Abstract
Objective
Trivalent chromium (Cr3+) is an essential micronutrient. Findings since the 1950s suggest that Cr3+ might benefit cholesterol homeostasis. Here we present mechanistic evidence in support of this role of Cr3+.
Method and Results
High-density lipoprotein cholesterol generation in 3T3-L1 adipocytes, rendered ineffective by hyperinsulinemia, known to accompany disorders of lipid metabolism was corrected by Cr3+. Mechanistically, Cr3+ reversed hyperinsulinemia-induced cellular cholesterol accrual and associated defects in cholesterol transporter ABCA1 trafficking and apolipoprotein A1-mediated cholesterol efflux. Moreover, direct activation of AMP-activated protein kinase (AMPK), known to be activated by Cr3+, and/or inhibition of hexosamine biosynthesis pathway (HBP) activity, known to be elevated by hyperinsulinemia, mimics Cr3+ action.
Conclusion
These findings suggest a mechanism of Cr3+ action that fits with long-standing claims of its role in cholesterol homeostasis. Furthermore, these data implicate a mechanistic basis for the coexistence of dyslipidemia with hyperinsulinemia.
Keywords: Cholesterol, Chromium, Dyslipidemia, Hyperinsulinemia, Lipoprotein
Trivalent chromium (Cr3+) is classified as an essential micronutrient for optimal carbohydrate and lipid metabolism. Although evidence relating Cr3+ deficiency and cardiovascular disease (CVD) is fragmentary, deficiency has been linked to reduced high-density lipoprotein cholesterol (HDL-C)1. A rate-limiting step in HDL-C generation entails cholesterol transporter ABCA1-mediated cholesterol efflux to lipid-poor apolipoprotein A1 (ApoA1). The HDL-C particle formed is preβ-1 HDL-C, a subclass which removes cholesterol from macrophages2, a cardioprotective event. These findings raise the question of whether an essential mechanism of Cr3+ action involves ABCA1/ApoA1-mediated preβ-1 HDL-C generation. Importantly, ABCA1/ApoA1 dysregulation may represent an unappreciated basis of low HDL-C coexisting with metabolic derangements (e.g., hyperinsulinemia).
Methods
Insulin-sensitive and hyperinsulinemia-induced insulin-resistant 3T3-L1 adipocytes and Cr3+ in the picolinate form (CrPic) at a 1 μM dose were used as previously described3-4. Detailed methods and information on Cr3+ doses/forms tested are provided in online material (http://www.atcb.ahahournals.org).
Results
Examination of ABCA1 trafficking revealed that plasma membrane (PM) ABCA1 was diminished by hyperinsulinemic conditions relevant to disease3, yet in the presence of Cr3+ this was prevented (Fig. 1A). Endosomal membrane (EM) ABCA1 was elevated by hyperinsulinemia and normalized by Cr3+ (Fig. 1B). Total ABCA1 protein was not changed (Fig. S1A). Mechanistically, ABCA1 is regulated by the EM-to-cytosol (Cyto) cycling of the GTPase Rab8. Hyperinsulinemia increased and decreased EM- and Cyto-Rab8, respectively, and these changes were normalized by Cr3+ (Figs. 1C, D).
Fig. 1.
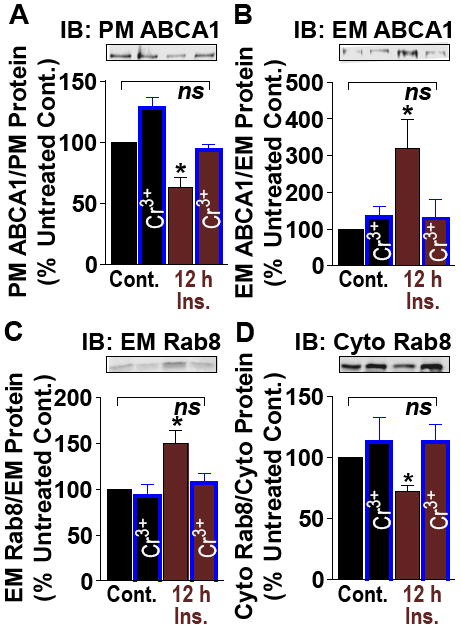
Control (Cont.) or hyperinsulinemic (12h Ins.) cells were treated without or with Cr3+. (A) Plasma membrane (PM) ABCA1, (B) Endosomal membrane (EM) ABCA1, (C) EM Rab8, and (D) Cytosolic Rab8. n = 4-11. *P < 0.05 versus untreated control.
