WHERE WE WERE
Reports of airway irritant effects from Florida red tide have been documented to since 1844 (Baden et al., 2005). In general, it is well established that persons inhaling marine aerosols containing polyether brevetoxins (PbTxs) produced by the dinoflagellate, Karenia brevis (K. brevis), can exhibit either upper and/or lower airway symptoms (see Backer and McGillicuddy, this issue). Upper airway symptoms include cough, sneezing, rhinorrhea (runny nose), a burning sensation in the nose and throat, and watery eyes (Kirkpatrick et al., 2004). Lower airway symptoms include chest tightness and wheezing and/or shortness of breath, all of which reflect difficulty in breathing (Kirkpatrick et al., 2004). Normal individuals, as well as those with pre-existing airway diseases such as asthma (i.e., susceptible populations), can be affected, although anecdotal evidence has indicated that susceptible populations are at greater risk (Asai et al., 1982).
While such accounts leave little doubt that aerosolized red tides can elicit adverse airway responses, until recently there was a void in our knowledge due to the inability to accurately quantify exposure assessment and associate the exposure with pulmonary consequences. Furthermore, there was a paucity of data detailing the mechanisms responsible for the adverse pulmonary events. To rectify this problem, an interdisciplinary group of scientists has engaged in studies that combine analytical marine natural products chemistry, quantitative air sampling, laboratory model studies of inhalation exposure, and human exposure and health assessment in areas of active red tide (Fleming et al., 2005a).
WHERE WE ARE
Data from field studies indicate that the severity of the pulmonary responses to aerosolized Florida red tide is dependent on the combined interaction of the total air toxin load, the specific toxins aerosolized, and the particle size of these airborne toxins. We have learned that the total air toxin load is dependent not only on the cell counts of K. brevis in the water, but also on the wind speed and the fraction of the time the wind is in the onshore direction (Cheng et al., 2005). Thus, high-water cell counts in the presence of off shore winds, resulting in minimal onshore aerosol exposures, can explain why no observable airway effects occur even with significant oceanic blooms of Florida red tide.
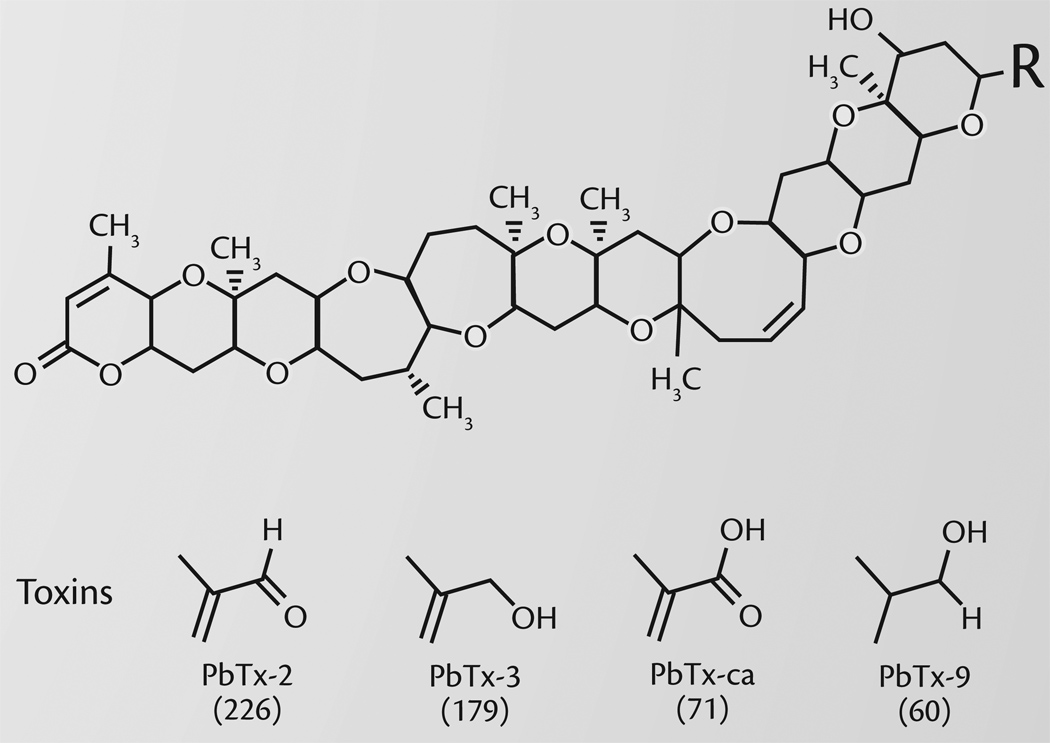
Currently, there are 12 known PbTx’s produced by K. brevis (Baden et al., 2005). Analysis of aerosols during different Florida red tide episodes indicate that PbTx-2, PbTx-3, and two metabolites PbTx-9 and PbTx-carboxylic acid (PbTx-ca), are the major toxins found in collected air samples (Cheng et al., 2005; D.G. Baden, University of North Carolina, Wilmington, personal communication, 2006). Figure 1 provides the chemical structures and Table 1 compares the relative constrictor effects of equivalent amounts of these toxins in a large animal model of asthma (Abraham et al., 2005a). As can be seen, PbTx-2 and PbTx-3 are the major toxins responsible for the constrictor effects of aerosolized Florida red tide. The decomposition products are less active than the primary toxins but are still potent enough to contribute to the airway irritant effects.
