Abstract
Within Galliformes, megapods (brush turkey, malleefowl, scrubfowl) exhibit unique forms of parental care and growth. Hatchlings receive no post-hatching parental care and exhibit the most exaggerated precocial development of all extant birds, hatching with fully developed, flight-capable forelimbs. Rather than flying up to safety, young birds preferentially employ wing-assisted incline running. Newly hatched Australian brush turkeys (Alectura lathami) are extraordinarily proficient at negotiating all textured inclined surfaces and can flap-walk up inclines exceeding the vertical. Yet, as brush turkeys grow, their forelimb-dependent locomotor performance declines. In an attempt to elucidate how hatchlings perform so well, we analysed hindlimb forces and forelimb kinematics. We measured ground reaction forces (GRFs) for animals spanning the entire growth range (110–2000 g) as they ascended a variably positioned inclined ramp that housed a forceplate. These data are compared with a similar dataset for a chukar partridge (Alectoris chukar) that exhibit a growth strategy typical of most other Galliformes and that demonstrate improved incline performance with increasing age. The brush turkeys' ontogenetic decline in incline running performance is accompanied by loss of traction at steep angles, reduced GRFs and increased wing-loading. We hypothesize that Australian brush turkeys, in contrast to other Galliformes, develop from forelimb-dominated young that exploit a variable terrain (e.g. mound nests, boulders, embankments, cliffs, bushes and trees) into hindlimb-dominated adults dependent on size and running speed to avoid predation.
Keywords: locomotor development, WAIR, over-ground performance, force-plate, precocial hatchlings
1. Introduction
Mammals and birds develop along a pronounced altricial to precocial axis. Altricial young (e.g. primates and songbirds) are born or hatch naked, with eyes closed, and are completely dependent on their parents for thermoregulation, feeding and protection. Juveniles of altricial species become independent only after they grow to near adult mass and have well-developed locomotor skills. In contrast, precocial species (e.g. ungulates and game birds) are born covered with hair or feathers, eyes open, thermally independent and ambulatory so that they can forage and flee danger. Members of the megapod family (Galliformes; e.g. Australian brush turkey, Alectura lathami, malleefowl, scrubfowl) of the Indo-Pacific region are at the extreme of the precocial spectrum in birds and, as such, are characterized as super-precocial [1]. Hatchlings emerge from the nest alone and completely independent of their parents, with wings fully functional and even capable of aerial flight [2]. No other avian clade exhibits such advanced hatchling development.
At birth, precocial young are confronted with immediate challenges, as they must escape predators, search for food and behaviourally thermoregulate. Locomotor capacity is therefore critical. However, the relatively small size and incomplete neuromuscular development in young animals is predicted to limit locomotor performance, even in precocial species [3]. Despite this paradox, quantitative studies of the ontogeny of locomotor performance are scarce. The limited available data demonstrate that among many precocial species, youngsters have similar or superior absolute escape performance when compared with adults (e.g. hares, Lepus [4,5]; lizards [6]; birds, Alectoris [7]. See [8] for review).
Despite excellent physiological and ecological studies (e.g. [2,9–13]), there are no quantitative investigations of megapod locomotor performance during growth. We found that Australian brush turkeys of all ages preferred to use wing-assisted incline running (WAIR; [14,15]), rather than flight, to reach an elevated refuge and to navigate their natural habitat. WAIR involves simultaneous use of running hindlimbs and flapping forelimbs [14,15]. Since WAIR requires less aerodynamic output than flight [16] and is exhibited by all ages of brush turkeys and other Galliformes, it offers a common metric of locomotor performance during avian development [7,14,15,17].
We quantified locomotor performance of Australian brush turkeys throughout development using the maximum angle ascended during bouts of WAIR and compared performance among age classes to measures of forelimb kinematics and hindlimb ground reaction forces (GRF). Many researchers have quantified GRF values for running on the level (e.g. [18–21]). However, studies of incline running are few and most involve studying animals ascending only shallow inclines (<15°) [22,23]. Here we study how brush turkeys negotiated inclines from horizontal to 110° (i.e. 20° past vertical).
2. Material and methods
Nine wild Australian brush turkeys were captured using hand nets 110 km northeast of Adelaide during January 2004. The youngest bird was captured the day it emerged from its nest mound. We estimated the ages (days post-hatching = dph) of the older birds (ranging from 116 to 1890 g) from growth curves described by Göth [24]. Every 3–5 days each animal was weighed and photographed with their wings outstretched against a gridded background in order to obtain wing length and wing area (analysed in ImageJ [25]) to calculate wing-loading (body mass divided by the surface area of both wings). Low wing-loading (large wings relative to body size) is generally associated with the ability to produce high aerodynamic forces relative to body weight, yet may be costly because of aerodynamic drag on the wings particularly at higher velocities. Every 2–3 days each bird was challenged to ascend a textured runway ramp outfitted with a forceplate flush with the ramp. The ramp was inclined over a broad array of angles including 0° (horizontal) and from 50° to 110° in 10° increments (figure 1). Each bird was tested over a two-week period (during January–February 2004). During that period the fastest growing individual was the youngest bird, which increased in mass by approximately 40 per cent (116–162 g). However, no birds demonstrated a change in performance ability during the two weeks, thus all data for each individual were pooled. After the experiment, all nine brush turkeys were released at their area of capture.
