Abstract
Growth cones at the tips of nascent and regenerating axons direct axon elongation. Netrin-1, a secreted molecule that promotes axon outgrowth and regulates axon pathfinding, elevates cyclic AMP (cAMP) levels in growth cones and regulates growth cone morphology and axonal outgrowth. These morphological effects depend on the intracellular levels of cAMP. However, the specific pathways that regulate cAMP levels in response to netrin-1 signaling are unclear. Here we show that ‘soluble’ adenylyl cyclase (sAC), an atypical calcium-regulated cAMP-generating enzyme previously implicated in sperm maturation, is expressed in developing rat axons and generates cAMP in response to netrin-1. Overexpression of sAC results in axonal outgrowth and growth cone elaboration, whereas inhibition of sAC blocks netrin-1–induced axon outgrowth and growth cone elaboration. Taken together, these results indicate that netrin-1 signals through sAC-generated cAMP, and identify a fundamental role for sAC in axonal development.
Axonal outgrowth is essential for the proper development of the nervous system as well as for axonal regeneration following injury. Axonal growth cones detect outgrowth factors in their environment and transduce these signals into rearrangements in the axonal cytoskeleton, ultimately resulting in axonal elongation1. Several families of extracellular molecules have been discovered that promote axon outgrowth, such as netrins2,3 and neurotrophins4. Members of the netrin family can trigger axonal outgrowth2 and can also elicit either attractive or repulsive turning responses that are fundamental for axonal pathfinding5-7. Netrin-1, the founding member of the netrin family2, has outgrowth and pathfinding roles in the development of many types of neurons, including cortical8, spinal commissural3,9 and peripheral neurons10.
Netrin-1 binds to the deleted in colorectal cancer (DCC) family of receptors, which include neogenin and DCC in vertebrates11, resulting in the reorganization of the growth cone cytoskeleton and subsequent axonal elongation12. This process involves the regulation of the activity of Rho family monomeric GTPases, including Cdc42 and Rac1 (ref. 13). Bath application of netrin-1 induces ‘growth cone elaboration’, characterized by significant increases in growth cone surface area and in the number of filopodia12,13—the F-actin–rich, finger-like protrusions at the leading edge of the growth cone.
cAMP has critical roles in mediating the responses of axons to netrin-1 and also has direct effects on axonal elongation. Application of netrin-1 increases cAMP levels in growth cones6, and blockade of cAMP signaling by inhibition of protein kinase A (PKA) or by growing axons on laminin-1, a substratum that reduces basal cAMP levels, blocks netrin-1–mediated attractive responses6,14. cAMP seems to signal outgrowth and attractive turning, as Xenopus laevis embryonic spinal axons turn and extend toward gradients of forskolin or dibutyryl cAMP (ref. 15), pharmacological agents that activate cAMP signaling pathways, and elevation of intracellular cAMP promotes outgrowth of injured spinal cord axons in the rat16. Although cAMP is a key mediator of netrin-1 signaling, the mechanisms by which the netrin-1 activation of DCC leads to increased cAMP levels in growth cones are still unclear. DCC does not seem to be linked to heterotrimeric G protein–responsive transmembrane adenylyl cyclases (tmACs), and attempts to link netrin-1 to G protein–coupled receptors (GPCRs) remain controversial. Additionally, although several proteins interact with DCC (ref. 13), none of these are known to be coupled to tmAC activation.
Mammalian cells possess a second source of cAMP, the evolutionarily conserved, bicarbonate- and calcium-responsive sAC. sAC was originally identified as a soluble activity in the testis that was not activated by forskolin17. Molecular cloning of the enzyme revealed that sAC does not contain transmembrane domains, is evolutionarily more related to bacterial cyclases than to tmAC (ref. 18), and is ubiquitously expressed19 with several alternatively spliced isoforms that exhibit tissue-specific expression patterns20. Isoforms enriched in the testis are involved in sperm motility21,22 and maturation22. In the testis, sAC activity is predominantly cytosolic, but in other cell types, sAC protein is particulate, with isoforms distributed to the nucleus, mitochondria and cytoskeletal structures23. sAC is not regulated by G protein pathways17,24; instead, it is modulated by calcium25,26.
Here we report that sAC is expressed in the axons and growth cones of developing neurons. The effects of sAC overexpression—axonal outgrowth and elaboration of growth cones—resemble morphological changes elicited by the treatment of axons with netrin-1. Using pharmacological and siRNA approaches, we found that sAC activity is required for netrin-1–induced cAMP generation and for netrin-1–mediated growth cone elaboration and axon outgrowth. Blockade of G protein–responsive tmACs had no effect on netrin-1 signaling, indicating that GPCR activation is not involved in these effects of netrin-1. Our data reveal a new role for sAC as a downstream effector of netrin-1 and demonstrate that sAC-mediated cAMP production accounts for the morphologic effects of netrin-1 on growth cones.
RESULTS
sAC expression in developing neurons
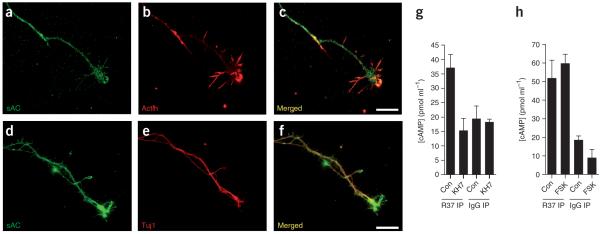
Although the function of sAC in sperm development is well defined21,22, its role outside the testis is largely unknown. To determine whether sAC might be involved in cAMP signaling in axons, we examined sAC immunoreactivity in several populations of developing neurons. At embryonic day (E) 14–15, sensory axons emerge from the dorsal root ganglia (DRG) and elongate toward various sensory targets27. Immunofluorescent labeling using an antibody (R21) that recognizes the first catalytic domain of sAC (ref. 23) resulted in the labeling of E15–16 DRG cell bodies and axons from rats at 3 days in vitro (DIV) (Fig. 1a–c). In growth cones, sAC was enriched in the microtubule-rich central part of the growth cone and axonal stalk, with lower levels of immunoreactivity in filopodia (Fig. 1a–c). Similar localizations were seen using a different antibody (R37) that recognizes the second catalytic domain of sAC (Supplementary Fig. 1 online). Similarly, sAC localization was also detected by immunofluorescence in the axons of rat E15 dorsal spinal cord (DSC) explants (Fig. 1d–f) and by immunohistochemistry in neuronal cell bodies of rat E15 DSC (Supplementary Fig. 1). sAC-expressing axons in DSC explant cultures also stained for TAG-1, indicating that they were commissural axons (ref. 28 and Supplementary Fig. 1). sAC immunoreactivity was also detected in cell bodies, dendrites and axons of stage III hippocampal neurons (DIV 4) (Supplementary Fig. 1).
