Abstract
The Cohort Consortium Vitamin D Polling Project of Rarer Cancers (VDPP ) study failed to find a beneficial role of prediagnostic serum 25-hydroxyvitamin D [25(OH)D] levels on risk of seven types of rarer cancer: endometrial, esophageal, gastric, kidney, ovarian and pancreatic cancer and non-Hodgkin's lymphoma (NHL). However, ecological studies and studies of oral vitamin D intake have generally found solar ultraviolet B (UVB) and oral vitamin D inversely correlated with incidence and/or mortality rates of these cancers. To explore the discrepancy, I conducted an ecological study of cancer mortality rates for white Americans in the United States for 1950–1994 with data for 503 state economic areas in multiple linear regression analyses with respect to UVB for July, lung cancer, alcohol consumption and urban/rural residence. UVB was significantly inversely correlated with six types of cancer (not pancreatic cancer) in both periods. However, the adjusted R2 values were much lower for cancers with lower mortality rates than those in an earlier ecological study that used state-averaged data. This finding suggests that the VDPP study may have had too few cases. Thus, the VDPP study should not be considered as providing strong evidence against the solar UVB-vitamin D-cancer hypothesis.
Key words: ecological study, cancer, ultraviolet-B, vitamin D, smoking, alcohol consumption, smoking
A recent pooled analysis of prediagnostic serum 25-hydroxyvitamin D [25(OH)D] levels and cancer incidence in the Cohort Consortium Vitamin D Polling Project of Rarer Cancers (VDPP) study failed to find a beneficial role in reducing the risk of any of seven types of rarer cancer: endometrial, esophageal, gastric, kidney, ovarian and pancreatic cancer and non-Hodgkin's lymphoma (NHL).1 However, considerable evidence exists, largely from ecological studies, that solar ultraviolet-B (UVB) irradiance and vitamin D reduce the risk of incidence and/or death from many types of cancer, including those that the VDPP study addressed. Ecological studies finding inverse correlations between indices for solar UVB and the rarer types of cancer as well as breast, colon and rectal cancer are listed in Table 1. Because similar findings have emerged for several different populations and no effect of solar UVB other than vitamin D production has been proposed, the ecological studies strongly support the UVB-vitamin D-cancer hypothesis.37–39 No other factor other than vitamin D production has been proposed to explain the results of many ecological studies. In addition to many ecological studies, several good observational studies of prediagnostic serum 25(OH)D level and incidence for breast and colorectal cancer have developed dose-response relations.40–43 Also, a recent observational study from Finland found a marginally insignificant inverse correlation between prediagnostic serum 25(OH)D and ovarian cancer incidence in combination with calcium.44 Observational evidence supporting UVB irradiance in reducing the risk of NHL is also part of the literature.45 A few observational studies of beneficial effects for oral intake of vitamin D for the rarer cancers also exist: endometrial,46 esophageal,47 kidney,48 ovarian,49 pancreatic cancer50,51 and NHL.52 No factor associated with solar UVB irradiance other than vitamin D has been suggested as a cancer risk-reduction factor, although solar radiation regulation of melatonin production seems to be involved in breast cancer, with benefits occurring largely in winter when there are fewer hours of sunlight.53
Table 1.
Evidence from ecological studies and one cohort study that solar UVB reduces the risk of the seven rarer types of cancer as well as breast, colon and rectal cancer.
| Cancer | United States, ecological | United States, cohort | Europe | Australia | Asia | Multicontinent |
| Breast | Refs. 2–10 | Refs. 11–13 | Ref. 14 | Ref. 15 | ||
| Colon | Refs. 3–7, 10, 16 | Ref. 17 | Refs. 12, 13, 18 | Refs. 14,* 19, 20 | Refs. 21, 22* | |
| Endometrial | Refs. 3, 6, 7, 9 | Refs. 23 | Ref. 24 | |||
| Esophageal | Refs. 3, 5–7, 9, 10 | Ref. 17 | Refs. 13 | Refs. 14, 19 | ||
| Gastric | Refs. 3, 5–7, 9 | Ref. 17* | Refs. 13 | Refs. 14, 19 | Ref. 22* | |
| Kidney | Refs. 3, 6, 7 | Ref. 17* | Refs. 18 | Refs. 21, 22,* 25 | ||
| NHL | Refs. 3, 6, 7, 26, 27 | Ref. 17* | Refs. 11, 12, 28 | Ref. 29 | Ref. 21 | |
| Ovarian | Refs. 3, 4, 6, 7, 9, 30, 31 | Refs. 12, 13 | Refs. 21, 22,* 32 | |||
| Pancreatic | Refs. 3, 6, 7, 9 | Ref. 17 | Refs. 12, 13 | Ref. 33 | Refs. 14, 34, 35 | Refs. 22,* 36 |
| Rectal | Refs. 3, 5–7, 9, 10 | Ref. 17 | Refs. 12, 18 | Refs. 14,* 19 | Ref. 22* |
*, Marginally insignificant, requires further study to confirm.
One reason why the VDPP study may have failed to find a beneficial effect of serum 25(OH)D level is that the study included very few cases with higher serum 25(OH)D levels; the 95% confidence intervals of the odds ratios were much larger than the anticipated risk reduction. Inspection of Figure 1 in reference 1 indicates that if lines were drawn across the graphs starting with the upper limit of the error bar for 25(OH)D <25 nmol/L and keeping the line within the error bars, there was generally at least a 50% reduction for >100 nmol/L compared to the value at <25 nmol/L.
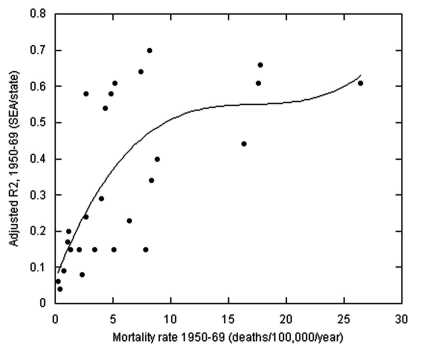
Figure 1.
Plot of the ratios of adjusted R2 for the regressions for mortality rates for various cancers for state economic area-averaged data to state-averaged data vs. the mortality rate for each cancer. A third order fit to the data is shown.
One can investigate the effect of number of cases by using the cancer mortality rate data for the United States from two periods, 1950–1969 and 1970–1994,54 employing either the state-averaged data6 or the state economic area (SEA) data, which include more than 500 geographical units comprised of one or more counties. One can use solar UVB doses along with the additional risk-modifying factors smoking, alcohol consumption and urban/rural residence, as well as being mindful of including those with Hispanic heritage in the category “white Americans.”6 The advantage of using SEA data is that the UVB dose and lung cancer mortality rate data, used as the index for the health effects of smoking, are more tightly linked to the populations in the cancer mortality rate data set than for state-averaged data.