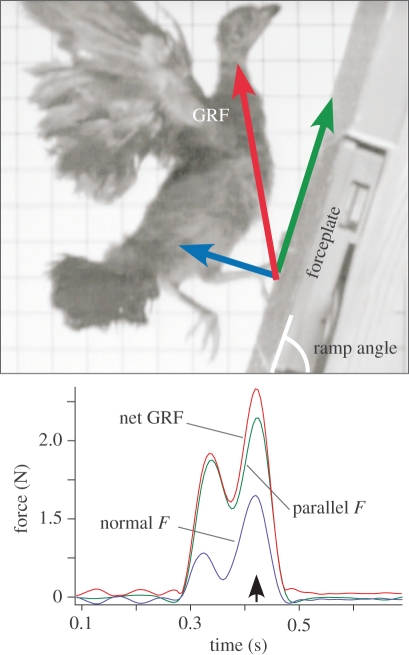
Figure 1.
Brush turkeys ran over a multi-axis forceplate that describes normal (blue arrow) and parallel (green arrow) forces. Bottom panel shows a representative force trace from a young brush turkey flap-running up a 70° ramp (pictured).
All brush turkeys readily performed WAIR when placed at the bottom of the inclined ramp (electronic supplementary material, video S1). Runs were recorded with a laterally placed high-speed digital video camera (250 Hz, Motionscope, Redlakes Inc.) and saved to digital videotape (mini-DV, Sony Camcorder). The ramp (15 cm wide by 2 m long) was covered in a 30-grit sandpaper covered with rubber carpet backing and fine hardware cloth in order to maximize traction. A 3-axis forceplate (15 × 15 cm, recording at 200 Hz via amplifier model PJB-101 to NetForce software, v. 2.2, AMTI, Watertown, MA, USA) was mounted flush with the ramp's surface at the midpoint. The forceplate top-sheet was also covered with sandpaper and hardware cloth.
The video served several functions. From it, we calculated wing beat frequency and stroke-plane angle (angle between the line swept by the wingtip at mid-downstroke and the global horizontal) for each run. We also scored each run as: (i) success (ascent without slipping), (ii) slipping (occasional foot slips while successfully ascending), (iii) treadmilling (running in place on the ramp with no forward progress), and (iv) failure (sliding backwards or abandoning the ramp) (table 1). Finally we used the video to confirm single foot-strikes on the forceplate.
Table 1.
Best performance for each bird on inclined substrates. Italic entries indicate successful ascents. Comparable behavioural data for Chukar available in Dial et al. [17].
| bird | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| mass (g) | 163 | 233 | 271 | 361 | 374 | 426 | 585 | 856 | 1861 |
| age (dph) | 2 | 43 | 56 | 82 | 84 | 99 | 138 | 180 | adult |
| category | hatchling | young | young | juvenile | juvenile | juvenile | sub-adult | sub-adult | adult |
| ramp angle | |||||||||
| 50 | walk/run | walk/run | walk/run | walk/run | walk/run | WAIR | WAIR | slip | slip |
| 60 | WAIR | WAIR | WAIR | WAIR | WAIR | WAIR | slip | slip | slip |
| 70 | WAIR | WAIR | WAIR | WAIR | WAIR | WAIR | slip | treadmill | treadmill |
| 80 | WAIR | WAIR | WAIR | WAIR | WAIR | WAIR | treadmill | treadmill | treadmill |
| 90 | WAIR | WAIR | WAIR | WAIR | WAIR | WAIR | treadmill | fail | fail |
| 100 | WAIR | slip | fail | fail | fail | fail | fail | fail | fail |
| 110 | WAIR | fail | fail | fail | fail | fail | fail | fail | fail |
Force plate recordings were filtered with a five-sample moving average and analysed in IGOR Pro (Wavemetrics, Inc., Portland, OR, USA). From the forceplate recordings, we determined foot-down time (force > 5% body mass), and toe-off time (force < 5% body mass). Between these times, we determined peak and average normal and parallel (in line with ramp direction) forces, and peak and average total GRFs. Peak transverse forces were typically less than 5 per cent of peak GRF and thus were not included in this analysis.
We present results for individual birds and for age categories. The nine brush turkeys were grouped based on similar mass and estimated age into five categories (hatchling, young, juvenile, sub-adult, adult; table 1). Age category means values are presented as the pooled mean of measurements among all individuals within a given category.
For comparative purposes, we present previously unpublished forceplate data for developing chukar (Alectoris chukar). Recording and analysis techniques were the same as for brush turkeys, except that chukars were recorded only at 50° and 70°. Chukar care, age and kinematic data are available in Jackson et al. [7].