Figure 1.
Expression of sAC in rat neurons. (a,b) Immunofluorescence localization of sAC in cultured DRG explant axons. E15 dissociated rat DRG neuronal cultures (DIV 3) were labeled with an anti-sAC antibody (R21) to aa 203–217 of human sAC, and an anti-actin antibody. sAC immunoreactivity (green) was detected throughout the axon and the central portion of the growth cone (a), but was less abundant in filopodia and in the actin-rich growth cone periphery (red) (b). (c) Overlay of a and b. Scale bar, 20 μm. (d,e) Immunofluorescence localization of sAC in cultured DSC explant axons. sAC (green) was detected using anti-sAC (R21), and axons were labeled with an antibody to Tuj1 (red). (f) Overlay of d and e. Scale bar, 20 μm. (g,h) sAC activity is present in the brain. Brain lysates were applied to control Ig or sAC Ig-Affigel 10 resin, and bound sAC was eluted and detected in a cAMP accumulation assay. Adenylyl cyclase activity was detected in the eluates from the sAC Ig-resin and was identified as sAC due to its sensitivity to KH7 (g), and its resistance to forskolin (h). Because forskolin stimulation of adenylyl cyclase activity requires MgCl2, forskolin-stimulation assays were performed in the presence of 5 mM MgCl2. Results presented in g and h are representative of experiments repeated at least five times. Error bars represent s.e.m.
To further confirm sAC expression in the brain, we immunoprecipitated sAC from brain extracts. Control antibodies did not precipitate adenylyl cyclase activity, whereas antibodies to sAC immunoprecipitated sAC activity (Fig. 1g,h), as defined as adenylyl cyclase activity that is resistant to forskolin and sensitive to KH7, a sAC-specific inhibitor22,29. A genome-wide microarray-based ‘gene atlas’ of rat tissues has indicated that sAC transcripts are enriched in several peripheral tissues and brain structures, with the highest transcript levels in the amygdala, the core nucleus accumbens, primary cortical neurons, bone marrow and DRG (ref. 30). Together, these data indicate that the mRNA encoding sAC as well as sAC protein are found in both central and peripheral neurons, and are expressed in dendrites, axons and growth cones.
sAC activity induces outgrowth and cAMP accumulation
To identify a potential role for sAC in developing axons, we infected dissociated DRG neurons with a sAC-expressing lentivirus (Fig. 2a). Expression of sAC in dissociated DRG cultures resulted in a 1.73-fold increase (36.53 ± 3.72% for LacZ-expressing neurons and 63.43 ± 3.58% for sAC-expressing neurons, n = 50 growth cones, P < 0.01) in the number of filopodia-type growth cones (Fig. 2b,c), as defined by growth cones that comprise more than two filopodia that are longer than 10 μm, as well as a 1.57 ± 0.89-fold (n = 27 growth cones, P < 0.01) increase in the size of growth cones above that in LacZ-expressing neurons. To determine if sAC affects axonal outgrowth, we used DRG pseudoexplants, which were prepared by plating dissociated DRG neurons in a 3-mm inner-diameter cloning cylinder. Pseudoex-plants are useful as they comprise dissociated neurons, which can be infected at high efficiency, and because they contain an unambiguous border, which is useful for accurately measuring axon lengths. Pseudoexplants were infected at DIV 1 with LacZ or sAC lentivirus, and axon lengths were measured 48 h later. Pseudoexplants overexpressing sAC had significantly longer axons than LacZ-expressing pseudoexplants (1330 μm ± 428.9 for sAC-expressing neurons and 837.8 μm±208.3 for LacZ-expressing neurons, n = 27 axons, P < 0.001). These effects of sAC overexpression resemble the effects seen after treatment of neurons with axon outgrowth–stimulating molecules, such as netrin-1 (refs. 2,13), raising the possibility that sAC has a role in axonal elongation.
Figure 2.
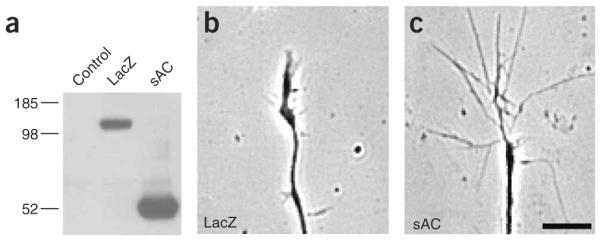
sAC overexpression induces axonal outgrowth and growth cone elaboration. (a) Characterization of sAC- and LacZ-expressing lentiviruses. HEK293T cells were infected with an identical titer of each virus, and the recombinant V5-tagged proteins were detected by western blotting using an anti-V5 antibody. (b,c) Lentivirus-mediated expression of sAC resulted in increased growth cone elaboration compared to that observed in LacZ-infected DRG neuronal cultures. Dissociated E15 DRG neurons were plated as pseudoexplants and infected at DIV 1. The percent of growth cones with a filopodial morphology and the average growth cone size were measured 48 h later. Representative phase contrast images of growth cones expressing LacZ and sAC lentiviruses are shown. Scale bar, 10 μm.
