Abstract
New neurons are incorporated into the adult brains of a variety of organisms, from humans and higher vertebrates, to non-vertebrates such as crustaceans. In virtually all of these systems serotonergic pathways appear to provide important regulatory influences over the machinery producing the new neurons. We have developed an in vitro preparation where adult neurogenesis can be maintained under highly controlled conditions, and are using this to test the influence of hormones on the production of neurons in the crustacean (Homarus americanus) brain. Serotonin levels have been manipulated in this in vitro preparation, and the resulting effects on the rate of neurogenesis have been documented. In addition we have compared in vitro influences of serotonin with results acquired from in vivo exposure of whole animals to serotonin. These experiments suggest that there are multiple mechanisms and pathways by which serotonin may regulate neurogenesis in the crustacean brain: (1) serotonin is effective in regulating neurogenesis at levels as low as 10−10M, suggesting that circulating serotonin may have hormonal influences on neuronal precursor cells residing in the proliferation zones; (2) contrasting effects of serotonin on neurogenesis (up- vs. down-regulation) at high concentrations (10−4M), dependent upon whether eyestalk tissue is present or absent, indicate that serotonin elicits the release of substances from the sinus glands that are capable of suppressing neurogenesis; (3) previously demonstrated (Beltz et al., 2001) serotonergic fibers from the dorsal giant neuron project directly into the proliferation zone in Cluster 10, suggest synaptic or local influences on neurogenesis in the proliferation zones where the final cell divisions and neuronal differentiation occur. Serotonin therefore regulates neurogenesis by multiple pathways, and the specific mode of influence is concentration-dependent.
Keywords: sinus gland, bromodeoxyuridine, BrdU, neurogenic niche, molt inhibiting hormone, crustacean hyperglycemic hormone
Introduction
Serotonergic pathways are important regulators of neuronal birth during embryogenesis and in adult organisms (Whitaker et al., 1996; Brezun and Daszuta, 1999; Benton and Beltz, 2001; Fricker et al., 2005). We are interested in how serotonergic mechanisms are involved in regulating adult neurogenesis in the crustacean brain, where serotonin (5-hydroxytryptamine; 5HT) is localized in a handful of neurons including the dorsal giant neuron (DGN), whose anatomy and physiological functions have been extensively examined (Sandeman R.E. and Sandeman D.C., 1987, Sandeman D.C. and Sandeman R.E., 1994; Sandeman, R.E. et al., 1995; Sandeman, D.C. et al., 1995; Benton and Beltz, 2001). The paired DGNs innervate (1) the ipsilateral primary olfactory centers of the crustacean brain, the olfactory lobes (OLs); (2) synaptic regions that process higher-order olfactory, visual and mechanosensory information, the accessory lobes (ALs) (Sandeman D.C. et al., 1995; Sullivan and Beltz, 2005a); and (3) the proliferation zone in Cluster 10 where new olfactory projection neurons are born throughout life (Fig. 1A) (Beltz et al., 2001; anatomical terminology from Sandeman et al, 1992). We have proposed that the DGN regulates adult neurogenesis in the crustacean brain via the release of serotonin directly into the Cluster 10 proliferation zone, thereby promoting cell division of the immediate precursors of the olfactory projection neurons (Fig. 1B) (Beltz et al., 2001). In the current paper, we explore serotonergic mechanisms further, with experiments demonstrating that multiple serotonergic pathways are likely to be involved in regulating neurogenesis in the adult crustacean brain.
Fig 1.
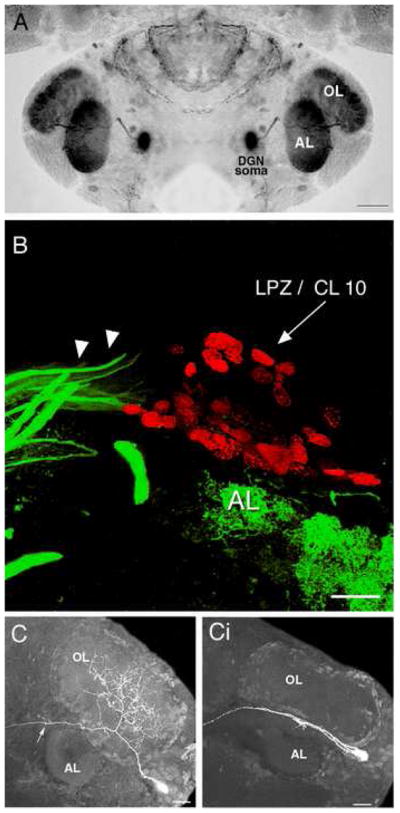
A. Serotonin immunostaining of an embryonic lobster brain at hatching. Each dorsal giant neuron (DGN) innervates the ipsilateral olfactory (OL) and accessory (AL) lobe. The intense labeling of the OL and AL is due to the massive DGN projection to these areas. B. Following a 6-hour survival time after BrdU injection, labeled nuclei (red) are found in the lateral proliferation zone (LPZ) immediately adjacent to the AL. Serotonin antibodies label a group of fibers (green; arrowheads) that terminate blindly at the proliferation zone. These fibers have been traced back to the DGN in the adult, juvenile, and larval brains (adapted from Beltz et al., 2001). C. Stacked confocal images illustrate the morphology of normal OL (Ci) when these late embryos are chemically depleted of serotonin. The cells were filled intracellularly with Lucifer Yellow. The arrow in C points to the primary neurite that courses anteriorly to innervate target regions in the medulla terminalis (adapted from Sullivan et al., 2000). Scale bars: A= 200 μm. B, C = 20 μm.