Results
The results of the multilinear regression analyses are given in Tables 2 and 3. For 1950–1969, 12 types of cancer were found to have both a reasonably high adjusted R2 value and a reasonably high normalized correlation coefficient: bladder, breast, colon, endometrial, esophageal, gastric, kidney, laryngeal, oral (males), ovarian, prostate and rectal. For 1970–1994, those 12 types of cancer plus gallbladder and vulvar cancer and NHL met the criteria. Sometimes, such as for thyroid cancer, determining whether UVB was associated with reduced risk was difficult since the mortality rate was so low.
Table 2.
Results of the regression analyses for cancer mortality rate data by state economic area for 1950–1960
| Cancer | Sex | Mortality rate, US average** | UVB dose (β, p) | Lung cancer (β, p) | Alcohol consumption (β, p) | Urban residence (β, p) | Adjusted R2, p |
| All less lung | M | 145.21 | −0.53* | 0.54* | 0.16* | 0.12, 0.003 | 0.66* |
| F | 130.37 | −0.72* | 0.30* | 0.07, 0.009 | 0.20* | 0.67* | |
| Bladder | M | 7.38 | −0.41* | 0.48* | 0.25* | 0.18* | 0.64* |
| F | 2.64 | −0.36* | 0.36* | 0.008, 0.90 | 0.09, 0.11 | 0.24* | |
| Breast | F | 26.43 | −0.59* | 0.17* | 0.20* | 0.23* | 0.61* |
| Cervical | F | 7.87 | −0.08, 0.08 | 0.34* | −0.32* | −0.02, 0.80 | 0.15* |
| Colon | M | 17.79 | −0.65* | 0.33* | 0.05, 0.22 | 0.32* | 0.66* |
| F | 14.97 | −0.67* | 0.17* | 0.06, 0.15 | 0.29* | 0.61* | |
| Endometrial | F | 6.46 | −0.51* | 0.04, 0.34 | −0.32* | 0.40* | 0.23* |
| Esophageal | M | 4.34 | −0.36* | 0.55* | 0.19* | 0.10, 0.03 | 0.54* |
| F | 1.24 | −0.002, 0.96 | 0.42* | −0.12, 0.005 | 0.16* | ||
| Gastric*** | M | 16.34 | −0.47* | 0, 0.99 | 0.33* | −0.09, 0.12 | 0.44* |
| F | 8.37 | −0.44* | −0.04, 0.38 | 0.29* | −0.15, 0.02 | 0.34* | |
| Hodgkin's lymphoma | M | 2.33 | −0.30* | 0.07, 0.14 | −0.03, 0.68 | 0.04, 0.50 | 0.08* |
| F | 1.36 | −0.30* | 0.09, 0.05 | 0.21, 0.002 | −0.10, 0.11 | 0.15* | |
| Laryngeal | M | 2.67 | −0.14* | 0.75* | −0.01, 0.78 | 0.06, 0.17 | 0.58* |
| F | 0.25 | 0.05, 0.27 | 0.26* | 0.02, 0.76 | −0.09, 0.17 | 0.06* | |
| Melanoma | M | 1.57 | 0.47* | 0.24* | −0.19, 0.04 | 0.05, 0.36 | 0.37* |
| F | 1.13 | 0.46* | 0.18* | −0.21* | 0.07, 0.23 | 0.34* | |
| Multiple myeloma | M | 1.84 | −0.12, 0.01 | 0.10, 0.03 | 0.11, 0.10 | −0.17, 0.009 | 0.03* |
| F | 1.30 | −0.17, 0.001 | 0.13, 0.004 | 0.09, 0.19 | −0.11, 0.01 | 0.04* | |
| NHL | M | 5.05 | −0.31* | 0.20* | 0.02, 0.70 | 0.13, 0.04 | 0.15* |
| F | 3.38 | −0.31* | 0.06, 0.16 | 0.20* | 0.14* | ||
| NMSC | M | 1.17 | 0.34* | 0.13, 0.001 | −0.41* | 0.04, 0.45 | 0.35* |
| F | 0.43 | 0.21* | −0.04, 0.32 | −0.32* | −0.02, 0.80 | 0.26* | |
| Other oral and pharynx | M | 4.80 | −0.18* | 0.75* | −0.06, 0.16 | 0.10, 0.002 | 0.58* |
| F | 1.17 | 0.20* | 0.21* | −0.22, 0.001 | −0.14, 0.02 | 0.20* | |
| Ovarian | F | 8.84 | −0.50* | 0.11, 0.003 | 0.13, 0.02 | 0.21* | 0.40* |
| Pancreatic | M | 10.20 | 0.02, 0.70 | 0.51* | 0.10, 0.12 | −0.17, 0.003 | 0.25* |
| F | 6.26 | −0.14, 0.005 | 0.25* | 0.09, 0.19 | −0.09, 0.19 | 0.07* | |
| Prostate | M | 20.15 | −0.33* | 0.05, 0.25 | 0.07, 0.32 | −0.15, 0.02 | 0.12* |
| Rectal | M | 8.18 | −0.61* | −0.22* | 0.23* | 0.25* | 0.70* |
| F | 5.20 | −0.58* | 0.17* | 0.23* | 0.20* | 0.61* | |
| Renal | M | 3.99 | −0.35* | 0.18* | 0.26* | 0.04, 0.45 | 0.29* |
| F | 2.07 | −0.26* | 0.03, 0.45 | 0.31* | −0.22* | 0.15* | |
| Thyroid | M | 0.45 | −0.16, 0.001 | 0.04, 0.40 | −0.01, 0.85 | 0.17, 0.01 | 0.04* |
| F | 0.73 | −0.23* | 0.06, 0.16 | 0.12, 0.07 | 0.03, 0.67 | 0.09* |
M, male; F, female. *p < 0.001; **, deaths/100,000/year; ***, UVB dose limited to <7.5 kJ/m2; β, normalized regression coefficient.
Table 3.