Treatment of cultured neurons with netrin-1 results in increases in cAMP in growth cones, as assessed by cAMP immunofluorescence6. To determine if netrin-1–mediated elevations in cAMP are mediated by activation of sAC, we examined cAMP accumulation in netrin-1–treated neuronal cultures. DRG neurons expressed low levels of the netrin-1-receptor DCC, which rose by 3 DIV (Supplementary Fig. 2 online). Treatment of dissociated cultured DRG neurons with netrin-1 resulted in a 1.4 ± 0.5-fold (n = 3 experiments, P < 0.01) increase in cAMP levels as compared to that in vehicle-treated cultures (Fig. 3). Pretreatment of the cultures with KH7 blocked netrin-1–dependent increases in cAMP, consistent with direct activation of sAC activity by netrin-1. KH7.15, a structurally related control compound, did not affect netrin-1–induced elevations in cAMP (Fig. 3). The catechol estrogen 2-hydroxy estradiol, a structurally unrelated inhibitor that is selective for sAC at low concentrations31, also blocked netrin-1–induced increases in cAMP (Fig. 3). The effects of netrin-1 were not due to the modulation of cyclic nucleotide phosphodiesterase activity as 3-isobutyl-1-methylxanthine (IBMX), a nonselective phosphodiesterase inhibitor, was used in all assays. Thus, netrin-1–induced cAMP accumulation is mediated by activation of sAC.
Figure 3.
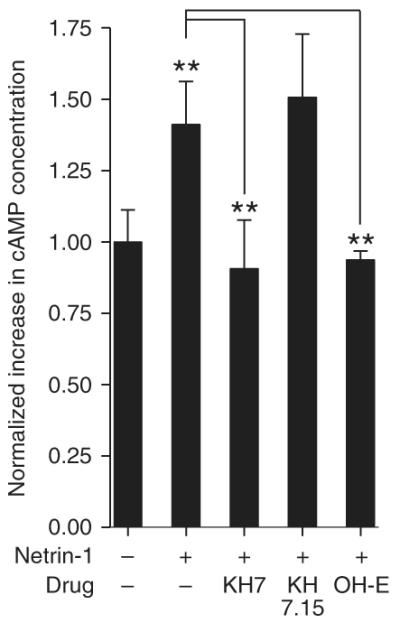
Netrin-1 induction of cAMP is blocked by sAC inhibitors. Netrin-1 treatment elevated cAMP levels in a sAC-dependent manner. Dissociated DRG neurons were preincubated with KH7, KH7.15, 2-hydroxy-estradiol (OH-E, a catechol estrogen) or vehicle in the presence of IBMX, a nonselective phosphodiesterase inhibitor. cAMP levels were measured by enzyme-linked immunosorbent assay (ELISA) after treatment with vehicle or netrin-1 (300 ng ml−1) for 15 min. Netrin-1 increased cAMP levels; this increase was blocked by KH7 and OH-E but not KH7.15. Results shown are the average of three separate experiments. Error bars represent s.e.m.
Netrin-1–induced elaboration requires sAC activity
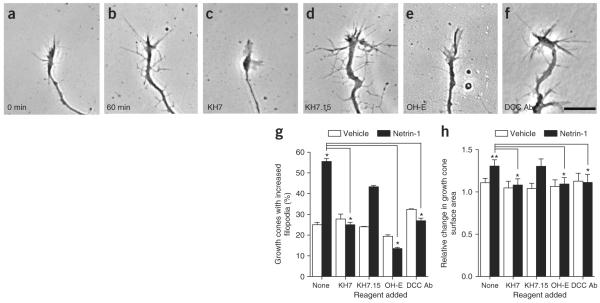
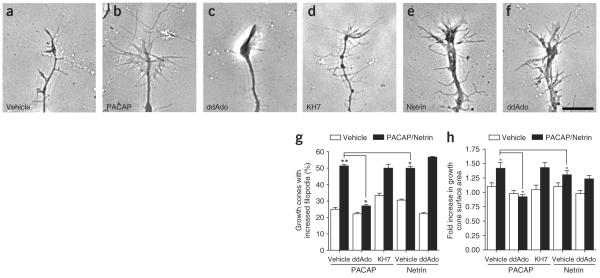
Netrin-1 induces axonal outgrowth2 and morphologic changes in growth cones13 in a cAMP-dependent manner5,14,32, although the pathways that regulate cAMP in growth cones are unclear. To test whether sAC-derived cAMP participates in netrin-1 signaling, we examined the effects of selective sAC inhibitors on netrin-1–mediated growth cone elaboration in E15 DSC explant axons (Fig. 4a,b). Application of netrin-1 resulted in an increase in both filopodia formation and growth cone size (Fig. 4a–h). These increases were blocked by KH7, whereas KH7.15 did not affect netrin-1–induced growth cone elaboration (Fig. 4c,d,g,h). 2-Hydroxy-estradiol also blocked the effects of netrin-1 on filopodia formation and growth cone size (Fig. 4e,g,h). These effects were mediated by DCC, as they were blocked by a DCC-neutralizing anti-body (Fig. 4f–h). We also examined the role of sAC in netrin-1 signaling in E15 DRG explant cultures. DCC is expressed in DRG neurons10, and its expression appeared in DRG explant cultures by 3 DIV (Supplementary Fig. 2). As with DSC explant axons, netrin-1–induced increases in filopodia formation and growth cone size were blocked by KH7 and catechol estrogen, but not by the corresponding control compounds (Supplementary Fig. 2).
Figure 4.
Netrin-1–induced growth cone elaboration is blocked by sAC inhibitors. (a–h) Netrin-1 mediates growth cone elaboration through sAC. E15 DSC explant cultures (DIV 4) were pretreated with the indicated drug for 30 min, and the morphology of individual growth cones were measured at 0 min and 60 min after treatment with netrin-1 (75 ng ml−1) or vehicle. The percent of growth cones that showed an increase in filopodia number and the relative increase in growth cone size were quantified. (a,b) Phase-contrast images of DSC growth cones at 0 min and 60 min after the addition of netrin-1. (c,d) Growth cone elaboration after 60 min of netrin-1 treatment was blocked by pretreatment with KH7 (3 μM), but not by KH7.15 (3 μM), a structurally similar control compound. (e,f) Growth cone elaboration after 60 min of netrin-1 treatment was also blocked by pretreatment with OH-E (5 μM), a structurally unrelated sAC inhibitor, and with a DCC-blocking antibody (1 μg ml−1). Scale bar, 10 μm. (g) Quantification of percent of growth cones with increased filopodia after 60 min for the conditions in a–f. n = 36 growth cones from three different experiments. (h) Quantification for of fold increase in growth cone size after 60 min for the conditions in a–f. n = 36 growth cones from three different experiments. *P < 0.01, **P < 0.001. Error bars represent s.e.m.