3. Results
(a). Performance
Incline locomotor performance, determined by maximum ascent angle, decreased with age and size (table 1, figure 2 and electronic supplementary material, video S2). The 2 dph ‘hatchling’ easily ascended 110° inverted inclines without slipping. In contrast, the two oldest birds could negotiate inclines only up to 60° but lost traction and slipped during most steps (table 1). On the 70° ramp, these oldest birds were unable to gain traction and eventually slipped-off the ramp sideways or backwards. The oldest bird to successfully ascend a 90° incline was estimated to be 99 dph and had less than 25 per cent of adult mass (bird 6, table 1). Older birds progressively failed as ramp angle increased: clean footsteps were followed by occasional slips, then protracted failure with treadmilling (i.e. hindlimb slipping or running in place) and finally complete inability to maintain position on the ramp (table 1).
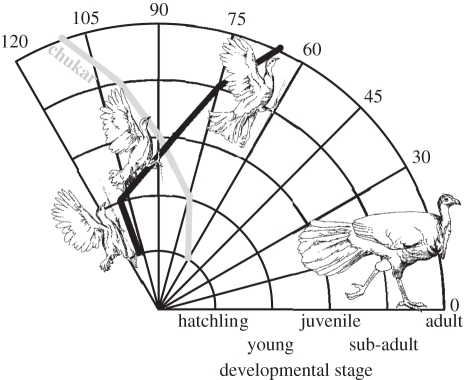
Figure 2.
Maximal locomotor performance, measured as the steepest angle successfully ascended, decreased with age in brush turkeys (this study) in sharp contrast to the increase observed in chukars (data from Jackson et al. [7]).
(b). Ground reaction forces
The hindlimbs functioned primarily in weight-support against gravity and propulsion up the inclines (figure 3). On shallower inclines (level through 70°), average and peak forces were oriented within 15° of vertical. On 80–90° inclines, slipping and treadmilling birds produced average and peak forces oriented greater than 30° from vertical. At 80° and 90°, the angle between the ramp (or vertical) and the GRF increased with age (figure 3). The youngest bird produced peak forces of 1.8 times body weight (×mg, where m is body mass in kilogram and g is the acceleration owing to gravity) on the level, and 1.1 × mg (1.6 N), oriented nearly in line with the 100° incline (average forces of 0.9 × mg and 0.6 × mg, respectively) (figure 4). Thus, even on an inverted (i.e. overhanging) substrate, the legs produced significant propulsive forces. Generally, individuals maintained parallel forces but reduced normal forces with increasing incline angle, resulting in lower peak and average GRF at steeper inclines (figure 4). For example, in the youngest bird, average GRF during stance decreased from 0.8 × mg on 50° inclines to 0.2 × mg on 110° inclines. This trend corresponded with a change in gait from running at 50° to ‘walking’ (duty factor >0.5) at inclines steeper than 70°.
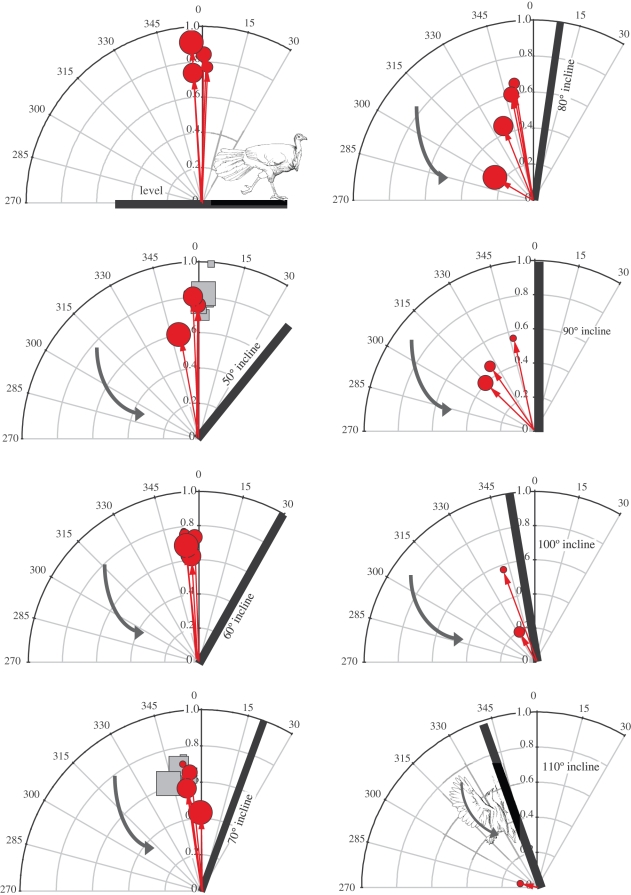
Figure 3.