Our understanding of serotonergic influences on neuronal development in the crustacean brain began with a series of studies in embryonic lobsters where serotonin levels were depleted using the pharmacological agent 5,7-dihydroxytryptamine (5,7-DHT). The brains of these embryos were reduced in size and structurally immature compared to the brains of embryos that developed without serotonin intervention (Benton et al., 1997). These abnormalities were reflected in reduced volumes of the OLs, ALs and olfactory globular tract neuropils (Benton et al., 1997). Subsequent studies in embryonic and juvenile lobsters demonstrated that this growth retardation following chronic 5,7-DHT treatment was associated with reduced neurogenesis among the olfactory interneurons (Beltz et al., 2001; Benton and Beltz, 2001), as well as a failure of these interneurons to branch and grow into the OLs and ALs (Fig. 1C, Ci) (Sullivan et al., 2000). Serotonin therefore influences both the proliferation and differentiation of neurons in the crustacean brain.
Most of the neurons in the brains of adult decapods are born during embryonic development and are the progeny of large precursor cells, known as neuroblasts (for review see Harzsch, 2003). Neuroblasts arise during early embryonic development and divide asymmetrically, generating specific lineages of neurons before degenerating during late embryonic or early postembryonic development. Proliferation in most regions of the decapod brain ceases, therefore, in the period around hatching. The exception to this, however, is in the central olfactory pathway where mitotic activity resumes after hatching among a group of glial cells that reside in a vascular niche (Sullivan et al., 2007a, b). The daughters of these glial precursors migrate along glial processes towards clusters 9 and 10, the soma clusters containing the olfactory interneurons (Fig. 2). Proliferation within these cell clusters occurs in restricted regions, known as proliferation zones. Pulse-chase BrdU experiments show that newborn cells are translocated away from the proliferation zones over time (Harzsch et al., 1999), become dispersed amongst the other cells in the clusters (Beltz et al., 2001), differentiate into neurons (Sullivan and Beltz, 2005b), are wired into the appropriate circuitry (Sullivan and Beltz, 2005b), and survive for at least a year (Beltz et al., 2001; Schmidt, 2001; Sullivan and Beltz, 2005b; Sullivan et al., 2007b). We are using this pathway as a model for examining mechanisms underlying neurogenesis as well as the regulation of these processes. The crustacean niche and migratory streams are readily accessible on the ventral surface of the brain and continue to be viable when brains are placed in organ culture, providing a convenient and reliable assay for examining mechanisms underlying neurogenesis.
Fig 2.
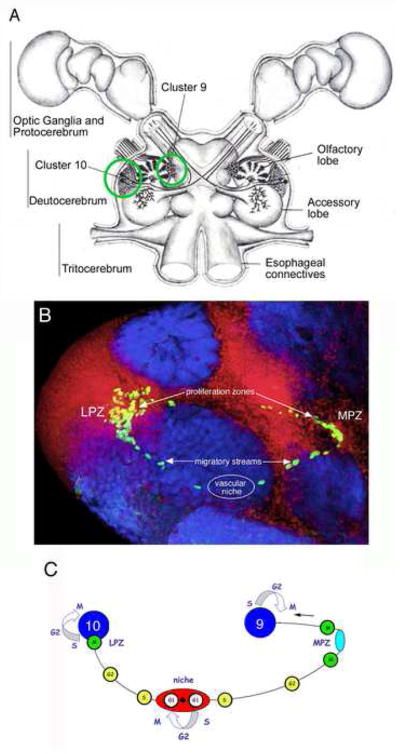
A. Diagram of the eureptantian (crayfish/lobster) brain including the optic ganglia, and showing the locations of the cell body clusters and neuropils. The bilateral soma clusters 9 and 10 (circled, left hemi-brain) are the locations where neurogenesis persists in the adult brain. These cell clusters flank two prominent neuropil regions of the deutocerebrum, the olfactory (OL) and accessory (AL) lobes. B.The proliferative system maintaining adult neurogenesis in the central olfactory pathway; shown here in images from the crayfish, Procambarus clarkii. Left side of the brain of P. clarkii labeled immunocytochemically for BrdU (green). Labeled cells are found in the lateral proliferation zone (LPZ) contiguous with Cluster 10 and in the medial proliferation zone (MPZ) near Cluster 9. The two zones are linked by a chain of labeled cells in a migratory stream that originates in the oval region labeled “niche”. Labeling for Drosophila synapsin (blue) and propidium iodide (red) is shown. C. Model summarizing our current view of events leading to the production of new olfactory interneurons in adult crayfish, clawed lobsters and crabs. Relatively quiescent precursor cells exhibiting glial characteristics reside within a neurogenic niche where they divide asymmetrically, resting in G1 phase of the cell cycle between divisions, to produce one self-renewed precursor cell and one daughter cell. Daughter cells begin migrating to the proliferation zones (lateral, LPZ, which contributes cells to Cluster 10; medial, MPZ, which contributes cells to Cluster 9), progressing from S phase of the cell cycle to the G2 phase as they migrate. Close to the proliferation zones these cells undergo mitosis to become third generation progenitors; one or more divisions of these cells will generate immature neurons in clusters 9 or 10. Many of these cells subsequently differentiate into olfactory interneurons and become incorporated into the nervous system as functional units. (Adapted from Sullivan et al., 2007b). Scale bar: B=100μm.
Our previous 5,7-DHT depletion studies in lobsters suggested that increased levels of serotonin should stimulate the production of new neurons, however, this has not been tested directly by increasing serotonin levels and observing the resulting effect on adult neurogenesis. Therefore, the present study asks how increased serotonin levels influence the rate of neurogenesis in both the isolated brain preparation and in whole animals exposed to this amine. Experiments addressing this initial question led to a second area of interest relating to the possible influences of sinus gland hormones on adult neurogenesis. An abstract describing some of these studies has been published (Benton and Beltz, 2007), and the primary findings in lobsters presented here also have been confirmed in the crayfish, Cherax destructor (Rogan, 2004).