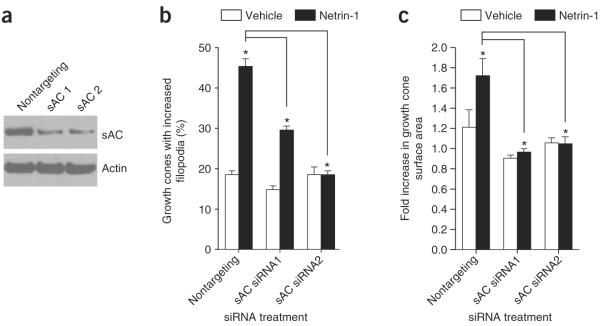
To further examine the role of sAC in netrin-1 signaling, we examined the effect of two sAC-specific siRNAs on netrin-1–mediated growth cone elaboration in dissociated DRG neuronal cultures. DRG neurons are susceptible to highly efficient siRNA-mediated mRNA knockdown (refs. 33,34 and Supplementary Fig. 3 online). Transfection of siRNA directed against exon 4 (sAC1) or exon 5 (sAC2) induced marked knockdown of sAC protein (Fig. 5a). Neurons transfected with sAC-specific siRNA showed a substantial reduction in netrin-1–induced filopodia formation and growth cone size (Fig. 5b,c). DRG cultures transfected with nontargeting control siRNA showed no knockdown of endogenous sAC and had normal responses to netrin-1 (Fig. 5a–c).
Figure 5.
siRNA-mediated knockdown of sAC reduces netrin-1–dependent growth cone elaboration. (a) sAC-specific siRNAs reduced sAC levels in DRG neurons. Top, E15 dissociated DRG neuronal cultures were transfected with control or sAC-specific siRNAs on DIV 1, and sAC protein levels were detected 48 h later by immunoblotting with a sAC-specific antibody (R21). Bottom, the same blot was probed with anti-actin to confirm equal loading in all lanes. (b,c) DRG neurons were transfected as in a and then assayed for netrin-1–dependent growth cone elaboration 48 h later. We measured the morphology of individual growth cones at 0 min and 60 min after treatment with netrin-1 or vehicle, and quantified the percent of growth cones showing an increase in filopodia number (n = 50 growth cones from three different experiments) and the fold increase in growth cone size (n = 27 growth cones from 3 different experiments) after 60 min. siRNA-mediated knockdown of sAC blocked netrin-1–mediated outgrowth. *P < 0.01. Error bars represent s.e.m.
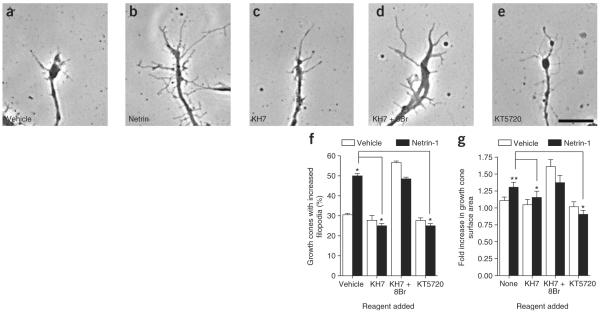
To confirm that the actions of KH7 were due to reductions in cAMP, we assessed the ability of 8-bromoadenosine-cAMP (8-Br-cAMP), a nonhydrolyzable cAMP analog, to rescue KH7-mediated inhibition of netrin signaling. In DSC explant cultures incubated with KH7 and netrin-1, 8-Br-cAMP treatment resulted in increased filopodia formation and growth cone size (Fig. 6a–g), consistent with KH7 mediating its inhibitory effects on growth cone formation through sAC-generated cAMP. 8-Br-cAMP seems to function in the same pathway as netrin-1 as the coapplication of 8-Br-cAMP and netrin-1 did not induce further growth cone elaboration (Fig. 6f,g). To determine whether the effects of cAMP are mediated by PKA, we pretreated DRGs with KT5720, a PKA inhibitor, and found that KT5720 also blocked netrin-1–mediated growth cone elaboration (Fig. 6e–g). Similar effects were seen in DRG explant cultures (Supplementary Fig. 4 online). Thus, the activity of both sAC and PKA are required for the effects of netrin-1 on growth cone morphology. Together, these pharmacological and siRNA experiments indicate that sAC, by means of its generation of cAMP and subsequent modulation of PKA, is required for netrin-1–mediated growth cone elaboration.
Figure 6.
Netrin-1–mediated growth cone elaboration requires cAMP and PKA. E15 DSC explant cultures (DIV 4) were pretreated with the indicated drug for 30 min, and the morphology of individual growth cones was measured at 0 min and 60 min after treatment with netrin-1 (75 ng ml−1) or vehicle. (a,b) Phase-contrast images of DRG growth cones 60 min after the addition of vehicle and netrin-1. (c,d) Netrin-1–induced growth cone outgrowth at 60 min was blocked by pretreatment with KH7 (3 μM) and was rescued by simultaneous treatment with 8-bromo-cAMP (8Br, 3 μM). (e) Netrin-1–induced growth cone outgrowth at 60 min was also blocked by pretreatment with the PKA inhibitor KT5720 (1 μM). Scale bar, 10 μm. (f,g) Quantification for a–e of percent of growth cones with increased filopodia and fold increase in growth cone size, after 60 min. n = 36 growth cones from three different experiments. *P < 0.01, **P < 0.001. Error bars represent s.e.m.
sAC and tmAC are involved in distinct signaling pathways
In addition to sAC, roles for tmACs in regulating growth cone morphology have been demonstrated in experiments using pituitary adenylyl cyclase–activating polypeptide (PACAP), which regulates growth cone morphology through a GPCR/tmAC pathway35,36. To differentiate the roles for sAC and tmAC in growth cone signaling, we used the P-site inhibitor 2′,5′-dideoxyadenosine (2′,5′-ddAdo; ref. 37), which is selective for tmACs (ref. 38). Treatment of DSC explant cultures with PACAP resulted in a marked increase in filopodial formation and growth cone size, consistent with the previously reported chemoattractive turning effects of this molecule (ref. 36 and Fig. 7a–h). Application of 50 μM2′,5′-ddAdo resulted in a marked reduction in PACAP-mediated increases in filopodial formation and growth cone size (Fig. 7c,h), suggesting a role for tmAC in PACAP signaling. Unlike netrin-1, the application of KH7 had no effect on PACAP-mediated growth cone elaboration (Fig. 7d,h), indicating that KH7 does not have a general inhibitory effect on growth cone morphology. On the other hand, netrin-1–mediated growth cone elaboration was not affected by 2′,5′-ddAdo, indicating that tm AC is not required for these effects of netrin-1 (Fig. 7e–h). Similar results were seen in DRG explant cultures (Supplementary Fig. 5 online). Together, these results indicate that cAMP generated by either sAC or tmACs can elicit similar morphologic changes in growth cones, but that specific adenylyl cyclases are used by different signaling pathways.