Cholesterol accumulation has been implicated in EM ABCA1 sequestration in Niemann-Pick disease, type C (NPC)5. Like NPC, a substantial increase in EM cholesterol was found in cells cultured under hyperinsulinemic conditions that Cr3+ prevented (Fig. 2A). Interestingly, exercise is recognized to increase HDL-C levels, and like exercise, Cr3+ increases AMP-activated protein kinase (AMPK) activity4, known to suppress cholesterol synthesis6. AICAR (5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, an AMPK activator) and Cr3+ stimulated AMPK (Fig. 2B), and similarly to Cr3+, AICAR lowered EM cholesterol (Fig. 2C) and corrected membrane Rab8/ABCA1 levels (Figs. S1B-E); however, a gain in Cyto-Rab8 was not seen; likely due to a shorter AICAR treatment duration not permitting a detectable level of Rab8 to accumulate in the dilute cytosol fraction. Importantly, Cr3+ and AICAR both prevented hyperinsulinemia-impaired ApoA1-mediated cholesterol efflux (Fig. 2D).
Fig. 2.
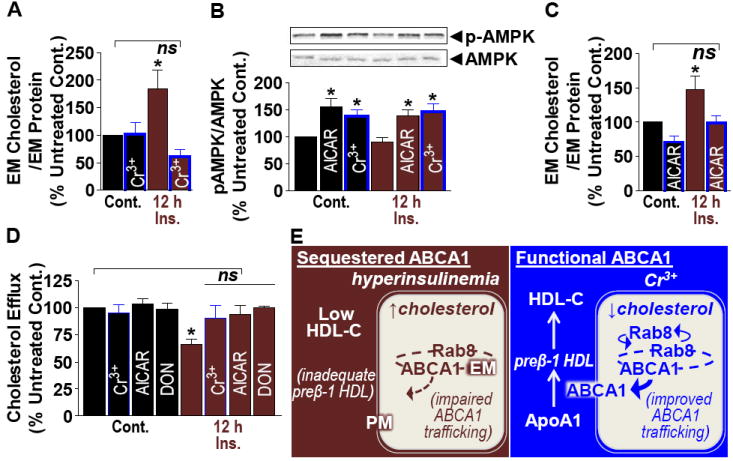
Control (Cont.) or hyperinsulinemic (12h Ins.) cells were treated without or with Cr3+, AICAR, or DON. (A, C) EM cholesterol, (B) pAMPK, and (D) ApoA1-mediated cholesterol efflux. n = 4-11. *P < 0.05 versus untreated control. (E) Model of Cr3+ protection against (Blue) hyperinsulinemia-induced cholesterol-associated impairment in (Red) Rab8/ABCA1 trafficking and ApoA1-mediated cholesterol efflux.
Contrasting AMPK, increased hexosamine biosynthesis pathway (HBP) activity has been implicated in cholesterol accrual induced by hyperinsulinemia7. Testing the effect of the HBP inhibitor 6-Diazo-5-Oxo-L-Norleucine (DON) revealed Cr3+- and AICAR-like effects (Fig. 2D). Neither agent nor Cr3+ displayed any effect on control cells. Also, cholesterol lowering with methyl-β-cyclodextrin mimicked the protective effect on ApoA1-mediated cholesterol efflux (Fig. S1F).
Discussion
The role of Cr3+ in health and disease is complex. While patients with diabetes on Cr3+ supplementation see improvement in hyperglycemia, benefits on raising HDL-C remain unclear8. An emerging appreciation is that total HDL-C measurements are misleading in understanding its cardioprotective actions, as the ABCA1-generated preβ-1 HDL-C particle likely represents the “functional” subfraction2. Therefore, study demonstrating that Cr3+ enhances this ABCA1-mediated event in cells cultured in a diabetic milieu is significant.
As the serum concentration of the preβ-1 HDL-C accounts for only a small fraction of total HDL-C, trials designed to assess the benefits of Cr3+ on total HDL-C may have had an inherent flaw in understanding Cr3+’s effect. In addition, Cr3+ deficiency in humans is expected to be slight, if any9, thus measurement of a supplemental effect may be negligible. Nevertheless, analyses reveal popular weight loss diets provide Cr3+ at suboptimal levels10.