Results of the regression analyses for cancer mortality rate data by state economic area for 1970–1994
| Cancer | Sex | Mortality rate, US average** | UVB dose (β, p) | Lung cancer (β, p) | Alcohol consumption (β, p) | Urban residence (β, p) | Adjusted R2, p |
| All less lung | M | 140.07 | −0.50* | 0.55* | 0.27* | 0.17* | 0.52* |
| F | 111.95 | −0.68* | 0.38* | 0.09, 0.005 | 0.20* | 0.67* | |
| Bladder | M | 6.56 | −0.51* | 0.33* | 0.31* | 0.21* | 0.47* |
| F | 1.87 | −0.37* | 0.45* | 0.09, 0.04 | 0.07, 0.09 | 0.36* | |
| Breast | F | 26.89 | −0.57* | 0.28* | 0.15* | 0.20* | 0.57* |
| Cervical | F | 3.22 | −0.10, 0.02 | 0.26* | −0.22* | −0.02, 0.76 | 0.09* |
| Colon | M | 20.13 | −0.65* | 0.32* | 0.06, 0.09 | 0.33* | 0.50* |
| F | 14.97 | −0.68* | 0.23* | −0.07, 008 | 0.23* | 0.50* | |
| Endometrial | F | 3.72 | −0.58* | −0.04, 0.26 | −0.05, 0.29 | 0.38* | 0.42* |
| Esophageal | M | 4.80 | −0.49* | 0.33* | 0.33* | 0.16* | 0.44* |
| F | 1.24 | −0.14* | 0.54* | 0.22* | 0.03, 0.48 | 0.42* | |
| Gallbladder | M | 0.57 | −0.28* | −0.16* | 0.01, 0.82 | 0.19* | 0.16* |
| F*** | 1.10 | −0.56* | −0.25* | −0.08, 0.12 | 0.14, 0.003 | 0.41* | |
| Gastric*** | M | 7.33 | −0.41* | −0.04, 0.46 | 0.14, 0.007 | 0.14, 0.009 | 0.33* |
| F | 3.41 | −0.29* | −0.001, 0.98 | 0.17, 0.003 | 0.10, 0.08 | 0.20* | |
| Hodgkin's | M | 1.10 | −0.29* | 0.01, 0.78 | −0.10, 0.07 | 0.08, 0.13 | 0.07* |
| F | 0.67 | −0.31* | 0.08, 0.06 | 0.05, 0.28 | 0.09* | ||
| Laryngeal | M | 2.49 | −0.20* | 0.67* | 0.14, 0.001 | 0.10, 0.02 | 0.41* |
| F | 0.42 | −0.09, 0.11 | 0.56* | 0.02, 0.67 | 0.05, 0.25 | 0.33* | |
| Leukemia | M | 8.80 | −0.18* | 0.11, 0.03 | −0.12, 0.02 | 0.15, 0.005 | 0.04* |
| F | 5.16 | −0.17* | −0.01, 0.82 | −0.14, 0.01 | 0.13, 0.01 | 0.03, 0.001 | |
| Melanoma | M | 2.96 | 0.42* | 0.28* | 0.02, 0.62 | 0.07, 0.14 | 0.27* |
| F | 1.61 | 0.39* | 0.13, 0.002 | −0.14, 0.001 | 0.18* | ||
| Multiple myeloma | M | 3.10 | −0.18* | −0.10, 0.03 | −0.05, 0.36 | −0.05, 0.34 | 0.04* |
| F | 2.08 | −0.22* | 0.008, 0.86 | 0.07, 0.18 | −0.05, 0.39 | 0.05* | |
| NHL | M | 7.03 | −0.41* | 0.11, 0.01 | 0.03, 0.55 | 0.17, 0.001 | 0.18* |
| F | 4.76 | −0.40* | 0.08, 0.05 | −0.06, 0.23 | 0.12, 0.02 | 0.15* | |
| NMSC | M | 1.17 | 0.37* | 0.47* | −0.02, 0.59 | −0.11, 0.009 | 0.44* |
| M*** | 1.17 | 0.36* | 0.41* | 0.04, 0.41 | −0.11, 0.01 | 0.46* | |
| F | 0.43 | 0.15, 0.001 | 0.23* | −0.16, 0.002 | −0.13, 0.01 | 0.12* | |
| F*** | 0.43 | 0.27* | 0.16, 0.001 | −0.13, 0.03 | −0.04, 0.44 | 0.16* | |
| Other oral and pharynx | M | 3.99 | −0.10, 0.007 | 0.61* | 0.24* | 0.10, 0.02 | 0.34* |
| F | 1.41 | 0.12, 0.001 | 0.60* | 0.16* | −0.10, 0.01 | 0.41* | |
| Ovarian | F | 8.38 | −0.59* | 0.08, 0.02 | 0.10, 0.02 | 0.22* | 0.43* |
| Pancreatic | M | 10.21 | 0 | 0.36* | 0.15, 0.005 | −0.11, 0.04 | 0.13 * |
| F | 6.84 | −0.05, 0.24 | 0.28* | 0.27* | 0.01, 0.77 | 0.13* | |
| Prostate | M | 22.01 | −0.21* | −0.17* | 0.30* | −0.23* | 0.18* |
| Rectal | M | 4.40 | −0.64* | 0.14* | 0.25* | 0.24* | 0.57* |
| F | 2.54 | −0.59* | 0.19* | 0.19,* | 0.24* | 0.57* | |
| Renal | M | 4.90 | −0.35* | 0.20* | 0.05, 0.34 | 0.07, 0.18 | 0.14* |
| F | 2.24 | −0.38* | 0.08, 0.06 | 0.04, 0.41 | −0.02, 0.66 | 0.15* | |
| Thyroid | M | 0.33 | −0.11, 0.02 | −0.11, 0.02 | −0.09, 0.09 | 0.11, 0.04 | 0.02* |
| F | 0.42 | −0.14, 0.002 | −0.08, 0.06 | 0.19* | 0.05* | ||
| Vulva | F | 0.34 | −0.43* | −0.05, 0.27 | 0.10, 0.01 | 0.20* |
M, male; F, female. *p < 0.001; **, deaths/100,000/year; ***, UVB dose limited to <7.5 kJ/m2; β, normalized regression coefficient.
One can compare the effects of using SEA data vs. state-averaged data by looking at the adjusted R2 vs. mortality rate for each type of cancer. The data were processed with nearly the same set of risk-modifying factors;6 the SEA data analysis did not include poverty, but it generally had little significance in the analyses, and it did not include Hispanic heritage, which was relevant only for endometrial, gallbladder, and rectal cancer and was dealt with by also running the analysis for UVB <7.5 kJ/m2 for those cancers. Figure 1 compares the two analyses for 1950–1969. The third order fit to the adjusted R2 vs. mortality rate data increases from an adjusted R2 value near 0.05 to near 0.8 for the SEA data and from near 0.4 to near 0.85 for the state-averaged data. The variation is due to the difference in mean number of deaths in each area.
An important difference between the two periods is that the adjusted R2 generally decreased in the second period compared with the first period, with the effect more pronounced for males than females.
Discussion
This analysis reconfirms the inverse correlations between solar UVB and mortality rates for about 15 types of cancer, depending on the criteria based on normalized regression coefficient and adjusted R2. This analysis also finds that smoking is correlated with nearly all types of cancer but is inversely correlated with gallbladder and prostate cancer. The direct correlation with lung cancer is consistent with the journal literature for many types of cancer.54,55 In addition, the index of smoking, lung cancer mortality rate, could also be in part an index of diet since a high-fat, low-vegetable diet is also a risk factor for lung cancer.56 The inverse correlation with prostate cancer could be due to the fact that prostate cancer mortality rates increase more rapidly than age-adjusted data normally account for. The finding for gallbladder cancer differs from the finding using state-averaged data and is not supported by the journal literature.57 The finding that alcohol consumption is also directly correlated with several cancers, including breast, esophageal, laryngeal and rectal cancer, is supported in the journal literature.58 However, for some cancers for which alcohol is also considered a risk factor, such as colon cancer, this analysis found no correlation.