Methods and Materials
Animals
Juvenile lobsters (1–2 cm total body length; stages 5–8) were obtained from the Lobster Rearing Facility at the New England Aquarium (Boston, MA), where they were reared in filtered seawater at 16–18 C on a 12:12 light cycle. At Wellesley College, lobsters were maintained in the same temperature and lighting conditions, but in recirculating, filtered artificial seawater (SW). Animals from these stocks were manipulated in one of three ways (in vivo, in vitro and eyestalk-ablated in vivo preparations), and then were treated with serotonin or not (the control condition). Adult neurogenesis in these animals was defined as the time when only the adult mechanisms generating new neurons (i.e., the niche and streams) were present.
In vivo preparation (Figs. 3, 4)
Fig 3.
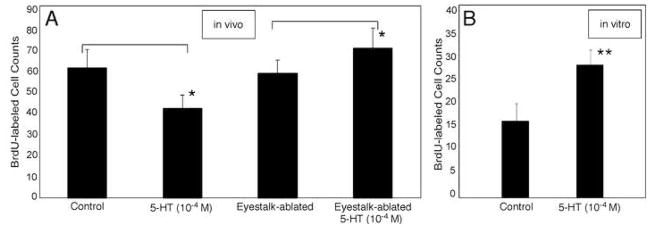
Effects of serotonin (10−4M) incubation on cell proliferation in Cluster 10 depends upon whether serotonin was administered to the in vivo (n=14) (A, left pair of histograms) or eyestalk-ablated in vivo (n=10) (A, right pair of histograms), or (B) in vitro (n=14), preparation. In the in vivo preparation, serotonin exposure results in decreases in neurogenesis in CL10. Serotonin increases neurogenesis in the eyestalk-ablated in vivo preparation (A) and the in vitro brains (B). Histograms represent mean BrdU-labeled cell counts ± standard deviations. Asterisks (*) indicate statistical significance for each serotonin incubated group relative to control, for Student T tests on the paired data, p = < 0.001 level. Double asterisks, p =<0.0001.
Fig 4.
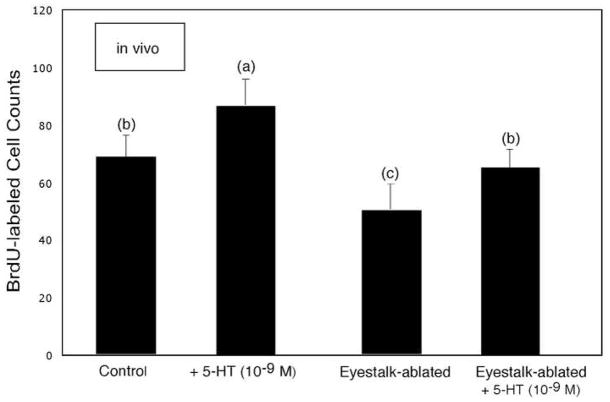
Incubation in serotonin (10−9M for 6hr) produces an increase in the rate of cell proliferation in Cluster 10 in the in vivo (left pair of histograms) and eyestalk-ablated in vivo (right pair of histograms) preparations (n=8 for each group). Unlike the differential effects seen at higher levels of serotonin (Fig. 3), neurogenesis in Cluster 10 increased in both groups of animals relative to their respective controls. Statistical similarity is indicated by the same letters. ANOVA (F=25.5: p < 0.0001) followed by Tukey-Kramer HSD reveals pairs of means that are significantly different.
Groups of live juvenile lobsters were placed directly in one of three treatment solutions, all of which contained 2 mg/ml bromodeoxyuridine (BrdU; Sigma) in SW: (1) BrdU alone; (2) BrdU + 5HT creatinine sulfate (Sigma; 10−4M); (3) BrdU + 5HT creatinine sulfate (10−9M). These free-swimming lobsters were incubated in treatment solutions for 6 hr at 16 C. Brains were then dissected and fixed in 4% paraformaldehyde (pH 7.4) in 0.1M phosphate buffer (PB) at 4 C.
In vitro preparation (Figs. 3, 5, 6)
Fig 5.
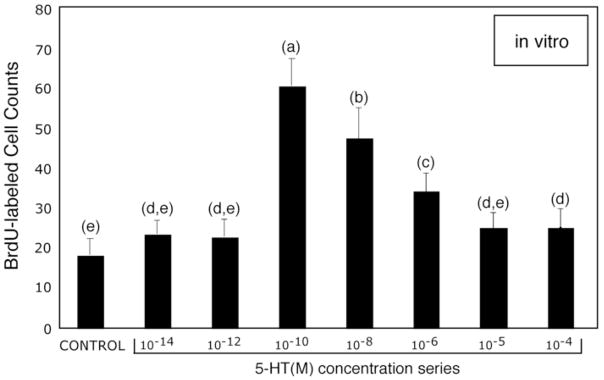
Dose-response curve for serotonin and levels of adult neurogenesis in Cluster 10 of in vitro lobster brains. The graph of BrdU-labeled cell counts vs. serotonin levels (10−14M – 10−4M) demonstrates that the rate of cell proliferation in Cluster10 is most sensitive to neurohormonal levels (10−8M – 10−10M) of this amine. ANOVA (F=78: p = < 0.0001) followed by Tukey-Kramer HSD (p= <0.05), reveals significant differences at serotonin levels 10−10 – 10−6 M relative to control brains. Statistical similarity is indicated by the same letters. n=10 per group for each serotonin concentration and control.
Fig 6.
