sEHi
soluble epoxide hydrolase inhibitor
- TUPS
1-(1-methylsulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea
References
- Aglah C, Gordon T, Posse de Chaves EI. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology. 2008;55:8–17. doi: 10.1016/j.neuropharm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Banker G, Goslin K. Culturing Nerve Cells. The MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Bianco RA, Agassandian K, Cassell MD, Spector AA, Sigmund CD. Characterization of transgenic mice with neuron-specific expression of soluble epoxide hydrolase. Brain Res. 2009;1291:60–72. doi: 10.1016/j.brainres.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Brady MT, Rosolowsky LJ, Falck JR. Metabolism of arachidonic acid by rat adrenal glomerulosa cells: synthesis of hydroxyeicosatetraenoic acids and epoxyeicosatrienoic acids. Endocrinology. 1991;128:2183–2194. doi: 10.1210/endo-128-4-2183. [DOI] [PubMed] [Google Scholar]
- Campello-Costa P, Fosse-Junior AM, Oliveira-Silva P, Serfaty CA. Blockade of arachidonic acid pathway induces sprouting in the adult but not in the neonatal uncrossed retinotectal projection. Neuroscience. 2006;139:979–989. doi: 10.1016/j.neuroscience.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, Gill SS, Hammock BD, Estabrook RW. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223:639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- Chen CH, Chen YJ, Jeng CJ, Yang SH, Tung PY, Wang SM. Role of PKA in the anti-Thy-1 antibody-induced neurite outgrowth of dorsal root ganglionic neurons. J Cell Biochem. 2007;101:566–575. doi: 10.1002/jcb.21217. [DOI] [PubMed] [Google Scholar]
- Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46:842–848. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol. 1990;259:H1171–1177. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- Enayetallah AE, French RA, Barber M, Grant DF. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J Histochem Cytochem. 2006;54:329–335. doi: 10.1369/jhc.5A6808.2005. [DOI] [PubMed] [Google Scholar]
- Higgins D, Lein PJ, Osterhout DJ, Johnson MI. Tissue culture of mammalian autonomic neurons. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT Press; Cambridge, MA: 1991. pp. 177–205. [Google Scholar]
- Hiruma H, Ichikawa T, Kobayashi H, Hoka S, Takenaka T, Kawakami T. Prostaglandin E(2) enhances axonal transport and neuritogenesis in cultured mouse dorsal root ganglion neurons. Neuroscience. 2000;100:885–891. doi: 10.1016/s0306-4522(00)00347-x. [DOI] [PubMed] [Google Scholar]
- Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, Lein PJ. Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol. 2003;190:72–86. doi: 10.1016/s0041-008x(03)00156-x. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010;91:68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang R, Zeldin DC, Alkayed NJ. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol. 2009;296:H1352–1363. doi: 10.1152/ajpheart.00950.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Ulu A, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein P, Guo X, Hedges AM, Rueger D, Johnson M, Higgins D. The effects of extracellular matrix and osteogenic protein-1 on the morphological differentiation of rat sympathetic neurons. Int J Dev Neurosci. 1996;14:203–215. doi: 10.1016/0736-5748(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Leu BH, Schmidt JT. Arachidonic acid as a retrograde signal controlling growth and dynamics of retinotectal arbors. Dev Neurobiol. 2008;68:18–30. doi: 10.1002/dneu.20561. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci U S A. 1999;96:8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- Snyder GD, Capdevila J, Chacos N, Manna S, Falck JR. Action of luteinizing hormone-releasing hormone: involvement of novel arachidonic acid metabolites. Proc Natl Acad Sci U S A. 1983;80:3504–3507. doi: 10.1073/pnas.80.11.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GD, Yadagiri P, Falck JR. Effect of epoxyeicosatrienoic acids on growth hormone release from somatotrophs. Am J Physiol. 1989;256:E221–226. doi: 10.1152/ajpendo.1989.256.2.E221. [DOI] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Tam J, Rosenberg L, Maysinger D. Neurite outgrowth in dorsal root ganglia induced by islet neogenesis-associated protein peptide involves protein kinase A activation. Neuroreport. 2006;17:189–193. doi: 10.1097/01.wnr.0000198948.08068.7e. [DOI] [PubMed] [Google Scholar]
- Teng FY, Tang BL. Axonal regeneration in adult CNS neurons--signaling molecules and pathways. J Neurochem. 2006;96:1501–1508. doi: 10.1111/j.1471-4159.2006.03663.x. [DOI] [PubMed] [Google Scholar]
- Tsai HJ, Hwang SH, Morisseau C, Yang J, Jones PD, Kasagami T, Kim IH, Hammock BD. Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. European Journal of Pharmaceutical Sciences. 2010;40:222–238. doi: 10.1016/j.ejps.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Morisseau C, Newman JW, Hammock BD. In vitro metabolism of the mammalian soluble epoxide hydrolase inhibitor, 1-cyclohexyl-3-dodecyl-urea. Drug Metab Dispos. 2003;31:846–853. doi: 10.1124/dmd.31.7.846. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Walsh FS, Doherty P. The production of arachidonic acid can account for calcium channel activation in the second messenger pathway underlying neurite outgrowth stimulated by NCAM, N-cadherin, and L1. J Neurochem. 1994;62:1231–1234. doi: 10.1046/j.1471-4159.1994.62031231.x. [DOI] [PubMed] [Google Scholar]
- Wong PY, Lai PS, Falck JR. Mechanism and signal transduction of 14 (R), 15 (S)-epoxyeicosatrienoic acid (14,15-EET) binding in guinea pig monocytes. Prostaglandins Other Lipid Mediat. 2000;62:321–333. doi: 10.1016/s0090-6980(00)00079-4. [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, et al. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]