The generally decreased inverse correlation between solar UVB and cancer mortality rates in the second period compared with the first period seems to be tied largely to changes in attitudes toward solar UV irradiance and increased use of sunscreen. Evidence in support of this hypothesis is that nonmelanoma skin cancer (NMSC) rates decreased from at least two sources. For one, NMSC mortality rates decreased from 1950–1954 to 1970–1974 and then increased for males but remained constant for females through the period 1990–1994.59 On the other hand, melanoma rates increased monotonically throughout the period for males and through 1985–1989 for females. Since death from NMSC is linked to integrated UVB irradiance,60 whereas sunburns and UVA without sunburn are the important risks for melanoma risk,61,62 these results are consistent with increased use of sunscreen and shifting from midday solar UV irradiance to morning or afternoon irradiance. A second line of evidence is that mean serum 25(OH)D levels have declined in the US on the basis of data from cross-sectional studies for two periods, 1988–1994 and 2001–2004.63 Although this finding is for a period later than those for the cancer data that this study used, this finding probably represents a continuation of a trend that began during the time of the cancer data. However, the fact that many people moved from the northeast to the Sunbelt starting in the 1960s could also have contributed to the reduced inverse correlation between solar UVB and cancer mortality rates. Some studies indicate that UVB irradiance early in life reduces the risk of cancer later in life, as for prostate cancer.64
Specific cancer risk factors other than UVB affect the geographical variation of cancer mortality rates in the US for several of the rarer cancers. Examples include pesticides (including herbicides) for kidney cancer65 and NHL,66 giving rise to high mortality rates in the upper Midwest; Helicobacter pylori infection for gastric cancer among immigrants from Mexico and Central America,67 giving rise to high rates in states near the US-Mexico border; smoking for pancreatic cancer;50,51 and oral contraceptives, which reduce the risk of endometrial and ovarian cancer but not breast cancer.68 Thus, such factors may explain in part why the correlations of some of the rarer types of cancer do not correlate as highly as others with breast, colon and rectal cancers.
Although an ecological study in 1990 identified prostate cancer as UVB- and vitamin D sensitive,69 later studies failed to find that prediagnostic serum 25(OH)D was inversely correlated with incidence rate.42,70,71 Prostate cancer differs from the generally accepted vitamin D-sensitive cancers in that the highest mortality rates are in the northwest and the lowest in the southeast.59 However, vitamin D increases survival for those diagnosed with prostate cancer.72 The features of the atlas of prostate cancer mortality rates in the US match those in the map of greatest ancestry by county in 2000.73 A multicountry ecological study found that prevalence of the apolipoprotein E ε4 allele, cereal/grain supply (inverse) and per capita gross domestic product were significantly correlated with prostate cancer mortality rates.74
Several reasons may explain why the VDPP study may not have observed an inverse correlation between prediagnostic serum 25(OH)D level and incidence of any of the seven rarer cancers. One is that a single serum 25(OH)D level measurement may not be representative of serum 25(OH)D levels related to cancer risk reduction, i.e., that the serum 25(OH)D level at the time when it could have most reduced the risk of cancer may not correspond to the time of serum draw and the serum 25(OH)D levels could have changed in time.75 It is noted that inverse correlations were much stronger for case-control studies with serum 25(OH)D level measurement made at time of diagnosis than for nested case-control studies with serum 25(OH)D level measurement made several years prior to cancer diagnosis (see, e.g., ref. 41). A second reason is that the study identified few cases for any cancer: from 516 for ovarian cancer to 1,353 cases to lymphoma.1 The 95% confidence interval was generally 0.3-0.5 for the various ranges of 25(OH)D level, which is generally larger than the expected effect. On the basis of an updated version of the analysis using a power law fit to the data in reference 43, going from 25 nmol/L to 75 nmol/L would reduce the odds ratio by 0.37 and 0.41 for breast and colorectal cancer incidence, respectively. From multiplying the standardized regression coefficients by the adjusted R2 for the various types of cancer in the ecological study for US mortality rate data in reference 6, it appears that the dose-response relations would range from 50% to 90% of those for breast and colorectal cancer. As seen in Figure 1, having few cases or deaths leads to large 95% confidence intervals. On the other hand, a recent nested case-control study of ovarian cancer incidence from Finland found an odds ratio = 0.54 (0.28–1.02) for the 30 cases with serum 25(OH)D levels >57.8 nmol/L vs. the 138 cases with 25(OH)D <57.8 nmol/L.44 Also, many of the observational studies of breast and colorectal cancer incidence had only a few hundred cases yet managed to find significant inverse correlations with respect to prediagnostic serum 25(OH)D levels,40–43 although more so for case-control studies than nested case-control studies (e.g., ref. 41). A third reason is that some of the important risk-modifying factors may not have been included, such as pesticide exposure, as discussed for kidney cancer65 and NHL.66 It is curious why the researchers in the VDPP study did not seek to validate their approach of pooling ten independent studies by verifying that the often repeated inverse correlation between serum 25(OH)D and breast or colorectal cancer. Doing so would have either exposed a weakness of the approach or provided a contrast to the results for the seven rarer types of cancer. In light of these concerns, the VDPP study should not be considered as providing strong evidence against the solar UVB-vitamin D-cancer hypothesis.
The fundamental question that this study addressed is whether the role of vitamin D in reducing risk for 15–20 types of cancer can be considered causal. The criteria for causality in a biological system outlined by A. Bradford Hill,76 and extended subsequently,77 are useful in doing so. This was done for cancer recently,78 which I briefly repeat here. The primary criteria and how they are largely satisfied is indicated in Table 4. Bias warrants further discussion. In 2008, the International Agency for Research on Cancer issued its Working Group Report on vitamin D and cancer.79 After reviewing 1,368 papers, the Working Group concluded that the evidence was convincing only for colorectal cancer and that for the two other most studied cancers, breast and prostate, as well as the minor cancers, the evidence was not convincing. Convincing observational evidence now exists for a beneficial role of vitamin D in reducing the risk of breast cancer.41–43 As explained in a critique of the IARC report, the Working Group consisted largely of dermatologists more concerned about protecting the public from UV irradiance in order to reduce the risk of NMSC and melanoma than in finding a beneficial role for solar UVB and vitamin D.80
Table 4.