Figure 1.
Structures of the brevetoxins discussed in this article. These brevetoxins, derived from the brevetoxin B backbone (top), are found in air samples collected during active Florida red tides and show constrictor activity in the laboratory. The various toxins are based on alteration of the R-side chain. The values in parentheses correspond to the activity of each toxin in Table 1. PbTx-2 and PbTx-3 are the most potent of the brevetoxins. PbTx-ca and PbTx-9 are metabolites of PbTx-2 and PbTx-3; their constrictor activity, although diminished with respect to the parent compound, is still significant.
Table 1.
Airway Constrictor Responses to Brevetoxins
| Toxins* (n) | % Increase in pulmonary resistance from baseline |
|---|---|
| PbTx-2 (n=7) | 226 ± 21 |
| PbTx-3 (n=10) | 179 ± 22 |
| PbTx-ca (n=4) | 71 ± 3 |
| PbTx-9 (n=2) | 60 ± 7 |
Sheep were challenged with 20 breaths of 10 pg/ml of each toxin. Values are mean ± standard error of the mean (s.e.m.) Number of animals (n).
Such structure-function studies are important for understanding the mechanisms involved in toxin-induced airway effects. Furthermore, they are highly pertinent because they are conducted with environmentally relevant concentrations of individual toxigenic agents and/or natural brevetoxins (Abraham et al., 2005a, 2005b). To complicate matters further, K. brevis also produces its own natural antagonist—brevenal (Bourdelais et al., 2004). Brevenal has been identified in collected field air samples (Cheng et al., 2005), and when aerosolized, brevenal can block the constrictor effects of crude brevetoxins, PbTx-2, and PbTx-3 (Abraham et al., 2005a). Thus, the relative concentrations of the toxins and antagonist will influence the overall pulmonary effect of an aerosol exposure, another reason why some Florida red tide blooms have greater respiratory effects than do others.
Finally, even though there may be a significant toxin aerosol burden, the particle size of the toxin will determine the major site of action. Cheng et al. (2005) found that only 2–6 percent of the aerosolized toxin was sufficiently small (i.e., the respirable fraction) to allow effective deposition in the pulmonary (lower) airways. Thus, the majority of the aerosol toxin load impacts the upper airways, which explains the relative ease in demonstrating upper-airway effects in human field studies. It follows, then, that the severity of the lower-airway effects will depend on the concentration of the respirable fraction of the total aerosol burden.