Mechanistically, observation that AMPK stimulation ramps up ABCA1/ApoA1 functionality is interesting, given the appreciated benefits of exercise, a known stimulant of AMPK activity, on the prevention of metabolic syndrome and its consequences. In this regard, skeletal muscle and adipose tissue contain more cholesterol than any other organ11. In fact, a new importance of adipose tissue cholesterol in the generation of HDL-C has recently been recognized12-13. In particular, the generation of preβ-1 HDL-C appears critical in mediating cholesterol efflux from cholesterol-laden macrophages. The idea Cr3+ could have an indirect effect on cholesterol handling by macrophages is of interest. Testing this possibility as well as characterizing any direct effect Cr3+ may have on macrophage cholesterol metabolism is warranted.
In closing, these data suggest low circulating HDL-C, resulting from metabolic disorder, may arise from hyperinsulinemia/HBP-mediated peripheral tissue cholesterol accrual (Fig. 2E). This is associated with an EM sequestration of Rab8/ABCA1, and low preβ-1 HDL-C. Data also implicate that Cr3+ suppresses cholesterol synthesis/accrual via AMPK and this improves Rab8/ABCA1 functionality and HDL-C generation. Whether this cell-based model explains the benefits of Cr3+ and/or exercise in humans with diabetes remains to be validated.
Supplementary Material
Acknowledgments
National Institutes of Health AT001846, DK082773 and DK082773-01S1 (JSE), and the Indiana Center for Vascular Biology HL079995 (WS). We thank Nutrition 21 for providing the CrPic.
References
- 1.Simonoff M. Chromium deficiency and cardiovascular risk. Cardiovasc Res. 1984;18:591–596. doi: 10.1093/cvr/18.10.591. [DOI] [PubMed] [Google Scholar]
- 2.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The Ability to Promote Efflux Via ABCA1 Determines the Capacity of Serum Specimens With Similar High-Density Lipoprotein Cholesterol to Remove Cholesterol From Macrophages. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, Raman P, Bhonagiri P, Strawbridge AB, Pattar GR, Elmendorf JS. Protective effect of phosphatidylinositol 4,5-bisphosphate against cortical filamentous actin loss and insulin resistance induced by sustained exposure of 3T3-L1 adipocytes to insulin. J Biol Chem. 2004;279:39705–39709. doi: 10.1074/jbc.C400171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Liu P, Pattar GR, Tackett L, Bhonagiri P, Strawbridge AB, Elmendorf JS. Chromium activates glucose transporter 4 trafficking and enhances insulin-stimulated glucose transport in 3T3-L1 adipocytes via a cholesterol-dependent mechanism. Mol Endocrinol. 2006;20:857–870. doi: 10.1210/me.2005-0255. [DOI] [PubMed] [Google Scholar]
- 5.Linder MD, Uronen RL, Holtta-Vuori M, van der Sluijs P, Peranen J, Ikonen E. Rab8-dependent recycling promotes endosomal cholesterol removal in normal and sphingolipidosis cells. Mol Biol Cell. 2007;18:47–56. doi: 10.1091/mbc.E06-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 7.Pattar GR, Habegger KM, Bhonagiri P, Elmendorf JS. Plasma Membrane Cholesterol Accrual Elicited by Increased Hexosamine Biosynthesis Promotes Insulin Resistance in Fat and Skeletal Muscle Cells. Diabetes. 2009;58:A333–A333. [Google Scholar]
- 8.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes NR, McAdory D, Love S, Di Bona KR, Chen Y, Ansorge K, Hira J, Kern N, Kent J, Lara P, Rasco JF, Vincent JB. Urinary chromium loss associated with diabetes is offset by increases in absorption. J Inorg Biochem. 2010;104:790–797. doi: 10.1016/j.jinorgbio.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Calton JB. Prevalence of micronutrient deficiency in popular diet plans. J Int Soc Sports Nutr. 2010;7:24. doi: 10.1186/1550-2783-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause BR, Hartman AD. Adipose tissue and cholesterol metabolism. J Lipid Res. 1984;25:97–110. [PubMed] [Google Scholar]
- 12.Petremand J, Widmann C. The HDL: adipocyte connection. Curr Opin Lipidol. 2010;21:388–389. doi: 10.1097/MOL.0b013e32833c2227. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, McGillicuddy FC, Hinkle CC, O’Neill S, Glick JM, Rothblat GH, Reilly MP. Adipocyte Modulation of High-Density Lipoprotein Cholesterol. Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.109.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