How Hill's criteria for causality in a biological system are satisfied for solar UVB and vitamin D and cancer
| Factor | How satisfied | References |
| Strength of association | Ecological studies; observational studies of breast and colorectal cancer | 3–43 |
| Consistency | Ecological studies | 3–39 |
| Temporality | Observational studies | 17, 39–43 |
| Biological gradient | Dose-response for serum 25OHD for breast and colorectal cancer | 40–43 |
| Coherence (mechanisms) | Effects on cells, antiangiogenesis, antimetastasis, calcium absorption, immune system strengthening | 81–83 |
| Experiment | Randomized controlled trial | 85 |
| Analogy | Geographical variation of mortality rates for various cancers | 3–39 |
| Rule out confounding factors | Ecological studies | 3–39 |
| Avoid bias | IARC | 79, 80 |
The evidence is stronger for mortality rate than for incidence rate. In ecological studies that studied both, inverse correlations were generally higher for mortality rate than incidence rate for vitamin D sensitive cancers. In the United States, that was the case for other biliary, bladder, colon, esophageal (males), rectum and vulvar cancer.7 In China, that was the case in urban counties for all cancers, bladder, breast and lung cancer, with no difference for colorectal cancer.14 The likely reason is that many cancer risk factors affect cancer initiation but few agents affect angiogenesis and metastasis. Vitamin D reduces the risk of cancer by regulating cellular differentiation and apoptosis, progression by reducing angiogenesis around tumors and reducing metastasis.81–83
Although some researchers call for randomized, controlled trials (RCTs) to determine whether vitamin D can reduce the risk of cancer,79 one can argue that such trials are not required before public health policy can be changed since the ecological and case-control study evidence is very strong for breast and colorectal cancer and reasonably strong for several other cancers. In addition, conducting RCTs is becoming increasingly difficult with the widespread publicity on the health benefits of vitamin D, and other sources of vitamin D are available, including solar and artificial UVB, diet and supplements. A recent RCT on vitamin D among school children in Japan found a reduced risk of type A influenza but not type B influenza for those taking vitamin D supplements compared with those not taking supplements.84 Although RCTs investigating the effect of vitamin D on cancer risk85 (J.M. Lappe, personal communication) are ongoing, there does not appear to be any justification in delaying public-health policies regarding increasing vitamin D supplementation since the measure has many benefits and few adverse effects.86 However, those with granulomatous diseases and some with lymphoma might not want to exceed 2,000–4,000 IU/day and monitor their serum 1,25-dihydroxyvitamin D and calcium levels to guard against hypercalcemia.87
Despite the VDPP study's lack of confirmation of the UVB-vitamin D-cancer hypothesis, ample evidence from ecological and observational studies supports the hypothesis, and it satisfies the Hill criteria for causality in a biological system.76 People wanting to reduce their risk of cancer should consider increasing serum 25(OH)D levels to 40–80 ng/mL through a combination of UVB irradiance and supplements.88 Each 1,000 IU/day of vitamin D3 increases serum 25(OH)D levels by 6–10 ng/mL.85,89 Obtaining vitamin D from solar UVB irradiance is generally safe if done without sunburning. Few people experience harm from oral intake of vitamin D. Increasing country mean serum 25(OH)D levels to 40–45 ng/mL would reduce all-cause mortality rates by an estimated 15–20%.90–93
Data and Methods
The mortality rate data are from the website for the Atlas of Cancer Mortality Rates in the United States 1950–94.59 The data are age adjusted to the US population in 1990 for white Americans, which includes those of Hispanic heritage, for 1950–1969 and 1970–1994, averaged at the SEA. A total of 508 SEAs exist; however, this study omitted those for Alaska and Hawaii since the populations are low and serum 25(OH) D levels do not necessarily correspond to solar UVB doses. The analysis, in general, omits SEAs with zero values of mortality rate. The UVB data were determined at the surface from measurements made using the NASA satellite instrument Total Ozone Mapping Spectrometer (TOMS) for July 1992.94 This index is asymmetrical. The highest doses occur in the southwest and lowest doses in the northeast because of generally higher surface elevation in the western US and thinner stratospheric ozone layer in the southwest due to the prevailing westerly winds pushing up the tropopause height as the air masses cross the Rocky Mountains. Latitude in the US serves as the index for wintertime UVB dose because solar elevation angle becomes the most important factor then.95 The data were digitized to the center of population of each SEA, as discussed in Grant.3 Lung cancer is used as the index of the health effects of smoking, on the basis of findings of the strong correlation between lung cancer and rates of other cancers among African Americans.96 For 1950–1969, data on alcohol consumption (gallons of ethanol per capita of the drinking-age population) for 1960–1962 were obtained from Hyman and colleagues.97 Additional data, starting from 1970, were available from the National Institute of Alcohol Abuse and Alcoholism.98 The 1970 data were used for 1970–1994, in view of the fact that alcohol consumption rates vary slowly and the relative consumption rates stay relatively constant. Because men drink more alcohol than women, the findings for alcohol consumption for women are often not meaningful.
The cross-correlation coefficients for the four risk-modifying factors are given in Table 5. Only alcohol consumption and urban residence have significant overlap of concern. At times, including both factors in the analysis makes determining which is more important difficult; however, including both does not affect the outcome for either UVB or lung cancer. Sometimes alcohol was omitted for clarity.
Table 5.
Cross-correlation coefficients for the factors used in this study
| Factor | Lung cancer M (β, adjusted R, p) | Lung cancer F (β, adjusted R, p) | Urban residence (β, adjusted R, p) | UVB dose (β, adjusted R, p) |
| 1950–1969 | ||||
| Alcohol | 0.14, 0.02, 0.002 | 0.21, 0.04* | 0.71, 0.51* | −0.30, 0.09* |
| Lung, M | 0.71, 0.51* | 0.21, 0.04* | 0.14, 0.02, 0.002 | |
| Lung, F | 0.26, 0.07* | 0.19, 0.03* | ||
| Urban | ȡ0.006, 0.00, 0.88 | |||
| 1970–1994 | ||||
| Alcohol | −0.30, 0.09* | 0.22, 0.05* | 0.54, 0.29* | −0.15, 0.02, 0.001 |
| Lung, M | 0.59, 0.35* | −0.27, 0.07* | 0.12, 0.01, 0.006 | |
| Lung, F | 0.16, 0.02* | 0.09, 0.006, 0.05 | ||
| Urban | 0.07, 0.001, 0.11 |
M, male; F, female.
p < 0.001; β, normalized regression coefficient.
Since the category “white Americans” included those with Hispanic heritage, types of cancer for which Mexicans and Central Americans have higher incidence rates than other white Americans are elevated near the US-Mexico border. Such cancers include gallbladder and gastric cancer.99 Although obtaining data on the fraction of the population with Hispanic heritage at the state level is easy, doing so at the SEA level is harder. Thus, where lack of such data might affect the outcome, the UVB doses were restricted to those with 7.5 kJ/m2 or less.
In this work, it is assumed that optimal serum 25(OH)D level is greater than 40 ng/mL. That is the value above which incidence rates for breast and colorectal cancer decrease at a reasonably slow rate.43 It is also the value “found in humans living naturally in a sun-rich environment.”100 The value of 40–45 ng/mL was used in calculation of health benefits for western Europe,90 the United States,91 Canada92 and the Netherlands.93 This value is higher than the 30–32 ng/mL stated in other recent reviews.101,102 They define mild insufficiency in the range of 20–30 ng/mL and deficiency <20 ng/mL.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/13812
Note
The Institute of Medicine issued new guidelines on dietary vitamin D and calcium on November 30, 2010.103,104 These guidelines are based solely on demonstrated benefits for bones and recommend 600 IU/d of vitamin D for those aged 3 to 70 years and 800 IU/d for those aged 71+ years. The committee was restrained by the federal agency sponsors to consider only RCTs and prospective studies with a time lag between serum 25(OH)D measurement and disease outcome and to ignore ecological and other studies using solar UVB indices of vitamin D production as well as casecontrol studies in which serum 25(OH)D level was measured at the time of disease diagnosis. This report was prepared behind closed doors, overlooks much of the strong evidence of beneficial effects of vitamin D for 100 types of disease, and has set the public understanding of vitamin D back five years.