The conclusions outlined above have been gleaned, in part, from the results of three human-exposure field studies where environmental sampling and respiratory responses have been evaluated simultaneously (Backer et al., 2003; Backer et al., 2005; Fleming et al., 2005b). In general, these studies used questionnaires to identify symptoms, with objective pulmonary function tests (PFTs) and field aerosol data for the purposes of identifying human health effects. Data were collected in periods of little or no exposure and during separate periods of different levels of exposure. These studies confirmed that 28 percent of recreational beach goers exposed to high levels of brevetoxin (20–93 ng m−3) reported significant upper- and lower-respiratory symptoms (Backer et al., 2003) and that healthy lifeguards, who are exposed to red tide because of their occupation, reported significant upper-airway symptoms when exposed in the range of 0.5–26 ng m−3 total toxin (Backer et al., 2005). Of note, no objective changes were documented in the PFTs with the lifeguards during Florida red tide exposure, despite their increased reported symptoms. Collectively, these studies support the aforementioned arguments that toxin effects are, in part, related to the aerosol burden and the population studied.
A third study examined the effects of red tide toxins in persons with asthma (Fleming et al., 2005b). During the exposure period (mean brevetoxin concentration 36 ng m−3), there were significant increases in both upper- and lower- airway symptoms, which correlated with objective adverse changes in PFTs. The most severe changes in symptoms and PFTs were reported by a subgroup of the more severe asthmatics. This finding is consistent with the response to inhaled brevetoxin in allergic sheep whose lungs were inflamed because of a recently induced asthma attack (Abraham et al., 2005b). Collectively, these data suggest that the severity of the response to toxin exposure may be dependent on the pre-existing inflammatory condition of the airways. Furthermore, the data indicate that asthmatics experience these effects even when taking their normal medications.
WHERE WE ARE GOING
The doctrine for many years has been that symptoms of exposure to aerosolized Florida red tide would diminish when people leave the beach (Baden et al., 1982). Kirkpatrick et al. (2006) recently reported a significant (54 percent) increase in the rate of Emergency Room admissions for respiratory diseases (pneumonia, bronchitis, and asthma and upper-airway problems) among persons living in the coastal areas of Sarasota (Florida) during an active Florida red tide compared to the same time period a year later with no red tide. These data are consistent with experimental findings indicating that inhaled brevetoxins reduce normal mucociliary clearance, an innate host defense mechanism (Abraham et al., 2005a). This latter effect, similar to the airway constrictor effects of inhaled toxin, is concentration-dependent as well. Thus, increased toxin residence times in the airway (resulting from the delayed clearance), as well as the adverse impact of abnormal mucociliary clearance itself, could contribute to more chronic deleterious health effects than previously recognized.
Recent animal studies support this conclusion. Rats inhaling brevetoxin for four weeks demonstrated evidence of immune suppression (Benson et al., 2005), and sheep inhaling brevetoxin for four days showed decreased lung macrophage function (Zaias et al., 2004). Both of these responses would be consistent with increased incidence of disease. Further studies will be necessary to confirm these observations and identify the mechanisms responsible.
Animal studies have not only been useful in identifying potential harmful effects of toxin, but also have provided some data on the medications that can prevent and/or reverse the airway effects of Florida red tide (Abraham et al., 2005b). While confirmation of these results must await clinical trials, anecdotal evidence for the use of bronchodilators and antihistamines to treat and/or prevent the symptoms in humans support the laboratory findings (Fleming et al., 2005b).
Finally, the discovery of brevenal, the natural brevetoxin antagonist, has not only led to studies designed to counteract the effects of brevetoxin, but also to the discovery that this agent may have potential therapeutic value in combating airway diseases such as cystic fibrosis and chronic obstructive lung disease (Abraham et al., 2005a). As such, brevenal and/or other toxin congeners will be the focus of ongoing research. Toward this goal, the recent availability of radio-labeled brevenal provides a new potential probe for identifying drug targets in the airways.
ACKNOWLEDGEMENTS
This work was supported in part by NIEHS grant support (P01 ES 10594).
Contributor Information
William M. Abraham, Email: abraham@msmc.com, Mount Sinai Medical Center and Professor of Medicine, Miller School of Medicine, University of Miami, Miami Beach, FL, USA..
Daniel G. Baden, University of North Carolina, Center for Marine Science, Office of the Director, Wilmington, NC, USA..