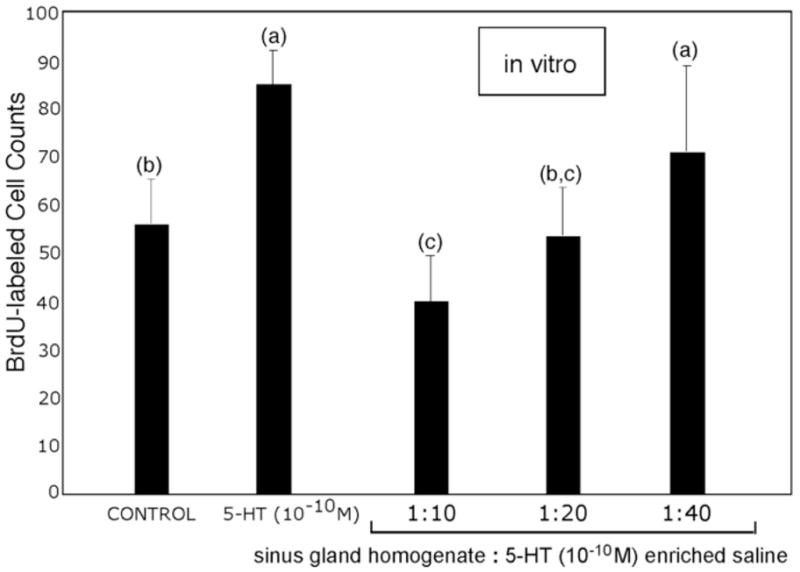
Suppression of neurogenesis in the in vitro brain preparation as a result of adding sinus gland homogenate. In this assay, sinus gland homogenate was tested at three dilutions: 1:10, 1:20 and 1:40 v/v in incubation medium that contained serotonin (10−10M). Decreases in the rate of BrdU incorporation are roughly linear with the strength of sinus gland homogenate. ANOVA (F=24: p= < 0.0001) reveals significant differences that were confirmed by Tukey-Kramer HSD. Statistical similarity is indicated by the same letters. n=10 per group.
Brains were dissected from juvenile lobsters and the lateral protocerebra (eyestalks) were removed. These brains were incubated on a shaker at 10–12 C for 4 hrs in one of several treatment solutions, all of which contained 0.2 mg/ml BrdU (Amersham) in lobster medium (45 mg bovine serum albumin [BSA])/ml lobster saline [462mM NaCL, 15.96mM KCL, 26mM CaCl2, 8 mM MgClβ, 11.11 mM Glucose and 10 mM Hepes, pH 7.4]): (1) BrdU alone; (2) BrdU + 5HT creatinine sulfate (Sigma; at specified concentrations from 10−4M to 10−14M). Brains were then fixed as for the in vivo preparations.
Eyestalk-ablated in vivo preparation (Figs. 3, 4)
Eyestalks were excised from juvenile lobsters at least one day before the experimental incubations; eyestalk ablation did not affect mortality of the lobsters. Eyestalk-ablated in vivo lobsters were incubated for 6 hrs at 16 C in one of three treatments: (1) BrdU (Sigma; 2mg/mL SW), (2) BrdU (2 mg/mL SW) + 5HT creatinine sulfate (10 −4M in SW or (3) BrdU (2 mg/mL SW) + 5HT creatinine sulfate (10 −9M in SW). Following these treatments, brains were dissected and fixed as for the in vivo preparations.
Sinus gland experiments (Fig. 6)
Adult lobsters were obtained from local vendors and their sinus glands were removed. These 12 sinus glands were macerated with a mortar and pestle, resulting in ~300μl sample volume. In this experiment using in vitro preparations, 6 brains were dissected from juvenile lobsters for each of 5 treatment groups, all of which contained 0.2 mg/mL BrdU (Amersham) in lobster culture medium: (1) BrdU alone; (2) BrdU + 5HT creatinine sulfate (Sigma; 10−10M); (3,4,5) BrdU + 5HT creatinine sulfate (Sigma; 10−10M) + sinus gland homogenate added at three strengths: (3) 1:10 homogenate in BrdU solution; (4) 1:20 homogenate in BrdU solution; and (5) 1:40 homogenate in BrdU solution. Following a 4-hour incubation period at 12°C, brains were fixed as for the in vivo preparations, and processed immunocytochemically for BrdU.
Immunocytochemistry to detect BrdU
After fixation, all preparations were rinsed in PB containing 0.3% Triton X-100 (PBTx), then incubated in 2N HCL for 30 minutes. After rinsing in PBTx, the dissected brains were incubated for 3 hr at room temperature in mouse anti-BrdU (1:50 in PBTx; Becton Dickinson), rinsed over several hours in PBTx, and incubated overnight at 4°C in Alexa 488-conjugated goat anti-mouse (1:50 in PBTx; Invitrogen). After final rinses in PB, brains were mounted in Gelmount (Biomeda) and viewed using a Leica laser confocal microscope.
Confocal microscopy
To determine the extent of BrdU labeling, serial optical sections were taken through the proliferation zone of Cluster 10 at 1μm and were saved as three-dimensional stacks. With a single confocal image projected on the monitor, BrdU-labeled cell profiles in Cluster 10 were traced onto a transparent sheet, and then counted. Final counts are presented as numbers of labeled cell profiles per hemi-brain. Student-t tests or post-hoc tests (Tukey-Kramer HSD) were performed after an ANOVA analysis. Labeled cells in Cluster 9 were not assessed in the present studies because the quality of labeling in this area is less reliable than in Cluster 10.
Results
Serotonin levels and the rate of adult neurogenesis
The in vivo preparation
Juvenile lobsters in which only the “adult” progenitors and patterns of neurogenesis persist (Sullivan et al., 2007a, b) were exposed to BrdU and serotonin at levels consistent with synaptic release of this amine (10 −4M in sea water). Unexpectedly, increased levels of serotonin resulted in decreased neurogenesis (Fig. 3A).
The in vitro preparation
In order to limit the influence of serotonin to direct effects in the brain alone, we used the in vitro preparation (see Methods). Neurons continue to proliferate in these isolated brains, although the basal levels of neurogenesis are reduced relative to in vivo brains from animals of the same size. Basal levels of neurogenesis in all brains are variable between individuals and slows with increasing in animal size/age (Sullivan et al., 2007b). We find that while high levels (10−4M) of serotonin resulted in decreased neurogenesis in the in vivo preparations, this same level of serotonin resulted in increased neurogenesis (nearly a doubling over 4 hours) in the in vitro brain (Fig 3B). Our subsequent experiments have been directed at understanding these contradictory results.