Average GRF never exceeded body weight during successful ascents, independent of age (age indicated by size of shapes) in brush turkeys (red circles) or chukar (grey squares), but generally was oriented against gravity so as to provide weight support. With increasing ramp angle, the larger (older) brush turkeys failed, and only the hatchling was able to ascend the 110° ramp. Curved arrows represent average stroke-plane angle (measured at mid-downstroke) for the youngest bird.
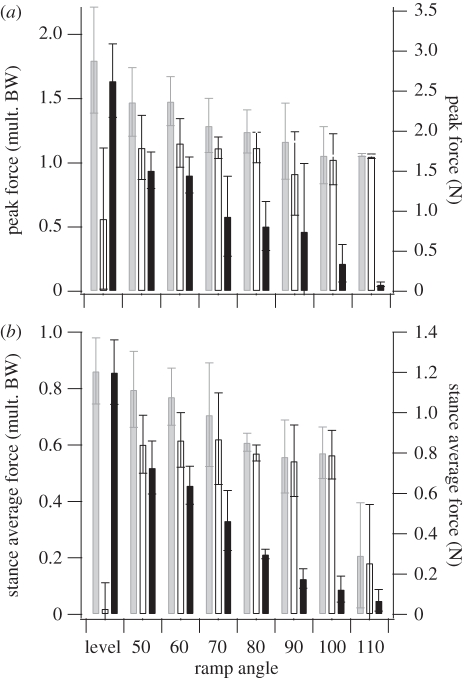
Figure 4.
(a) Peak and (b) average GRFs for the hatchling, the only bird to successfully ascend all ramp angles. Total (net, grey bars) GRF decreased with increasing ramp angle, driven by decreasing normal GRF (black bars) while parallel GRF (open bars) remained nearly constant.
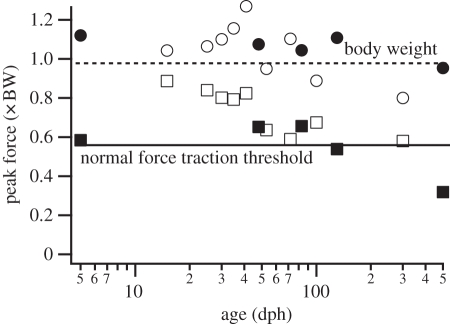
Chukar produced peak and average GRFs similar in magnitude and orientation to those produced by brush turkeys (figure 5). Peak forces, as multiples of body mass, decreased with age at 70° (the only angle that nearly all ages of both species can ascend). Peak parallel forces of sub-adults of both species averaged just above 1 × mg. Peak normal forces in both species decreased with age, from near 1.0 to 0.6 × mg in chukars, and 0.7 to 0.3 × mg in turkeys (figure 5), with similar patterns for average forces. The two oldest age categories of turkeys slipped and treadmilled at 70°, suggesting that normal forces between 0.5 and 0.6 × mg are necessary to provide the traction required for WAIR at 70° (threshold line in figure 5).
Figure 5.
Peak normal (squares) and parallel (circles) GRF for age groupings of brush turkeys (closed symbols) and chukar (open symbols) performing WAIR on a 70° incline. At 70°, the two oldest age categories of brush turkeys slipped, and they produced the lowest peak normal forces. All other birds produced normal forces above 0.55 × body weight during successful ascents, suggesting a possible normal force threshold to produce sufficient traction for ascent.
(c). Wing-loading, frequency, stroke-plane angle
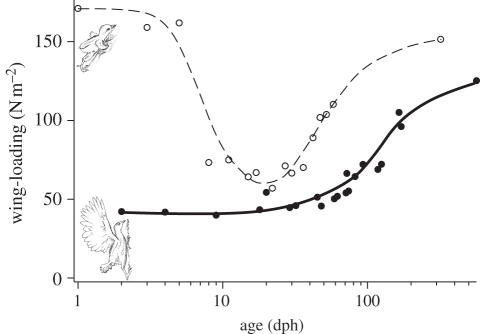
Wing-loading was lowest in the youngest birds and more than doubled during growth. Wing-loading remained essentially unchanged over the first 30 days post-hatching (40–46 N m−2; figure 6). Further, feather growth was minimal, and as body mass increased wing-loading grew to 70 N m−2 at 100 dph, and 100 N m−2 at 200 dph.
Figure 6.
Wing-loading trends during development for brush turkeys (filled circle, this study) and chukar (circle, [7]). Brush turkeys hatch with full wings in an advanced stage of development, leading to the lowest wing-loadings during their growth.
Wingbeat frequency generally decreased with age, but increased with ascent angle (figure 7). The turkeys beat their wings intermittently at the lowest ascent angles (i.e. 50° and 60°), usually pausing between mid-upstroke and end-upstroke, resulting in the lowest frequencies at a given age. When wingbeats were more consistent at steeper inclines, wingbeat frequency ranged from 9.2 to 10.3 Hz in the youngest bird, and from 6.9 to 7.8 Hz in the largest (figure 7). Maximum wingbeat frequency scaled as m−0.10.
Figure 7.