Figure 7.
Differentiation of the roles of tmAC and sAC in growth cone signaling. The tmAC inhibitor 2′,5′-ddAdo did not affect netrin-1–mediated growth cone elaboration, and KH7 did not block PACAP-mediated growth cone elaboration. E15 DSC explant cultures (DIV 4) were pretreated with the indicated drug for 30 min, and the morphology of individual growth cones was measured at 0 min and 60 min after treatment with netrin-1 (75 ng ml−1), PACAP (10 nM) or vehicle. (a,b) Phase-contrast images of DRG growth cones 60 min after the addition of vehicle or PACAP. (c,d) PACAP-induced growth cone outgrowth was blocked by 2′5′-ddAdo (50 μM), but not by pretreatment with KH7 (3 μM). (e,f) Netrin-1–induced growth cone elaboration was not blocked by pretreatment with 2′5′-ddAdo. (g,h) Quantification for a–f of percent of growth cones with increased filopodia and of fold increase in growth cone size, after 60 min. n = 36 growth cones from three different experiments. *P < 0.01, **P < 0.001. Error bars represent s.e.m.
sAC regulates netrin-1–induced axonal outgrowth
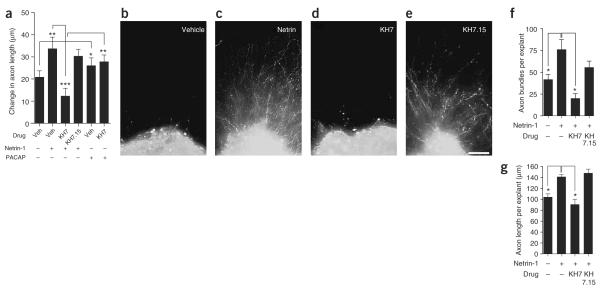
Netrin-1 was first discovered as a secreted protein with axon outgrowth–promoting activity in the spinal cord3. To determine the role of sAC in netrin-1–induced axonal outgrowth, we next examined the effects of sAC inhibitors on axonal outgrowth of cultured E15 DSC explants. DIV 4 explants were incubated with either vehicle, netrin (75 ng ml−1) or PACAP (10 nM), and changes in axon length were measured after 3 h. Netrin-1 treatment resulted in a marked increase in axonal growth relative to that in vehicle-treated explants (Fig. 8a). As shown in a previous study11, netrin-1–induced axon outgrowth in DSC explant cultures was dependent on DCC, as it was blocked by a DCC-specific antibody (Supplementary Fig. 6 online). Netrin-1–induced axonal outgrowth was blocked by KH7 but not KH7.15 (Fig. 8a). The effects of KH7 seem to be specific, as axonal elongation elicited by PACAP was not affected by KH7 (Fig. 8a).
Figure 8.
Netrin-1–induced axonal outgrowth is blocked by sAC inhibitors. (a) E15 DSC explants cultured on PDL/fibronectin substratum (DIV 4) were pretreated with the indicated drug for 30 min, and axon outgrowth was measured at 0 h and 3 h after treatment with netrin-1 (75 ng ml−1), PACAP (1 nM) or vehicle. Netrin-1–induced axon outgrowth was blocked by pretreatment with KH7 (3 μM), but not by KH7.15 (3 μM). PACAP-induced axon outgrowth was not blocked by pretreatment with KH7. n = 36 spinal cord axons. *P < 0.01, **P value < 0.001. (b–g) E15 DSC explants were cultured in collagen gels and axonal outgrowth was elicited axons. *P < 0.01, **P value < 0.001. (b–g) E15 DSC explants were cultured in collagen gels and axonal outgrowth was elicited by treatment with netrin-1 (300 ng ml−1). Axon growth was measured at 20 h using anti-Tuj1 immunofluorescence to detect axons. (b,c) Images of DSC explant 20 h after the addition of vehicle and netrin-1. (d,e) Netrin-1–induced axon outgrowth was blocked by treatment with KH7 (30 μM), but not by KH7.15 (30 μM). Scale bar, 200 μm. (f,g) Quantification of axon bundles and axon length, per explant, for the conditions in b–e. n = 6 spinal explants from two different experiments. * P < 0.01. Error bars represent s.e.m.
We also examined axonal elongation in DSC explants prepared in collagen gels. Consistent with previous studies2,3, little axonal outgrowth was seen in the absence of netrin at 20 h (Fig. 8b). However, explants cultured in the presence of netrin-1 stimulated a marked increase in both the length of axons and the number of axon bundles, and this effect was blocked by KH7 but not KH7.15 (Fig. 8c–g). Together, these results support the idea that sAC activity is required for netrin-1–induced axonal elongation and outgrowth.