The eyestalk-ablated in vivo preparation
One likely target of serotonin in vivo is the sinus gland, a major neuroendocrine control center located in the eyestalks of decapod crustaceans, which contains the terminals of neurohormonal cells located in the nearby X-organ (Cooke and Sullivan, 1982; Beltz, 1988). Prior studies have demonstrated that serotonergic projections from the midbrain project to the sinus gland (Sandeman et al., 1988), and also that serotonin induces the release of specific hormones from this region (Mattson and Spaziani, 1986). The X-organ-sinus gland complex is located in the eyestalks (lateral protocerebrum), which are removed in the in vitro preparation. To determine whether the presence of the eyestalks is responsible for the differential response to serotonin, the eyestalks were removed from juvenile lobsters prior to immersing them in 10−4M serotonin. In these animals, we find an increase in neurogenesis similar to that seen in the in vitro preparation (Fig. 3A). The suppression of neurogenesis by 10−4M serotonin in the intact animal therefore suggests that factor(s) released from the eyestalks by the synaptic action of serotonin may antagonize the stimulatory influence of serotonin on neurogenesis seen in the brains of eyetalk-ablated juvenile lobsters and in the in vitro brain preparation.
We then tested the influence of much lower concentrations of serotonin (10−9M) on both the in vivo and eyestalk-ablated preparations and found increases in neurogenesis in both cases (Fig. 4), indicating that at concentrations consistent with hormonal levels, serotonin does not result in the release of neurogenesis-depressing factors from the eyetalks. This possibility is intriguing because it suggests opposing, concentration-dependent mechanisms involving serotonergic regulation of adult neurogenesis. The idea here is that serotonin circulating in the hemolymph at low levels may act directly on neuronal precursor cells residing in the niche or proliferation zones, while serotonin released synaptically from terminals in the eyestalk may stimulate the release of sinus gland hormones that depress neurogenesis. It is also notable in these experiments that the levels of neurogenesis in the brain were significantly lower in the eyestalk-ablated animals, a common observation during these experiments. In these cases, the addition of serotonin (10−9M) then brings the level of neurogenesis back to control levels (see Fig. 4).
To explore the hypothesis that serotonin acts hormonally to regulate neurogenesis, we next conducted a dose-response study using the in vitro brain preparation. No significant differences in the numbers of BrdU-labeled profiles were observed compared to controls when brains were incubated in 10−14 and 10−12 M serotonin in culture medium (Fig. 5). However, 10−10 M serotonin resulted in roughly a tripling in the numbers of BrdU-labeled cells over the 4-hour testing period. Concentrations of serotonin ranging from 10−10-10−4M caused statistically significant increases in the numbers of BrdU-labeled cells compared to controls (with the exception of 10−5M, see Fig. 5), although the sensitivity to the amine decreased with increasing serotonin concentrations. These data suggest that the machinery generating new neurons is highly sensitive to this monoamine, and that serotonin circulating in the hemolymph may directly influence neurogenesis.
We next pursued the idea that sinus gland hormones are capable of antagonizing the direct, stimulatory effect of serotonin on neurogenesis. Groups of in vitro brains were incubated either in saline, serotonin (10 −10M) in lobster saline, or sinus gland homogenate diluted 1:10, 1:20 or 1:40 in serotonin (10−10M) in lobster saline (Fig. 6). Basal levels of neurogenesis were higher than in previous in vitro studies (e.g., Figs. 3, 5), as these were smaller and younger juvenile lobsters than in prior experiments; animal size is known to influence levels of neurogenesis (Sullivan et al., 2007b). While serotonin alone caused large increases in neurogenesis over the 4-hour test period, consistent with previous experiments (Figs. 4 and 5), brains incubated in serotonin and sinus gland homogenate showed reduced levels of BrdU incorporation (Fig. 6). The suppression of neurogenesis was roughly linear to the dilution of the homogenate.
Discussion
Three serotonergic pathways influence adult neurogenesis
Our results suggest that serotonin influences the machinery producing new neurons in the crustacean brain through multiple pathways: through direct effects of serotonin on precursor cells in the neurogenic lineage, or indirect influences via a serotonin-mediated release of hormones from the sinus gland, a neurohemal organ that contains a variety of crustacean hormones (Skinner, 1985; Beltz, 1988; Fu et al., 2005; Stemmler et al., 2007). The sinus gland is composed of the terminals of a tract of axons that link it to a dense cluster of small somata, the X-organ. The X-organ-sinus gland complex, which is functionally analogous to the vertebrate hypothalamo-neurohypophyseal system, forms the primary neuroendocrine control center in crustaceans (Cooke and Sullivan, 1982). The sinus gland abuts the major hemolymph sinus of the eyestalk and receives a branch from one of the eyestalk blood vessels, thereby allowing easy release and circulation of its hormones (Skinner, 1985).
Proliferation of neuron precursors is occurring in at least three sites in the crustacean brain: among the precursors residing in the neurogenic niche, and in the proliferation zones of Clusters 9 and 10 to which the daughters of the niche cells (the “immediate precursors” of neurons) migrate (Sullivan et al., 2007b). These precursors may be targeted by the same and/or different serotonergic delivery systems. The first pathway suggested by our studies is the stimulatory influence of low levels of serotonin (10−10M) on neurogenesis, a concentration consistent with circulating levels of amines (Beltz et al., 1984), and therefore suggesting a hormonal influence for serotonin on adult neurogenesis. The source of the circulating serotonin has not been identified, but potential sites of release are the sinus glands and/or pericardial organs, both of which contain this amine (Beltz 1988, 1995, 1999; Fu et al., 2005).