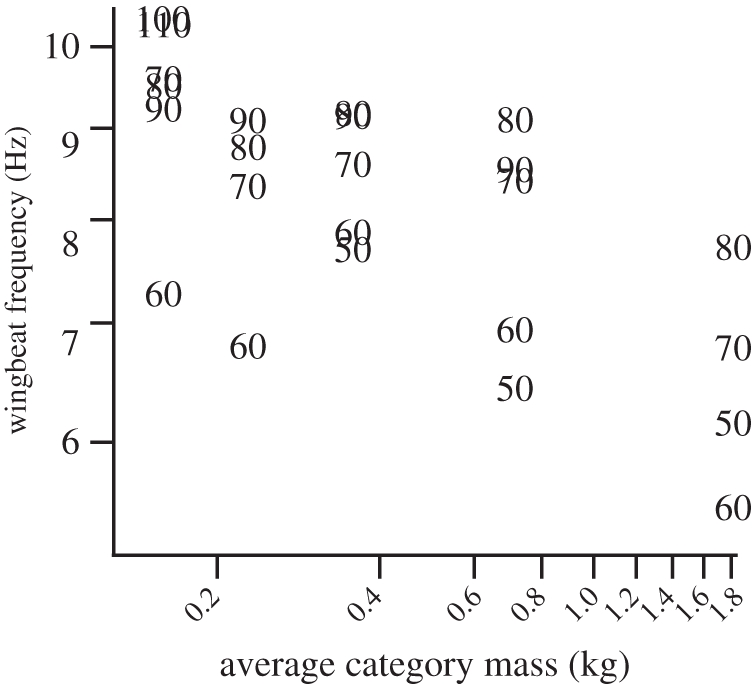
Average wingbeat frequency for each age category at each ramp angle (50°–110°). Intermittent flapping on the lowest ramp angles led to variable and low wingbeat frequencies. At higher angles (>70°), frequency generally increased with ramp angle. Maximum frequency among age categories scaled as m−0.10.
Stroke-plane angle changed very little with age or ramp angle (figure 3). Because of the intermittent wingbeats at 50° and 60° incline angles, variation in stroke-plane angles was high. At steeper inclines, mean stroke-plane angle ranged from 115° to 128° among all ages, and showed no trend with changes in incline angle or bird age.
4. Discussion
Hatchling Australian brush turkeys consistently out-performed older birds as they climbed a variable-pitched inclined ramp, built to emulate inclined objects that birds encounter in nature (e.g. earthed mounds, trees, boulders, cliffs). Although capable of true flight, the day they emerge from their ground nest, brush turkeys, like other Galliformes, preferentially used WAIR to ascend to an elevated refuge. Ricklefs [3] argued that precocial species should be adept at burst locomotion. In the wild, young brush turkeys are often exposed to intense predation (<15% may survive the first three weeks), and they seek elevated refuges when threatened or for roosting [2]. As adults, brush turkeys and other Galliformes can outrun many predators and use their wings only for brief escape flights. To avoid predators, young brush turkeys must negotiate a highly variable terrestrial environment on their own. Our data suggest that one trait promoting early incline-running performance is their extraordinarily low wing-loading—the lowest of their lives. This low wing-loading is achieved because hatchlings have very large wings, the development of which probably explains their prolonged egg stage (approx. 50-day incubation; [26]). This is in stark contrast to other Galliformes (20–25-day incubation) that cannot generate useful aerodynamic forces for several days after hatching and lack the running speeds of the adults [7], but enjoy the protection of their parents.
In brush turkeys, declining performance during development coincided with increasing wing-loading and decreasing relative normal GRF. All else equal, high wing-loading implies low aerodynamic force production relative to body weight. During WAIR in chukars, the wings produce forces that accelerate the bird towards the inclined surface [16,27]. Thus, even on a vertical incline, the hindlimbs can develop traction (via normal GRF) and accelerate the bird's centre of mass (via parallel GRF; [27]). In turkeys, both peak and average normal forces systematically declined with incline angle (hatchlings: peak normal GRF from 1.7 × mg in level movement to 0.5 × mg at 90°, figures 3 and 4). By contrast, peak parallel forces did not change (hatchlings: 1.0 × mg; figure 4). Similarly in adult chukars, normal GRF decreased from 0.7 to 0.6 × mg, and parallel GRF remained near 0.8 × mg (60–90° inclines). The only other forceplate data for running at extreme angles [27] reported peak forces from adult chukar partridge well above our brush turkey and chukar data (see electronic supplementary material for discussion). Older turkeys produced lower relative normal GRF at all angles, and lost traction at shallower angles than younger birds. Thus, the high wing-loading of older turkeys may therefore preclude them from maintaining hindlimb traction. However, changes in foot and claw morphology with age may modify the interface with the substrate and the animals may simply loose their behavioural propensity to employ WAIR with age. Additionally, as WAIR requires a small fraction of the aerodynamic forces of level flight [16] and adult brush turkeys are capable of powered level flight, it is unlikely that increased wing-loading is the sole limiting factor on WAIR performance.