DISCUSSION
In this study we presented evidence that sAC is a component of the netrin-1 signaling pathway and is required for netrin-1–mediated effects on axon outgrowth and growth cone morphology. Netrin-1 directly increases cAMP levels6, which promotes its ability to elicit axonal outgrowth, growth cone elaboration and attractive turning7. However, the mechanisms by which cAMP is regulated in growth cones has remained elusive, in part because DCC, the netrin-1 receptor, does not have sequence motifs suggestive of a mechanism by which it could activate tmAC. sAC was originally identified as a testis-enriched soluble adenylyl cyclase that was evolutionarily distinct from canonical tmACs (ref. 39). We found that sAC is expressed in neurons and is found in axons and growth cones. sAC overexpression in neurons induces axon outgrowth and growth cone elaboration, consistent with the idea that sAC-generated cAMP can regulate the growth cone cytoskeleton. These morphologies are reminiscent of those in netrin-1–treated neurons. Furthermore, inhibition of sAC by pharmacologic agents or by siRNA results in substantially attenuated netrin-1 responses as measured by filopodial changes, which largely reflect Cdc42-dependent regulation of actin polymerization in filopodia40 and of growth cone size, which is affected by Rac1-dependent lamellipodia formation. Inhibition of sAC also blocks netrin-1–induced axonal outgrowth and elongation. Netrin-1 induces an increase in cAMP levels and this increase is blocked by inhibitors of sAC. This observation suggests that activation of DCC by netrin-1 leads to sAC activation, which promotes growth cone elaboration and axonal outgrowth. These findings identify sAC as an effector of netrin-1 and raise the possibility that sAC may be a component of other cAMP-dependent signaling pathways in neurons.
Although the effects of netrin-1 on developing axons are directly affected by intracellular cAMP levels, a specific pathway for netrin-1–induced cAMP elevation has been unclear. It has been proposed that netrin-1 can activate the adenosine A2B receptor32, a GPCR. However, subsequent experiments have suggested that the A2B receptor is not involved in physiologic netrin-1–dependent guidance events, such as midline attraction by commissural neurons, and A2B receptor antagonists do not affect netrin-1–mediated turning41. The present data indicate that sAC, rather than tmAC, is downstream of netrin-1 activation of DCC. The possibility that a tmAC contributes to netrin-1 outgrowth, perhaps via a GPCR coreceptor, seems unlikely as 2′,5′-ddAdo does not affect netrin-1–mediated morphologic responses. However, the ability of PACAP, a tmAC-activating neuropeptide, to induce morphologic effects similar to those of netrin-1, indicates that tmAC-derived cAMP can also regulate growth cone signaling, and signaling pathways that affect either class of cyclase could affect axonal guidance. Unlike previous reports6,32 and data presented here, a recent study42 did not detect netrin-1–induced increases in cAMP. These data were generated without the use of the phospho-diesterase inhibitor IBMX, which permits the detection of transient increases in cAMP and also permits localized increases in cAMP23 to accumulate to detectable levels. To date, a role for cAMP in regulating the responses to outgrowth factors and guidance cues in invertebrates such as Caernohabditis elegans and Drosophila has not been definitively established. However, in these organisms, no sAC orthologs have yet been identified43, indicating that if cAMP has similar effects on axon outgrowth, these effects may be mediated by tmACs.
Unique sAC isoforms expressed in the brain seem to be critical for netrin-1 signaling. Genetic deletion of amino terminal–encoding exons of the gene encoding sAC, Sacy, abrogates the expression of the sAC isoforms present in the testis22, but sAC isoforms detected in the brain by immunohistochemistry and immunoprecipitation seem to be unaffected in Sacy knockout mice (M.K., D.R.H., J.B. and L.R.L., unpublished data). Sacy undergoes considerable alternative splicing in somatic tissues44, and many of these somatic isoforms take advantage of unique start sites. Thus, sAC activity from alternatively spliced isoforms expressed in the brain may account for the absence of any observed neuronal phenotype in Sacy knockout mice21.
sAC may be regulated by netrin-1–induced calcium fluxes in growth cones. Recent studies have suggested that netrin-1, through its receptor DCC, leads to phospholipase C activation and subsequent release of inositol 1,4,5-trisphosphate receptor (IP3R)-gated calcium stores, which, through an unknown mechanism, leads to the opening of transient receptor potential (TRPC) channels45, resulting in sodium and calcium influx. The depolarization induced by TRPC possibly leads to the opening of voltage-dependent calcium channels (VDCCs)5,45. The specific targets of IP3R, TRPC or VDCC-gated calcium are unknown, but may include sAC, which is known to be activated by calcium26. The activation of sAC may be restricted by these specific calcium sources, resulting in localized increases in cAMP that may be positioned for mediating specific cytoskeletal effects, such as actin-based filopodial changes or microtubule-based changes in axonal elongation.
METHODS
Cell cultures and elaboration/outgrowth assays
Reagents were from Invitrogen, unless otherwise indicated. E15–16 rat DRG and E15 DSC explants were cultured as described46. Explants were plated on glass-bottomed culture dishes (MatTek) or glass coverslips precoated with 33 μg ml−1 poly-d-lysine (PDL, Fisher) and 1 μg ml−1 fibronectin (ICN). For elaboration assays, DRG explants were cultured in Neurobasal supplemented with 1× B27, 100 ng ml−1 nerve growth factor (NGF), 2 mM glutamine and 20 μM 5-fluorodeoxyuridine (5-FdU, ICN). DSC explants were cultured in minimum essential media (MEM) supplemented with 2% fetal bovine serum (FBS), 1× N2, 2 mM glutamine, 100 IU penicillin and 100 μg ml−1 streptomycin. E15–16 dissociated DRG neurons were prepared as described34. For outgrowth assays involving lentiviral infection, we plated dissociated DRGs in cloning cylinders of diameter 3 mm for 3 d at 37 °C in 5% CO2. After 16 h, cloning cylinders were removed and DRGs were infected with sAC or LacZ lentivirus. Outgrowth assays were performed on DIV 3. E18 hippocampal neurons were cultured as described46 and plated on glass coverslips precoated with 33 μg ml−1 poly-d-lysine and 1 μg ml−1 fibronectin. The hippocampal neurons were cultured in Neurobasal supplemented with 1× B27, 0.5 mM glutamine and 25 μM glutamic acid. DSC explants in collagen gel matrices were cultured as described28.