Financial Disclosure
I receive or have received funding from the UV Foundation (McLean, VA), the Sunlight Research Forum (Veldhoven), Bio- Tech-Pharmacal (Fayetteville, AR), and the Vitamin D Council (San Luis Obispo, CA), and the Danish Sunbed Federation (Middelfart).
References
- 1.Helzlsouer KJ. For the VDPP Steering Committee. Overview of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epi. 2010;172:4–9. doi: 10.1093/aje/kwq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 3.Grant WB. An estimate of premature cancer mortality in the US due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DM, Dosemeci M, McGlynn K. Sunlight and mortality from breast, ovarian, colon, prostate and non-melanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med. 2002;59:257–262. doi: 10.1136/oem.59.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant WB. Lower vitamin-D production from solar ultraviolet-B irradiance may explain some differences in cancer survival rates. J Natl Med Assoc. 2006;98:357–364. [PMC free article] [PubMed] [Google Scholar]
- 6.Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26:2687–2699. [PubMed] [Google Scholar]
- 7.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States 1993-2000. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John EM, Schwartz GG, Koo J, Wang W, Ingles SA. Sun exposure, vitamin D receptor gene polymorphisms and breast cancer risk in a multiethnic population. Am J Epidemiol. 2007;166:1409–1419. doi: 10.1093/aje/kwm259. [DOI] [PubMed] [Google Scholar]
- 9.Grant WB. An ecological study of cancer mortality rates including indices for dietary iron and zinc. Anticancer Res. 2008;28:1955–1963. [PubMed] [Google Scholar]
- 10.Grant WB. Air pollution in relation to US cancer mortality rates: An ecological study; likely role of carbonaceous aerosols and polycyclic aromatic hydrocarbons. Anticancer Res. 2009;29:3537–3545. [PubMed] [Google Scholar]
- 11.Grant WB. An ecologic study of dietary and solar ultraviolet-B links to breast carcinoma mortality rates. Cancer. 2002;94:272–281. doi: 10.1002/cncr.10196. [DOI] [PubMed] [Google Scholar]
- 12.Grant WB. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003;164:371–377. doi: 10.1007/978-3-642-55580-0_27. [DOI] [PubMed] [Google Scholar]
- 13.Grant WB. An ecologic study of cancer mortality rates in Spain with respect to indices of solar UV irradiance and smoking. Int J Cancer. 2007;120:1123–1127. doi: 10.1002/ijc.22386. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Clements M, Rahman B, Zhang S, Qiao Y, Armstrong BK. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control. 2010;21:1701–1709. doi: 10.1007/s10552-010-9599-1. [DOI] [PubMed] [Google Scholar]
- 15.Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC. Relationship between low ultraviolet B irradiance and higher breast cancer risk in 107 countries. Breast J. 2008;141:255–260. doi: 10.1111/j.1524-4741.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- 16.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. JNCI. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 18.Bidoli E, Franceschi S, Dal Maso L, Guarneri S, Barbone F. Cancer mortality by urbanization and altitude in a limited area in Northeastern Italy. Rev Epidemiol Sante Publique. 1993;41:374–382. [PubMed] [Google Scholar]
- 19.Mizoue T. Ecological study of solar radiation and cancer mortality in Japan. Health Phys. 2004;87:532–538. doi: 10.1097/01.hp.0000137179.03423.0b. [DOI] [PubMed] [Google Scholar]
- 20.Grant WB. Does Solar Ultraviolet Irradiation affect Cancer Mortality Rates in China? Asian Pac J Cancer Prev. 2007;8:236–242. [PubMed] [Google Scholar]
- 21.Grant WB. The likely role of vitamin D from solar ultraviolet-B irradiance in increasing cancer survival. Anticancer Res. 2006;26:605–614. [PubMed] [Google Scholar]
- 22.Tuohimaa P, Pukkala E, Scelo G, Olsen JH, Brewster DH, Hemminki K, et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: Vitamin D as a possible explanation. Eur J Cancer. 2007;43:1701–1712. doi: 10.1016/j.ejca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Epstein E, Lindqvist PG, Geppert B, Olsson H. A population-based cohort study on sun habits and endometrial cancer. Br J Cancer. 2009;101:537–540. doi: 10.1038/sj.bjc.6605149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC. Is ultraviolet B irradiance inversely associated with incidence rates of endometrial cancer: an ecological study of 107 countries. Prev Med. 2007;45:327–331. doi: 10.1016/j.ypmed.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Mohr SB, Gorham ED, Garland CF, Grant WB, Garland FC. Are low ultraviolet B and high animal protein intake associated with risk of renal cancer? Int J Cancer. 2006;119:2705–2709. doi: 10.1002/ijc.22213. [DOI] [PubMed] [Google Scholar]
- 26.Freedman DM, Zahm SH, Dosemeci M. Residential and occupational exposure to sunlight and mortality from non-Hodgkin's lymphoma: composite (threefold) case-control study. BMJ. 1997;314:1451–1455. doi: 10.1136/bmj.314.7092.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu S, Ma F, Collado-Mesa F, Kirsner RS. Ultraviolet radiation and incidence of non-Hodgkin's lymphoma among Hispanics in the United States. Cancer Epidemiol Biomarkers Prev. 2004;13:59–64. doi: 10.1158/1055-9965.epi-03-0187. [DOI] [PubMed] [Google Scholar]
- 28.Smedby KE, Hjalgrim H, Melbye M, Torrang A, Rostgaard K, Munksgaard L, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97:199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 29.Hughes AM, Armstrong BK, Vajdic CM, Turner J, Grulich AE, Fritschi L, et al. Sun exposure may protect against non-Hodgkin lymphoma: a case-control study. Int J Cancer. 2004;112:865–871. doi: 10.1002/ijc.20470. [DOI] [PubMed] [Google Scholar]
- 30.Bakhru A, Mallinger JB, Buckanovich RJ, Griggs JJ. Casting light on 25-hydroxyvitamin D deficiency in ovarian cancer: A study from the NHANES. Gynecol Oncol. 2010;119:314–318. doi: 10.1016/j.ygyno.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Lefkowitz ES, Garland CF. Sunlight, vitamin D and ovarian cancer mortality rates in US women. Int J Epidemiol. 1994;23:1133–1136. doi: 10.1093/ije/23.6.1133. [DOI] [PubMed] [Google Scholar]
- 32.Garland CF, Mohr SB, Gorham ED, Grant WB, Garland FC. Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer. Am J Prev Med. 2006;31:512–514. doi: 10.1016/j.amepre.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Neale RE, Youlden DR, Krnjacki L, Kimlin MG, van der Pols JC. Latitude variation in pancreatic cancer mortality in Australia. Pancreas. 2009;38:387–390. doi: 10.1097/MPA.0b013e31819975f4. [DOI] [PubMed] [Google Scholar]
- 34.Kato I, Tajima K, Kuroishi T, Tominaga S. Latitude and pancreatic cancer. Jpn J Clin Oncol. 1985;15:403–413. [PubMed] [Google Scholar]