There is a rich vasculature in the proliferation zone of Cluster 10 and no blood-brain barrier (Abbott, 1971; 1972), and therefore serotonin circulating at low levels also has direct access to cells in the proliferation zone. The current experiments involving exposure of whole animals or isolated brains to serotonin increases the number of labeled neurons in Cluster 10 after only a few hours. This influence must therefore be localized to the immediate precursors of new neurons already present in the proliferation zone of Cluster 10, and not on hormonal effects on the niche pregenitor cells, because this would not be observed over a brief time course; the daughters of the niche progenitors require several days to navigate the migratory stream before reaching the proliferation zones of Clusters 9 or 10. The results from these short-term assays therefore suggest that the immediate precursors of neurons residing in the proliferation zones are stimulated by low levels of serotonin to enter S phase, and are thereby labeled with BrdU.
Also relevant to a potential role for circulating serotonin (or other hormones) are the neural progenitor cells that reside in a niche associated with the vasculature (Sullivan et al., 2007a, b). These niche progenitor cells, which have glial properties, have end feet that sit directly on a vascular cavity; these progenitors are therefore likely to be exposed to hormones, cytokines, neurotrophins and other molecules circulating in the hemolymph (Sullivan et al., 2007a, b). We therefore suggest that serotonin can also gain direct access to the niche progenitor cells, and thereby regulate the cell cycle of the first cells in the neurogenic lineage.
The second proposed pathway is indicated by the differences in response to high levels of serotonin (10−4 M), dependent upon the presence or absence of the eyestalks and associated sinus gland tissue. When eyestalk tissue is present or when sinus gland extract is added to the culture medium, neurogenesis is depressed compared with eyestalk-ablated or in vitro control preparations. These experiments suggest that serotonin can elicit the release of sinus gland hormones that suppress neurogenesis in this system. The response to serotonin is distinguished between the in vitro and in vivo preparations: (1) the basal level of neurogenesis in the in vitro brains is always lower than the in vivo situation; and (2) 10−4 M serotonin stimulates neurogenesis in the former, and suppresses neurogenesis in the latter situation. Ablating the eyestalks (along with the sinus glands) reverses the depressive influence of high levels of serotonin on neurogenesis in vivo, suggesting that hormones in the eyestalk may antagonize the stimulatory serotonergic influence. This suppression is not found at low concentrations of serotonin, but only is seen at higher levels, indicating that this influence is mediated by synaptic release of serotonin in the eyestalk. Such a pathway containing serotonergic fibers emerging from the midbrain that project to the sinus gland have been previously described in crayfish (Sandeman et al., 1988). We conclude that sinus gland hormone(s) oppose the direct stimulatory action of serotonin on neurogenesis at high levels of the amine because the application of sinus gland homogenates depresses neurogenesis in eyetalk-ablated in vitro preparations in comparison with the in vivo controls. In other words, in the presence of serotonin, the addition of the sinus gland homogenate has the same effect as retaining the eyestalks.
In addition to these proposed hormonal and synaptic pathways, fibers of the serotonergic DGNs terminate in the Cluster 10 proliferation zone, providing a third pathway by which serotonin may influence neurogenesis. Serotonin released by the DGNs into the vicinity of the immediate precursor cells in Cluster 10 may influence their cell cycle. The anatomical basis for this pathway was established in previous work (Beltz et al., 2001).
Serotonin and sinus gland hormones
Serotonin is known to enhance the release of sinus gland hormones (Gottfried et al., 1977), including molt inhibiting hormone (MIH; Mattson and Spaziani, 1985; 1986) and crustacean hyperglycemic hormone (CHH; Lee et al., 2001), both of which belong to the CHH family of peptides (Wang and Xiang, 2003; Montagné et al., 2008). These compounds are therefore potential candidates for the sinus gland component that influences neurogenesis.
The traditional model has been that MIH inhibits molting by directly suppressing the synthesis of steroid molting hormones (ecdysteroids) from the Y-organ, another major endocrine gland (Beltz, 1988; Wang and Xiang, 2003). Thus, the molt cycle is regulated by the balance of MIH and the Y-organ hormones. Variations in ecdysteroid levels over the course of the molt cycle have been documented in the American lobster (Snyder and Chang, 1991), where it was found that ecdysteroids peak just before the molt and gradually drop during postmolt to their intermolt levels. Since MIH directly suppresses ecdysteroid release, it would be expected that levels of MIH would be highest during intermolt, and lowest during premolt. Indeed, an inverse relationship between titers of these hormones has been found in the American crayfish, Procambarus clarkii (Nakatsuji and Sonobe, 2004) and in the blue crab, Callinectes sapidus (Lee et al., 1998). As the hormonal balance varies throughout the molt cycle, rates of neuronal proliferation in crustaceans also fluctuate. Harrison et al. (2001) and Gorrisen (2002) reported fluctuations in the rate of production of crustacean olfactory receptor neurons and olfactory interneurons, respectively, over the course of the molt cycle. The relationships between levels of neurogenesis and molting hormones have not, however, been specifically tested. While ecdysteroids are known to influence neurogenesis in the insect brain (Manduca sexta [Champlin and Truman, 1998]; Acheta domesticus [Malaterre et al., 2003]) brain, our experiments indicate that sinus gland hormones may have a direct influence on neurogenesis, because in the in vitro preparations exposed to sinus gland homogenate the Y organ (the source of ecdysteroids) was absent.
The primary known role of CHH is in regulating carbohydrate metabolism. There is evidence that CHH fluctuates on a circadian cycle (Fanjul-Moles and Prieto-Sagredo, 2003), as does serotonin (Wildt et al., 2004). The cyclical changes in these molecules are of interest in the context of neurogenesis, which in lobsters is under the control of an endogenous circadian clock (Goergen et al., 2002). In addition, CHH has been linked to stress responses in decapod crustaceans (Fanjul-Moles, 2006); stress is generally associated with decreases in neurogenesis in mammalian systems (Dranovsky and Hen, 2006). As with MIH, however, the specific role of CHH in regulating neurogenesis in crustaceans has not been experimentally tested.