REFERENCES
- Abraham WM, Bourdelais AJ, Sabater JR, Ahmed A, Lee TA, Serebriakov I, Baden DG. Airway responses to aerosolized brevetoxins in an animal model of asthma. American Journal of Respiratory and Critical Care Medicine. 2005ba;171:26–34. doi: 10.1164/rccm.200406-735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WM, Bourdelais AJ, Ahmed A, Serebriakov I, Baden DG. Effects of inhaled brevetoxins in allergic airways: Toxin-allergen interactions and pharmacologic intervention. Environmental Health Perspectives. 2005b;113:626–631. doi: 10.1289/ehp.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai S, Krzanowski JJ, Anderson WH, Martin DF, Polson JB, Lockey RF, Bukantz SC, Szentivanyi A. Effects of the toxin of red tide, Ptychodiscus brevis, on canine tracheal smooth muscle: A possible new asthma-triggering mechanism. Journal of Allergy and Clinical Immunology. 1982;69:418–428. doi: 10.1016/0091-6749(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Backer LC, Kirkpatrick B, Fleming LE, Cheng YS, Pierce R, Bean JA, Clark R, Johnson D, Wanner A, Tamer R, Baden DG. Occupational exposure to aerosolized brevetoxins during Florida red tide events: Impacts on a healthy worker population. Environmental Health Perspectives. 2005;113:644–649. doi: 10.1289/ehp.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden DG, Mende TJ, Bikhazi G, Leung I. Bronchoconstriction caused by Florida red tide toxins. Toxicon. 1982;20:929–932. doi: 10.1016/0041-0101(82)90081-2. [DOI] [PubMed] [Google Scholar]
- Baden DG, Bourdelais AJ, Jacocks H, Michelizza S, Naar J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environmental Health Perspectives. 2005;113:621–625. doi: 10.1289/ehp.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Hahn FF, March TH, McDonald JD, Gomez AP, Sopori MJ, Bourdelais AJ, Naar J, Zaias J, Bossart GD, Baden DG. Inhalation toxicity of brevetoxin 3 in rats exposed for four weeks. Environmental Health Perspectives. 2005;113:626–631. doi: 10.1289/ehp.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdelais AJ, Campbell S, Jacocks H, Naar J, Wright J, Carsi J, Baden DG. Brevenal is a natural inhibitor of brevetoxin action in sodium channel receptor binding assays. Cellular and Molecular Biology. 2004;24:553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YS, Zhou Y, Irvin CM, Pierces RH, Naar J, Backer LC, Fleming LE, Kirkpatrick B, Baden DG. Characterization of marine aerosol for assessment of human exposure to brevetoxins. Environmental Health Perspectives. 2005;113:638–643. doi: 10.1289/ehp.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Backer LC, Baden DG. Overview of aerosolized Florida red tide toxins: Exposures and effects. Environmental Health Perspectives. 2005a;113:618–620. doi: 10.1289/ehp.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Dalpra D, Tamer R, Zaias J, Cheng YS, Pierces R, Naar J, Abraham W, Clark R, Zhou Y, Henry MS, Johnson D, Van de Bogart G, Bossart GD, Harrington M, Baden DG. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Environmental Health Perspectives. 2005b;113:650–657. doi: 10.1289/ehp.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R, Zaias J, Bossart G, Baden DG. Literature review of Florida red tide: Implications for human health effects. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Backer LC, Bean JA, Tamer R, Kirkpatrick G, Kane T, Wanner A, Dalpra D, Reich A, Baden DG. Environmental exposures to Florida red tides: effects on emergency room respiratory diagnoses admissions. Harmful Algae. 2006 doi: 10.1016/j.hal.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaias J, Botvinnikova Y, Fleming LE, Bossart GD, Baden DG, Abraham WM. Aerosolized polyether brevetoxin (PbTx) causes airway hyperresponsiveness (AHR) and airway inflammation in both normal and allergic sheep. America Journal of Respiratory and Critical Care Medicine. 2004;169:A639. [Abstract] [Google Scholar]