KH7 (ref. 28, 3 μM), KH7.15 (ref. 22, 3 μM), KT5720 (1 μM), 2-hydroxy-estadiol (5 μM), 2-methoxy-estadiol (5 μM), DCC antibody (1 μg ml−1; clone AF5, Calbiochem), 8-bromo-cAMP (3 μM), 2′5′dideoxyadenosine (50 μM) or PACAP 1-27 (10 nM, AnaSpec) were bath-applied 30 min before the application of 300 ng ml−1 netrin-1 (R&D, for DRG experiments), 75 ng ml−1 netrin-1 (for DSC experiments) or vehicle (phosphate-buffered saline and 0.1% bovine serum albumin), for 60 min in growth cone elaboration and outgrowth assays and for 3 h in axon outgrowth assays. Netrin-1 concentrations were chosen as the highest concentration that did not elicit a desensitization response in growth cone elaboration assays (Supplementary Fig. 7 online) and are consistent with concentrations used previously47. Phase-contrast images of the same growth cones were taken with a Nikon TE2000-U microscope with a CoolSnapHQ CCD camera at 0 min and 60 min. A growth cone was considered to have an increase in filopodial content if it had an increase of more than two filopodia after 60 min and these filopodia were longer than 10 μm. Values are presented as the percent of growth cones showing elaborated morphologic criteria. For the measurement of growth cone size, the phase-contrast images were thresholded to define the borders of the growth cones. The thresholded areas were quantified using the MetaMorph (Universal Imaging) region measurement program. The thresholded area of a growth cone at 0 min and 60 min was acquired, and values are presented as relative increase in growth cone size (Areat = 60/Areat = 0). For the measurement of axon outgrowth, phase-contrast images of the same axons were taken at 0 h and 3 h, and changes in axon length were quantified.
Two-tailed Student’s t-test was used for all statistical analysis. Error bars represent s.e.m. from a minimum of three independent experiments. Unless otherwise noted, all comparisons are between the vehicle and the netrin or PACAP samples for each condition.
Immunofluorescence
DRGs, DSC explants and hippocampal neurons were fixed and processed for immunofluorescence as described34. DRGs were immunostained with antibody to sAC (anti-sAC, clone R21, 1:50; clone R37, 1:100) and antibody to actin (clone I-19, 1:1,000, Santa Cruz). R21 sAC antibody was characterized previously23. DSC explants were immunostained with anti-sAC (R21), antibody to TAG-1 (anti–TAG-1, clone 4D7, 1:500) and antibody to Tau (1:500, Sigma). Hippocampal neurons were immunostained with anti-sAC (clone R37, 1:100) and antibody to Tuj1 (1:1,000, Chemicon). R37 sAC antibody was generated against a glutathione-S-transferase fusion of sAC, corresponding to the 50 kDa splice variant of human sAC, and epitope-mapped to aa 435–466. Secondary antibodies used were Alexa Fluor 488 and 546 (Molecular Probes).
Immunohistochemistry
E15 rat embryos were fixed by immersion for 30 min with 4% paraformaldehyde (PFA) in cytoskeleton buffer at 4 °C and cryopro-tected with 30% sucrose for 48 h at 4 °C. Embryos were embedded in optimal cutting temperature (OCT) reagent (TissueTek), and 20-μm cryostat sections were collected on subbed slides. After a secondary fixation with 4% PFA for 30 min, sections were permeabilized with 0.5% Triton X-100 and Tris-buffered saline for 10 min and quenched with 0.5% hydrogen peroxide for 5 min. Sections were blocked in 1.5% goat serum for 30 min and then incubated with either anti-sAC (clone R37, 1:100) or anti–TAG-1 (clone 4D7, 1:100) antibody. The detection of anti-sAC and anti–TAG-1 were performed with a biotinylated secondary antibody (1:200, Santa Cruz) followed by streptavidin–horseradish peroxidase (HRP) complex using the ABC staining system (Santa Cruz).
sAC and cAMP accumulation assays
IgG and sAC (R37) antibodies (2 mg each) were crosslinked to 1 ml Affi-Gel 10 agarose (BioRad), followed by blocking in 0.1 M Tris-HCl buffer (pH 8.0) according to the manufacturer’s protocol. Mouse brains were homogenized and sonicated in NP-40 lysis buffer (50 mM Tris-HCl pH 7.5, 1% Nonidet P-40, 150 mM NaCl, protease inhibitor cocktail). Then high-speed supernatants were precleared over a mouse IgG Affi-Gel 10 agarose, then applied to either the IgG or sAC R37 mAb Affi-Gel 10 agarose. Resin was washed with 10 volumes of 100 mM Tris (pH 7.4), followed by 10 volumes of 100 mM Tris HCl (pH 7.4) and 0.1% NP-40. Protein was eluted with 100 mM glycine (pH 2.0) into neutralizing Tris buffer. Adenylyl cyclase activity in the eluates was measured in 50 mM Tris-HCl (pH 7.5), 5 mM MnCl2 and 2.5 mM ATP, with or without 50 μM KH7, in the presence of 0.5 mM IBMX, for 30 min at 30 °C. Because MgCl2 is required for forskolin-dependent activation of tmACs, experiments monitoring forskolin-activated cyclase activity in sAC immunoprecipitates used 5 mM MgCl2 in place of MnCl2. cAMP generated was quantitated using Correlate-EIA Direct cAMP Assay (Assay Designs).
Assays monitoring netrin-1–induced elevations in intracellular cAMP levels were performed with E15 dissociated DRG neurons plated in 24-well dishes at 3 × 106 cells ml−1. Cells were pretreated with various drugs for 15 min before the addition of 0.5 mM IBMX for 5 min. Netrin-1 was then applied for 15 min and the cells were lysed in 0.1 M HCl. cAMP levels were measured as above.
siRNA transfection
siRNA-mediated knockdown of sAC was achieved by transfecting either of two different siRNA oligonucleotides (sAC siRNA1 and sAC siRNA2) into E15–16 dissociated DRG neurons using GeneSilencer (Genlantis) as described previously33,34. sAC siRNA1 was directed against nts 692–719 of rat sAC (GenBank accession number AF081941), and sAC siRNA2 was directed against nts 900–920 of rat sAC. A commercially available Alexa 488–conjugated nontargeting siRNA was used as negative control (Qiagen). siRNA transfection was performed in dissociated DRG neurons at 1 DIV and outgrowth assays were performed after 48 h (3 DIV). The incorporation of siRNAs into DRGs was verified by fluorescence microscopy in neurons transfected with a nontargeting Alexa 488–conjugated siRNA (Supplementary Fig. 3). sAC expression in siRNA-transfected DRG neurons was detected by western blotting using the R21 sAC antibody (1:10,000)23. Protein concentration of lysates was determined using the BCA Protein Assay kit (Pierce), and equal amounts were loaded for SDS–polyacrylamide gel electrophoresis (SDS-PAGE).