Although the mechanisms by which sinus gland hormones suppress neurogenesis are not known, the anticipated release of MIH and CHH when serotonin is present in high concentrations is intriguing given the known roles of these compounds relative to circadian and molt cycles, and the relationships of these cycles to neurogenesis. From this perspective, serotonin could serve as a molecular link coordinating these cycles with neurogenesis.
Conclusions
Our results indicate multiple mechanisms and pathways by which serotonin regulates neurogenesis in the crustacean brain: (1) as a hormone circulating at low levels that influences the precursor cells residing in the proliferation zones and neurogenic niche; (2) at higher levels, by inducing the release of sinus gland hormones (e.g. MIH, CHH), which in turn antagonize the direct influence of low levels of serotonin on neurogenesis; and (3) through local release from the DGN directly into the proliferation zone (Beltz et al., 2001). We conclude that the opposite effect (depression) of serotonin at 10−4M on neurogenesis that is dependent on the presence or absence of the eyestalks, is due to the existence of an agent (or agents) in the eyestalks that has an inhibitory influence on neurogenesis. Further study with the in vitro preparation and neurogenesis may allow the isolation and identification of such substances and a clarification of the pathways that are involved.
Acknowledgments
We thank M. Tlusty and A. Kim of the New England Aquarium for lobster rearing, P. Carey and V. Quinan for technical assistance, and D.C. Sandeman for extensive discussions and review of this manuscript. Supported by NIH R01 MH67157, NSF-IBN 0344448 and The Maren Foundation, Mount Desert Island Biological Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abbott NJ. The organization of the cerebral ganglion in the shore crab, Carcinus maenas. II. The relation of intracellular blood vessels to other brain elements. Z Zellforsch. 1971;120:401–420. doi: 10.1007/BF00324900. [DOI] [PubMed] [Google Scholar]
- Abbott NJ. Access of ferritin to the interstitial space of Carcinus brain from intracerebral blood vessels. Tiss Cell. 1972;4:99–104. doi: 10.1016/s0040-8166(72)80010-7. [DOI] [PubMed] [Google Scholar]
- Beltz BS. Crustacean Hormones. In: Laufer H, Downer R, editors. Invertebrate Endocrinology. 2. Alan R Liss, Inc; NY: 1988. pp. 235–258. [Google Scholar]
- Beltz BS. Neurobiology and Neuroendocrinology. In: Factor JR, editor. Biology of the Lobster Homarus americanus. Chapter 11. Academic Press; 1995. [Google Scholar]
- Beltz BS. The distribution and functional anatomy of amine neurons in lobsters. Micros Res Tech. 1999;44:105–120. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<105::AID-JEMT5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Beltz B, Eisen JS, Flamm R, Harris-Warrick RM, Hooper SL, Marder E. Serotonergic innervation and modulation of the stomatogastric ganglion of three decapod crustaceans. J Exp Biol. 1984;109:35–54. doi: 10.1242/jeb.109.1.35. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Benton JL, Sullivan JM. Transient uptake of serotonin by newborn olfactory projection neurons. Proc Natl Acad Sci U S A. 2001;98:12730–12735. doi: 10.1073/pnas.231471298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton J, Beltz B. Effects of serotonin depletion on local interneurons in the developing olfactory pathway of lobsters. J Neurobiol. 2001;46:193–205. doi: 10.1002/1097-4695(20010215)46:3<193::aid-neu1002>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Benton J, Huber R, Ruchhoeft M, Helluy S, Beltz B. Serotonin depletion by 5,7-dihydroxytryptamine alters deutocerebral development in the lobster, Homarus americanus. J Neurobiol. 1997;33:357–373. doi: 10.1002/(sici)1097-4695(199710)33:4<357::aid-neu2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Benton J, Beltz BS. An in vitro approach sheds light on serotonergic influences on adult neurogenesis in Homarus americanus. Bull MDIBL. 2007;46:129–132. [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neurosci. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Champlin DT, Truman JW. Ecdysteroids govern two phases of eye development during metamorphosis of the moth, Manduca sexta. Development. 1998;125:2009–2018. doi: 10.1242/dev.125.11.2009. [DOI] [PubMed] [Google Scholar]
- Chang ES, Chang SA, Beltz BS, Kravitz EA. Crustacean hyperglycemic hormone in the lobster nervous system: localization and release from cells in the subesophageal ganglion and thoracic second roots. J Comp Neurol. 1999;414:50–56. [PubMed] [Google Scholar]
- Cooke IM, Sullivan KE. Hormones and neurosecretion. In: Atwood HH, Sandeman DC, editors. Biology of the Crustacea. Vol. 3. Academic Press; NY: 1982. pp. 205–290. [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanjul-Moles ML. Biochemical and functional aspects of crustacean hypterglycemic hormone in decapod crustaceans: Review and update. Comp Biochem Phys C. 2006;142:390–400. doi: 10.1016/j.cbpc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Fanjul-Moles ML, Prieto-Sagredo J. The circadian system of crayfish: a developmental approach. Microsc Res Tech. 2003;60:291–301. doi: 10.1002/jemt.10268. [DOI] [PubMed] [Google Scholar]
- Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res Mol Brain Res. 2005;138:228–235. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, de la Iglesia HO, Christie AE, Li L. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J Comp Neurol. 2005;493:607–26. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- Goergen EM, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. J Neurobiol. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Gorissen S. Honours Thesis. University of New South Wales; 2002. Endogenous control of neurogenesis in the juvenile crayfish brain. [Google Scholar]
- Gottfried E, Strolenberg C, Van Herp F. Demonstration of exocytosis in the sinus gland of Astacus leptodactylus (Nordmann) under the influence of serotonin injections. C R Acad Sci Hebd Seances Acad Sci D. 1977;284:57–59. [PubMed] [Google Scholar]