What does the ontogenetic increase in wing-loading indicate? Adult brush turkeys have stout hindlimbs that they use to construct and maintain their enormous (2000–6000 kg) mound nests [2]. Breeding males may move up to 50 kg of earth daily during incubation [28]. Adult turkeys also use their hindlimbs to flee from predators and in combat with other territorial males during the breeding period [2]. Thus, brush turkeys may experience a developmental and functional tradeoff between forelimb-dependent flap-running locomotor performance and development of stout hindlimbs for adult survival and reproductive success.
We hypothesize that Australian brush turkeys, in contrast to other Galliformes, develop from forelimb-dominated young that avoid predation by taking advantage of steep terrain and natural obstacles into hindlimb-dominated adults that have transitioned to an anti-predation strategy dependent on body size and running speed. We expect this developmental shift also to be marked by other morphological and physiological changes (e.g. in relative limb size and robustness, and in foot morphology for traction on incline versus level running). Compensating for their reduced inclined running locomotor performance, adults appear more adept at high-speed horizontal movement and are probably less naive of threatening situations (K. P. Dial 2004, personal observation); although currently no empirical data exist to substantiate this behavioural observation. Furthermore, the young of mound-builders (scrubfowl, malleefowl, and brush turkey) are far more capable of covering larger distances in the air than the adults [2]. It is the young birds, for the most part, that colonize new areas. In some cases the smaller, flight-capable kids cross considerable stretches of unsuitable habitat and thus are responsible for the majority of dispersal, particularly over water to other islands [29].
Locomotor performance develops quickly in many precocial species (for review, see [8]). In the only other study of locomotor ontogeny in birds (chukar partridges), WAIR performance increased with age and was correlated with morphological and kinematic changes early in development (8–20 days; [7]). However, there are few known examples where young outperform adults in escape-relevant behaviours: jackrabbits (Lepus; [4,5]), lizards (Anolis, [6]), crickets (Nemobius sylvestris [30]), brush turkeys (this study). It is likely that such developmental strategies evolved in response to high predation early in life (e.g. [8]), as is the case in brush turkeys (>85% juvenile mortality; [2]).
Understanding the relationship between locomotor ontogeny and degree of parental care of extant species may shed light on the historic ecological and evolutionary habits of extinct groups. For example, recent analyses suggest that many theropod dinosaurs grew over a period of several years, through a wide range of sizes, before reaching asymptotic size [31,32]. Small and immature members of a species, then, may have relied on different locomotor strategies than older and larger members. Some relatively small eumaniraptoran theropods, for instance, are preserved with fully feathered wings (e.g. Microraptor [33]; Anchiornis [34,35]). Yet larger and presumably flight-incapable adult theropods have been discovered with feathered forelimbs (e.g. Caudipteryx [36,37]; Velociraptor [38]) that perhaps generated useful aerodynamic forces only for the diminutive juveniles. Recently, Xu et al. [39] reported a dinosaur, Similicaudipteryx, that appears to exhibit changing feather morphology with age. This implies that different age classes may have enjoyed different locomotor capacities. Thus, just as brush turkeys begin life with aerodynamically functional forelimbs and become less aerial during development, some theropod dinosaurs may have exploited a more three-dimensional environment as juveniles, and become more obligately bipedal with age. The idea of immature animals exploiting unique ecological niches appears consistent with other forms exhibiting semi-indeterminate growth, as reported for locomotor development in crocodiles [40]. The ontogeny of locomotion in extant organisms can thus provide insight on the evolution of locomotion in extinct organisms.
Acknowledgements
Procedures were approved by IACUC of The University of Montana, Missoula, and the Animal Care Committee at the University of Adelaide.
The authors thank Terry Dial and Ross Randall for their assistance in data collection and analyses. This work is dedicated posthumously to Russell Baudinette (University of Adelaide) for his active support during his final months. Supported by NSF.