- 35.Kinoshita S, Wagatsuma Y, Okada M. Geographical distribution for malignant neoplasm of the pancreas in relation to selected climatic factors in Japan. Int J Health Geogr. 2007;6:34. doi: 10.1186/1476-072X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC. Ultraviolet B irradiance and vitamin D status are inversely associated with incidence rates of pancreatic cancer worldwide. 2010;39:669–674. doi: 10.1097/MPA.0b013e3181ce654d. [DOI] [PubMed] [Google Scholar]
- 37.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohr SB. A brief history of vitamin D and cancer prevention. Ann Epidemiol. 2009;19:79–83. doi: 10.1016/j.annepidem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Grant WB, Mohr SB. Ecological studies of ultraviolet B, vitamin D and cancer since 2000. Ann Epidemiol. 2009;19:446–454. doi: 10.1016/j.annepidem.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30:113–125. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 41.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: Serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46:2196–2205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 42.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2010 doi: 10.1002/ijc.25439. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 43.Grant WB. Relation between prediagnostic serum 25-hydroxyvitamin D level and incidence of breast, colorectal and other cancers. J Photochem Photobiol B. 2010;101:130–136. doi: 10.1016/j.jphotobiol.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Toriola AT, Surcel HM, Calypse A, Grankvist K, Tuohimaa P, Toniolo P, et al. Independent and joint effects of serum 25-hydroxyvitamin D and calcium on ovarian cancer risk: A prospective nested case-control study. Eur J Cancer. 2010;46:2799–2805. doi: 10.1016/j.ejca.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Kricker A, Armstrong BK, Hughes AM, Goumas C, Smedby KE, Zheng T, et al. Personal sun exposure and risk of non Hodgkin lymphoma: A pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122:144–154. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 46.Salazar-Martinez E, Lazcano-Ponce E, Sanchez-Zamorano LM, Gonzalez-Lira G, Escudero DE, Los Rios P, Hernandez-Avila M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int J Gynecol Cancer. 2005;15:938–945. doi: 10.1111/j.1525-1438.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 47.Launoy G, Milan C, Day NE, Pienkowski MP, Gignoux M, Faivre J. Diet and squamous-cell cancer of the oesophagus: a French multicentre case-control study. Int J Cancer. 1998;76:7–12. doi: 10.1002/(sici)1097-0215(19980330)76:1<7::aid-ijc2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 48.Bosetti C, Scotti L, Maso LD, Talamini R, Montella M, Negri E, et al. Micronutrients and the risk of renal cell cancer: A case-control study from Italy. Int J Cancer. 2007;120:892–896. doi: 10.1002/ijc.22374. [DOI] [PubMed] [Google Scholar]
- 49.Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Hernandez-Avila M. Nutritional determinants of epithelial ovarian cancer risk: a case-control study in Mexico. Oncology. 2002;63:151–157. doi: 10.1159/000063814. [DOI] [PubMed] [Google Scholar]
- 50.Skinner HG, Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Vitamin D intake and the risk of pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prevention. 2006;15:1688–1695. doi: 10.1158/1055-9965.EPI-06-0206. [DOI] [PubMed] [Google Scholar]
- 51.Bao Y, Ng K, Wolpin BM, Michaud DS, Giovannucci E, Fuchs CS. Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer. 2010;102:1422–1427. doi: 10.1038/sj.bjc.6605658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polesel J, Talamini R, Montella M, Parpinel M, Dal Maso L, Crispo A, et al. Linoleic acid, vitamin D and other nutrient intakes in the risk of non-Hodgkin lymphoma: an Italian case-control study. Ann Oncol. 2006;17:713–718. doi: 10.1093/annonc/mdl054. [DOI] [PubMed] [Google Scholar]
- 53.Oh EY, Ansell C, Nawaz H, Yang CH, Wood PA, Hrushesky WJ. Global breast cancer seasonality. Breast Cancer Res Treat. 2010;123:233–243. doi: 10.1007/s10549-009-0676-7. [DOI] [PubMed] [Google Scholar]
- 54.Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene. 2002;21:7307–7325. doi: 10.1038/sj.onc.1205807. [DOI] [PubMed] [Google Scholar]
- 55.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45:3–9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 56.De Stefani E, Brennan P, Boffetta P, Mendilaharsu M, Deneo-Pellegrini H, Ronco A, et al. Diet and adenocarcinoma of the lung: a case-control study in Uruguay. Lung Cancer. 2002;35:43–51. doi: 10.1016/s0169-5002(01)00281-1. [DOI] [PubMed] [Google Scholar]
- 57.Yagyu K, Kikuchi S, Obata Y, Lin Y, Ishibashi T, Kurosawa M, et al. Cigarette smoking, alcohol drinking and the risk of gallbladder cancer death: a prospective cohort study in Japan. Int J Cancer. 2008;122:924–929. doi: 10.1002/ijc.23159. [DOI] [PubMed] [Google Scholar]
- 58.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30:38–41. [PMC free article] [PubMed] [Google Scholar]
- 59.Devesa SS, Grauman DJ, Blot WJ, Pennello GA, Hover RN, Fraumeni JF., Jr Atlas of Cancer Mortality in the United States 1950–1994. 1999. [July 7, 2010]. NIH Publication No. 99-4564. http://www3.cancer.gov/atlasplus/ new.html. [DOI] [PubMed]
- 60.English DR, Armstrong BK, Kricker A, Fleming C. Sunlight and cancer. Cancer Causes Control. 1997;8:271–283. doi: 10.1023/a:1018440801577. [DOI] [PubMed] [Google Scholar]
- 61.Gorham ED, Mohr SB, Garland CF, Chaplin G, Garland FC. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Ann Epidemiol. 2007;17:956–963. doi: 10.1016/j.annepidem.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Moan J, Porojnicu AC, Dahlback A. Ultraviolet radiation and malignant melanoma. Adv Exp Med Biol. 2008;624:104–116. doi: 10.1007/978-0-387-77574-6_9. [DOI] [PubMed] [Google Scholar]
- 63.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.John EM, Koo J, Schwartz GG. Sun exposure and prostate cancer risk: Evidence for a protective effect of early-life exposure. Cancer Epidemiol Biomarkers Prev. 2007;16:1283–1286. doi: 10.1158/1055-9965.EPI-06-1053. [DOI] [PubMed] [Google Scholar]
- 65.Hu J, Mao Y, White K. Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup Med (Lond) 2002;52:157–164. doi: 10.1093/occmed/52.3.157. [DOI] [PubMed] [Google Scholar]
- 66.Chiu BC, Weisenburger DD, Zahm SH, Cantor KP, Gapstur SM, Holmes F, et al. Agricultural pesticide use, familial cancer and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:525–531. [PubMed] [Google Scholar]