Lentivirus production
Lentivirus production and tittering was performed using the Virapower Lentiviral production kit according to the directions of the manufacturer. Briefly, cDNAs encoding sAC (~50 kDa) or LacZ were cloned into the pLenti/D-TOPO vector (CMV promoter), and virus was generated in the 293FT viral packaging cell line. Equal titers of sAC and LacZ control virus were used in subsequent experiments. To verify expression, HEK293T cells were infected with equal amounts of LacZ or sAC virus for 48 h, and sAC and LacZ were detected by western blotting using an antibody to V5 (anti-V5, 1:1,000, Serotec).
Supplementary Material
ACKNOWLEDGMENTS
We thank L.J. Cox for his comments and suggestions. Supported by the US National Institutes of Health (NIH) MH066204 and the Christopher Reeve Paralysis Foundation (S.R.J.); NIH GM07739 and the Barbara and Stephen Friedman Fellowship Endowment (J.H.Z.); NIH 5T32 CA062948 (D.R.H.); NIH 2T32 DA07274 (M.K.); NIH HD38722, the American Diabetes Association and the Hirschl Weill-Caulier Trust (L.R.L.); and NIH HD42060, GM62328 and the Ellison Medical Foundation (J.B.).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Song H, Poo M. The cell biology of neuronal navigation. Nat. Cell Biol. 2001;3:E81–E88. doi: 10.1038/35060164. [DOI] [PubMed] [Google Scholar]
- 2.Serafini T, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 4.Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat. Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama M, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 6.Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 7.Hong K, et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 8.Metin C, Deleglise D, Serafini T, Kennedy TE, Tessier-Lavigne M. A role for netrin-1 in the guidance of cortical efferents. Development. 1997;124:5063–5074. doi: 10.1242/dev.124.24.5063. [DOI] [PubMed] [Google Scholar]
- 9.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 10.Seaman C, Anderson R, Emery B, Cooper HM. Localization of the netrin guidance receptor, DCC, in the developing peripheral and enteric nervous systems. Mech. Dev. 2001;103:173–175. doi: 10.1016/s0925-4773(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 11.Keino-Masu K, et al. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 12.Shekarabi M, Kennedy TE. The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell. Neurosci. 2002;19:1–17. doi: 10.1006/mcne.2001.1075. [DOI] [PubMed] [Google Scholar]
- 13.Shekarabi M, et al. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 2005;25:3132–3141. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ming GL, et al. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 15.Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer T, Filbin MT. A role for cAMP in regeneration of the adult mammalian CNS. J. Anat. 2004;204:49–55. doi: 10.1111/j.1469-7580.2004.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forte LR, Bylund DB, Zahler WL. Forskolin does not activate sperm adenylate cyclase. Mol. Pharmacol. 1983;24:42–47. [PubMed] [Google Scholar]
- 18.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinclair ML, et al. Specific expression of soluble adenylyl cyclase in male germ cells. Mol. Reprod. Dev. 2000;56:6–11. doi: 10.1002/(SICI)1098-2795(200005)56:1<6::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal BS, Conti M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J. Biol. Chem. 2001;276:31698–31708. doi: 10.1074/jbc.M011698200. [DOI] [PubMed] [Google Scholar]
- 21.Esposito G, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl. Acad. Sci. USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess KC, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zippin JH, et al. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 24.Braun T, Dods RF. Development of a Mn2+-sensitive, “soluble” adenylate cyclase in rat testis. Proc. Natl. Acad. Sci. USA. 1975;72:1097–1101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl. Acad. Sci. USA. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 27.Jackman A, Fitzgerald M. Development of peripheral hindlimb and central spinal cord innervation by subpopulations of dorsal root ganglion cells in the embryonic rat. J. Comp. Neurol. 2000;418:281–298. [PubMed] [Google Scholar]
- 28.Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- 29.Stessin AM, et al. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J. Biol. Chem. 2006;281:17253–17258. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su AI, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pastor-Soler N, et al. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corset V, et al. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi H, Yamashita T, Yoshikawa H, Tohyama M. Functional inhibition of the p75 receptor using a small interfering RNA. Biochem. Biophys. Res. Commun. 2003;301:804–809. doi: 10.1016/s0006-291x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 34.Wu KY, et al. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- 36.Guirland C, Buck KB, Gibney JA, DiCicco-Bloom E, Zheng JQ. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J. Neurosci. 2003;23:2274–2283. doi: 10.1523/JNEUROSCI.23-06-02274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson RA, et al. Isozyme-dependent sensitivity of adenylyl cyclases to P-site-mediated inhibition by adenine nucleosides and nucleoside 3′-polyphosphates. J. Biol. Chem. 1997;272:8962–8966. doi: 10.1074/jbc.272.14.8962. [DOI] [PubMed] [Google Scholar]
- 38.Gille A, et al. Differential inhibition of adenylyl cyclase isoforms and soluble guanylyl cyclase by purine and pyrimidine nucleotides. J. Biol. Chem. 2004;279:19955–19969. doi: 10.1074/jbc.M312560200. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 40.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multi-molecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 41.Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001;291:1976–1982. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- 42.Moore SW, Kennedy TE. Protein kinase A regulates the sensitivity of spinal commissural axon turning to netrin-1 but does not switch between chemoattraction and chemorepulsion. J. Neurosci. 2006;26:2419–2423. doi: 10.1523/JNEUROSCI.5419-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roelofs J, Van Haastert PJ. Deducing the origin of soluble adenylyl cyclase, a gene lost in multiple lineages. Mol. Biol. Evol. 2002;19:2239–2246. doi: 10.1093/oxfordjournals.molbev.a004047. [DOI] [PubMed] [Google Scholar]
- 44.Geng W, et al. Cloning and characterization of the human soluble adenylyl cyclase. Am. J. Physiol. Cell Physiol. 2005;288:C1305–C1316. doi: 10.1152/ajpcell.00584.2004. [DOI] [PubMed] [Google Scholar]
- 45.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- 46.Banker G, Goslin K. Culturing Nerve Cells. 2nd edn MIT Press; Cambridge, MA: 1998. [Google Scholar]
- 47.de la Torre JR, et al. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.