References
- 1.Starck J. M., Ricklefs R. E. 1998. Patterns of development: the altricial–precocial spectrum. Oxf. Ornithol. Ser. 8, 3–30 [Google Scholar]
- 2.Jones D. N., Göth A. 2008. Mound-builders: mallee fowl, brush turkeys and scrubfowl. Collingwood, Victoria, Australia: CSIRO Publishing [Google Scholar]
- 3.Ricklefs R. 1979. Adaptation, constraint, and compromise in avian postnatal development. Biol. Rev. 54, 269–290 10.1111/j.1469-185X.1979.tb01013.x (doi:10.1111/j.1469-185X.1979.tb01013.x) [DOI] [PubMed] [Google Scholar]
- 4.Carrier D. R. 1983. Postnatal ontogeny of the musculo-skeletal system in the black-tailed jack rabbit (Lepus californicus). J. Zool. 201, 27–55 10.1111/j.1469-7998.1983.tb04259.x (doi:10.1111/j.1469-7998.1983.tb04259.x) [DOI] [Google Scholar]
- 5.Carrier D. R. 1995. Ontogeny of jumping performance in the black tailed jack rabbit (Lepus californicus). J. Zool. 98, 309–313 [Google Scholar]
- 6.Toro E., Herrel A., Vanhooydonck B., Irschick D. J. 2003. A biomechanical analysis of intra- and interspecific scaling of jumping and morphology in Caribbean Anolis lizards. J. Exp. Biol. 206, 2641–2652 10.1242/jeb.00473 (doi:10.1242/jeb.00473) [DOI] [PubMed] [Google Scholar]
- 7.Jackson B. E., Segre P., Dial K. P. 2009. Precocial development of locomotor performance in a ground-dwelling bird (Alectoris chukar): negotiating a three-dimensional terrestrial environment. Proc. R. Soc. B 276, 3457–3466 10.1098/rspb.2009.0794 (doi:10.1098/rspb.2009.0794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrel A., Gibb A. C. 2006. Ontogeny of performance in vertebrates. Physiol. Biochem. Zool. 79, 1–6 10.1086/498196 (doi:10.1086/498196) [DOI] [PubMed] [Google Scholar]
- 9.Vleck D., Vleck C. M., Seymour R. S. 1984. Energetics of embryonic development in the megapode birds, mallee fowl Leipoa Ocellata and brush turkey Alectura lathami. Physiol. Zool. 57, 444–456 [Google Scholar]
- 10.Vleck C. M., Vleck D. 1987. Metabolism and energetics of avian embryos. J. Exp. Zool. Suppl. 1, 111–125 [PubMed] [Google Scholar]
- 11.Seymour R. S., Vleck D., Vleck C. M., Booth D. T. 1987. Water relations of buried eggs of mound building birds. J. Comp. Physiol. B: Biochem., Syst. Environ. Physiol. 157, 413–422 10.1007/BF00691824 (doi:10.1007/BF00691824) [DOI] [Google Scholar]
- 12.Jones D. N., Dekker R. W., Roselaar C. S. 1995. The megapodes. Oxford, UK: Oxford University Press [Google Scholar]
- 13.Göth A., Eising C. M., Herberstein M. E., Groothuis T. G. 2008. Consistent variation in yolk androgens in the Australian brush-turkey, a species without sibling competition or parental care. Gen. Comp. Endocrinol. 155, 742–748 10.1016/j.ygcen.2007.11.004 (doi:10.1016/j.ygcen.2007.11.004) [DOI] [PubMed] [Google Scholar]
- 14.Dial K. P. 2003. Wing-assisted incline running and the evolution of flight. Science 299, 402–404 10.1126/science.1078237 (doi:10.1126/science.1078237) [DOI] [PubMed] [Google Scholar]
- 15.Dial K. P., Jackson B. E., Segre P. 2008. A fundamental avian wing-stroke provides a new perspective on the evolution of flight. Nature 451, 985–989 10.1038/nature06517 (doi:10.1038/nature06517) [DOI] [PubMed] [Google Scholar]
- 16.Tobalske B. W., Dial K. P. 2007. Aerodynamics of wing-assisted incline running in birds. J. Exp. Biol. 210, 1742–1751 10.1242/jeb.001701 (doi:10.1242/jeb.001701) [DOI] [PubMed] [Google Scholar]
- 17.Dial K. P., Randall R. J., Dial T. R. 2006. What use is half a wing in the ecology and evolution of birds? BioScience 56, 437–445 10.1641/0006-3568(2006)056[0437:WUIHAW]2.0.CO;2 (doi:10.1641/0006-3568(2006)056[0437:WUIHAW]2.0.CO;2) [DOI] [Google Scholar]
- 18.Cavanagh P. R., Lafortune M. A. 1980. Ground reaction forces in distance running. J. Biomech. 13, 397–406 10.1016/0021-9290(80)90033-0 (doi:10.1016/0021-9290(80)90033-0) [DOI] [PubMed] [Google Scholar]
- 19.Biewener A. 1983. Locomotory stresses in the limb bones of two small mammals: the ground squirrel and chipmunk. J. Exp. Biol. 103, 131–154 [DOI] [PubMed] [Google Scholar]
- 20.Munro C. F., Miller D. I., Fuglevand A. J. 1987. Ground reaction forces in running: a reexamination. J. Biomech. 20, 147–155 10.1016/0021-9290(87)90306-X (doi:10.1016/0021-9290(87)90306-X) [DOI] [PubMed] [Google Scholar]