- 67.Travis PB, Goodman KJ, O'Rourke KM, Groves FD, Sinha D, Nicholas JS, et al. The association of drinking water quality and sewage disposal with Helicobacter pylori incidence in infants: the potential role of waterborne transmission. J Water Health. 2010;8:192–203. doi: 10.2166/wh.2009.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rice LW. Hormone prevention strategies for breast, endometrial and ovarian cancers. Gynecol Oncol. 2010;118:202–207. doi: 10.1016/j.ygyno.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–1311. [PubMed] [Google Scholar]
- 70.Gupta D, Lammersfeld CA, Trukova K, Lis CG. Vitamin D and prostate cancer risk: a review of the epidemiological literature. Prostate Cancer Prostatic Dis. 2009;12:215–226. doi: 10.1038/pcan.2009.7. [DOI] [PubMed] [Google Scholar]
- 71.Yin L, Raum E, Haug U, Arndt V, Brenner H. Metaanalysis of longitudinal studies: Serum vitamin D and prostate cancer risk. Cancer Epidemiol. 2009;33:435–445. doi: 10.1016/j.canep.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 72.Grant WB. Vitamin D may reduce prostate cancer metastasis by several mechanisms including blocking stat3. Am J Pathol. 2008;173:1589–1590. doi: 10.2353/ajpath.2008.080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brittingham A, de la Cruz GP US Dept. of Commerce CB, editor. Ancestry 2000. Census 2000 Brief CK2BR-35. Washington DC; 2004. [Google Scholar]
- 74.Grant WB. A multicountry ecological study of risk-modifying factors for prostate cancer: Apolipoprotein E ε4 as a risk factor and cereals as a risk reduction factor. Anticancer Res. 2010;30:189–199. [PubMed] [Google Scholar]
- 75.Grant WB. Re: “Overview of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epi. 2010;172:1210–1. doi: 10.1093/aje/kwq301. [DOI] [PubMed] [Google Scholar]
- 76.Hill AB. The Environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 77.Weed DL, Gorelic LS. The practice of causal inference in cancer epidemiology. Cancer Epidemiol Biomarkers Prev. 1996;5:303–311. [PubMed] [Google Scholar]
- 78.Grant WB. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer? An examination using Hill's criteria for causality. Dermato-Endocrinology. 2009;1:17–24. doi: 10.4161/derm.1.1.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.IARC Working Group Report 5: Vitamin D and Cancer. Lyon, France: IARC; 2008. Nov 25, [Google Scholar]
- 80.Grant WB. A critical review of Vitamin D and cancer: A report of the IARC Working Group on vitamin D. Dermato-Endocrinology. 2009;1:25–33. doi: 10.4161/derm.1.1.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008;24:139–149. doi: 10.1185/030079908x253519. [DOI] [PubMed] [Google Scholar]
- 82.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009;29:3687–3698. [PubMed] [Google Scholar]
- 83.Krishnan AV, Swami S, Feldman D. Vitamin D and breast cancer: inhibition of estrogen synthesis and signaling. J Steroid Biochem Mol Biol. 2010;121:343–346. doi: 10.1016/j.jsbmb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 85.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 86.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 87.Hewison M, Kantorovich V, Liker HR, Van Herle AJ, Cohan P, Zehnder D, et al. Vitamin D-mediated hypercalcemia in lymphoma: evidence for hormone production by tumor-adjacent macrophages. J Bone Miner Res. 2003;18:579–582. doi: 10.1359/jbmr.2003.18.3.579. [DOI] [PubMed] [Google Scholar]
- 88.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13:6–20. [PubMed] [Google Scholar]
- 89.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 90.Grant WB, Cross HS, Garland CF, Gorham ED, Moan J, Peterlik M, et al. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog Biophys Mol Biol. 2009;99:104–113. doi: 10.1016/j.pbiomolbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Grant WB. In defense of the sun: An estimate of changes in mortality rates in the United States if mean serum 25-hydroxyvitamin D levels were raised to 45 ng/mL by solar ultraviolet-B irradiance. Dermato-Endocrinology. 2009;1:207–214. doi: 10.4161/derm.1.4.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grant WB, Schwalfenberg GK, Genuis SJ, Whiting SJ. An estimate of the economic burden and premature deaths due to vitamin D deficiency in Canada. Molec Nutr Food Res. 2010;54:1127–1133. doi: 10.1002/mnfr.200900420. [DOI] [PubMed] [Google Scholar]
- 93.Grant WB, Schuitemaker G. Health benefits of higher serum 25-hydroxyvitamin D levels in The Netherlands. J Steroid Biochem Molec Biol. 2010;121:456–458. doi: 10.1016/j.jsbmb.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 94.Leffell DJ, Brash DE. Sunlight and skin cancer. [July 7, 2010];Sci Am. 1996 275:52–53. doi: 10.1038/scientificamerican0796-52. http://toms.gsfc.nasa.gov/ery_uv/dna_exp.gif. [DOI] [PubMed] [Google Scholar]
- 95.Grant WB. Hypothesis-Ultraviolet-B irradiance and vitamin D reduce the risk of viral infections and thus their sequelae, including autoimmune diseases and some cancers. Photochem Photobiol. 2008;84:356–365. doi: 10.1111/j.1751-1097.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- 96.Leistikow B. Lung cancer rates as an index of tobacco smoke exposures: validation against black male approximate non-lung cancer death rates 1969–2000. Prev Med. 2004;38:511–515. doi: 10.1016/j.ypmed.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 97.Hyman MM, Zimmermann MA, Gurioli C, Helrich A. Drinkers, Drinking and Alcohol-Related Mortality and Hospitalizations; A Statistical Compendium. 1980 Edition. New Burnswick NJ USA: Center of Alcohol Studies, Rutgers University; [Google Scholar]
- 98.Lakins NS, Williams GD, Li HY, Smothers BA. Surveillance Report #66: Apparent Per Capita Alcohol Consumption: National, State and Regional Trends 1977–2002. Washington, DC: NIAAA; 2004. [Google Scholar]
- 99.Canto MT, Chu KC. Annual cancer incidence rates for Hispanics in the United States: surveillance, epidemiology and end results 1992–1996. Cancer. 2000;88:2642–2652. doi: 10.1002/1097-0142(20000601)88:11<2642::aid-cncr29>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 100.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9:107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 101.Long AN, Ray MM, Nandikanti D, Bowman B, Khan A, Lamar K, et al. Prevalence of 25-hydroxyvitamin D deficiency in an urban general internal medicine academic practice. Tenn Med. 2010;103:51–52. [PubMed] [Google Scholar]
- 102.Holick MF. Vitamin D: Evolutionary, Physiological and Health Perspectives. Curr Drug Targets. 2010 doi: 10.2174/138945011793591635. In press. [DOI] [PubMed] [Google Scholar]
- 103.IOM (Institute of Medicine), author Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 104.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-2704. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]