- Harrison PJ, Cate HS, Swanson ES, Derby CD. Postembryonic proliferation in the spiny lobster antennular epithelium: rate of genesis of olfactory receptor neurons is dependent on molt stage. J Neurobiol. 2001;47:51–66. doi: 10.1002/neu.1015. [DOI] [PubMed] [Google Scholar]
- Harzsch S. Ontogeny of the ventral nerve cord in malacostracan crustaceans: a common plan for neuronal development in Crustacea, Hexapoda and other Arthropoda? Arthropod Struct Dev. 2003;32:17–37. doi: 10.1016/S1467-8039(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton J, Beltz B. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. J Neurosci. 1999;19:3472–3485. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Yang PF, Zou HS. Serotonergic regulation of crustacean hyperglycemic hormone secretion in the crayfish, Procambarus clarkii. Physiol Biochem Zool. 2001;74:376–382. doi: 10.1086/320430. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Watson RD, Roer RD. Molt-inhibiting hormone mRNA levels and ecdysteroid titer during a molt cycle of the blue crab, Callinectes sapidus. Biochem Biophys Res Commun. 1998;249:624–7. doi: 10.1006/bbrc.1998.9215. [DOI] [PubMed] [Google Scholar]
- Malaterre J, Strambi C, Aouane A, Strambi A, Rougon G, Cayre M. Effect of hormones and growth factors on the proliferation of adult cricket neural progenitor cells in vitro. J Neurobiol. 2003;56:387–397. doi: 10.1002/neu.10244. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Spaziani E. Characterization of molt-inhibiting hormone (MIH) action on crustacean Y-organ segments and dispersed cells in culture and a bioassay for MIH activity. J Exp Zool. 1985;236:93–101. doi: 10.1002/jez.1402360113. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Spaziani E. Regulation of the stress-responsive X-organ--Y-organ axis by 5-hydroxytryptamine in the crab, Cancer antennarius. Gen Comp Endocrinol. 1986;62:419–427. doi: 10.1016/0016-6480(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Montagné N, Soyez D, Gallois D, Ollivaux C, Toullec JY. New insights into evolution of crustacean hyperglycaemic hormone in decapods –first characterization in Anomura. FEBS J. 2008;275:1039–1052. doi: 10.1111/j.1742-4658.2007.06245.x. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Sonobe H. Regulation of ecdysteroid secretion from the Y-organ by molt-inhibiting hormone in the American crayfish, Procambarus clarkii. Gen Comp Endocrinol. 2004;135:358–64. doi: 10.1016/j.ygcen.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Rogan SC. Honors Thesis. Wellesley College; 2004. Effects of serotonin and sinus gland hormones on neurogenesis in the crustacean brain. [Google Scholar]
- Sandeman DC, Sandeman RE. Electrical responses and synaptic connections of giant serotonin-immunoreactive neurons in crayfish olfactory and accessory lobes. J Comp Neurol. 1994;341:130–144. doi: 10.1002/cne.903410111. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman RE, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: a common nomenclature for homologous structures. Biol Bull. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Beltz B, Sandeman RE. Crayfish brain interneurons that converge with serotonin giant cells in the accessory lobe glomeruli. J Comp Neurol. 1995;352:263–279. doi: 10.1002/cne.903520209. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Sandeman DC. Serotonin-like immunoreactivity of giant olfactory interneurons in the crayfish brain. Brain Res. 1987;403:371–374. doi: 10.1016/0006-8993(87)90078-3. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Watson AH, Sandeman DC. Ultrastructure of the synaptic terminals of the dorsal giant serotonin-IR neuron and deutocerebral commissure interneurons in the accessory and olfactory lobes of the crayfish. J Comp Neurol. 1995;361:617–632. doi: 10.1002/cne.903610406. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Neuronal differentiation and long-term survival of newly generated cells in the olfactory midbrain of the adult spiny lobster, Panulirus argus. J Neurobiol. 2001;48:181–203. doi: 10.1002/neu.1050. [DOI] [PubMed] [Google Scholar]
- Skinner DM. Effects of eyestalk ablation on larval molting rates and morphological development of the American lobster, Homarus americanus. Biol Bul. 1985;170:232–243. [Google Scholar]
- Snyder MJ, Chang ES. Ecdysteroids in relation to the molt cycle of the American lobster, Homarus americanus. I. Hemolymph titers and metabolites. Gen Comp Endocrinol. 1991;81:133–145. doi: 10.1016/0016-6480(91)90133-q. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Hsu YW, Cashman CR, Messinger DI, de la Iglesia HO, Dickinson PS, Christie AE. Direct tissue MALDI-FTMS profiling of individual Cancer productus sinus glands reveals that one of three distinct combinations of crustacean hyperglycemic hormone precursor-related peptide (CPRP) isoforms are present in individual crabs. Gen Comp Endocrinol. 2007;154:184–92. doi: 10.1016/j.ygcen.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Beltz BS. Serotonin depletion in vivo inhibits the branching of olfactory projection neurons in the lobster deutocerebrum. J Neurosci. 2000;20:7716–7721. doi: 10.1523/JNEUROSCI.20-20-07716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Integration and segregation of inputs to higher-order neuropils of the crayfish brain. J Comp Neurol. 2005a;481:118–126. doi: 10.1002/cne.20346. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J Neurobiol. 2005b;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis: a common strategy across diverse species. J Comp Neurol. 2007a;500:574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Sandeman DC, Benton JL, Beltz BS. Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons. J Mol Histol. 2007b;38:527–542. doi: 10.1007/s10735-007-9112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZZ, Xiang JH. Cloning and analysis of three genes encoding type II CHH family neuropeptides from Fennropenaeus chinensis. Yi Chuan Xue Bao. 2003;30:961–966. [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Wildt M, Goergen EM, Benton JL, Sandeman DC, Beltz BS. Regulation of serotonin levels by multiple light-entrainable endogenous rhythms. J Exp Biol. 2004;207:3765–74. doi: 10.1242/jeb.01205. [DOI] [PubMed] [Google Scholar]