- 21.Biewener A. A., Farley C. T., Roberts T. J., Temaner M. 2004. Muscle mechanical advantage of human walking and running: implications for energy cost. J. Appl. Physiol. 97, 2266–2274 10.1152/japplphysiol.00003.2004 (doi:10.1152/japplphysiol.00003.2004) [DOI] [PubMed] [Google Scholar]
- 22.Gottschall J. S., Kram R. 2005. Ground reaction forces during downhill and uphill running. J. Biomech. 38, 445–452 10.1016/j.jbiomech.2004.04.023 (doi:10.1016/j.jbiomech.2004.04.023) [DOI] [PubMed] [Google Scholar]
- 23.Roberts T. J., Belliveau R. A. 2005. Sources of mechanical power for uphill running in humans. J. Exp. Biol. 208, 1963–1970 10.1242/jeb.01555 (doi:10.1242/jeb.01555) [DOI] [PubMed] [Google Scholar]
- 24.Göth A. 2001. Survival, habitat selectivity and behavioural development of the Australian brush-turkey Alectura lathami. PhD thesis, Griffith University, Brisbane, Australia [Google Scholar]
- 25.Rasband W. S. 2009. ImageJ. Bethesda, MD, USA: U.S. National Institutes of Health; See http://rsb.info.nih.gov/ij/ [Google Scholar]
- 26.Göth A. 2002. Behaviour of Australian brush-turkey (Alectura lathami, galliformes: Megapodiidae) chicks following underground hatching. J. Ornithol. 143, 477–488 10.1007/BF02465603 (doi:10.1007/BF02465603) [DOI] [Google Scholar]
- 27.Bundle M. W., Dial K. P. 2003. Mechanics of wing-assisted incline running (WAIR). J. Exp. Biol. 206, 4553–4564 10.1242/jeb.00673 (doi:10.1242/jeb.00673) [DOI] [PubMed] [Google Scholar]
- 28.Jones D. N. 1988. Construction and maintenance of the incubation mounds of the Australian brush-turkey Alectura lathami. EMU 88, 210–218 10.1071/MU9880210 (doi:10.1071/MU9880210) [DOI] [Google Scholar]
- 29.Partridge T., Ward M. 2007. Nganamara in South Australia's aboriginal lands. In Proc. of the Malleefowl Forum 2007 (ed. Davies S. J. J. F.), Katanning, WA, 7–10 September, 2007, pp. 23–27 Perth: Edith Cowen University [Google Scholar]
- 30.Dangles O., Pierre D., Christides J. P., Casas J. 2007. Escape performance decreases during ontogeny in wild crickets. J. Exp. Biol. 210, 3165–3170 10.1242/jeb.004648 (doi:10.1242/jeb.004648) [DOI] [PubMed] [Google Scholar]
- 31.Padian K., de Ricqles A. J., Horner J. R. 2001. Dinosaurian growth rates and bird origins. Nature 412, 405–408 10.1038/35086500 (doi:10.1038/35086500) [DOI] [PubMed] [Google Scholar]
- 32.Erickson G. M., Makovicky P. J., Currie P. J., Norell M. A., Yerby S. A., Brochu C. A. 2004. Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature 430, 772–775 10.1038/nature02699 (doi:10.1038/nature02699) [DOI] [PubMed] [Google Scholar]
- 33.Xu X., Zhou Z., Wang X., Kuang X., Zhang F., Du X. 2003. Four-winged dinosaurs from China. Nature 421, 335–340 10.1038/nature01342 (doi:10.1038/nature01342) [DOI] [PubMed] [Google Scholar]
- 34.Hu D., Hou L., Zhang L., Xu X. 2009. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature 461, 640–643 10.1038/nature08322 (doi:10.1038/nature08322) [DOI] [PubMed] [Google Scholar]
- 35.Xu X., Zhao Q., Norell M., Sullivan C., Hone D., Erickson G., Wang X. L., Han F. L., Guo Y. 2009. A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chin. Sci. Bull. 54, 430–435 10.1007/s11434-009-0009-6 (doi:10.1007/s11434-009-0009-6) [DOI] [Google Scholar]
- 36.Qiang J., Currie P. J., Norell M. A., Shu-An J. 1998. Two feathered dinosaurs from northeastern China. Nature 393, 753–761 10.1038/31635 (doi:10.1038/31635) [DOI] [Google Scholar]
- 37.Zhou Z. H., Wang X. L., Zhang F. C., Xu X. 2000. Important features of Caudipteryx evidence from two nearly complete new specimens. Vertebr. PalAsiatica 38, 243–265 [Google Scholar]
- 38.Turner A. H., Makovicky P. J., Norell M. A. 2007. Feather quill knobs in the dinosaur Velociraptor. Science 317, 1721. 10.1126/science.1145076 (doi:10.1126/science.1145076) [DOI] [PubMed] [Google Scholar]
- 39.Xu X., Zheng X., You H. 2010. Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature 464, 1338–1341 10.1038/nature08965 (doi:10.1038/nature08965) [DOI] [PubMed] [Google Scholar]
- 40.Allen V., Elsey R. M., Jones N., Wright J., Hutchinson J. R. 2010. Functional specialization and ontogenetic scaling of limb anatomy in Alligator mississippiensis. J. Anat. 216, 423–445 10.1111/j.1469-7580.2009.01202.x (doi:10.1111/j.1469-7580.2009.01202.x) [DOI] [PMC free article] [PubMed] [Google Scholar]