Abstract
Neurobiological models of posttraumatic stress disorder (PTSD) suggest that altered activity in the medial temporal lobes (MTL) during encoding of traumatic memories contribute to the development and maintenance of the disorder. However, there is little direct evidence in the PTSD literature to support these models. The goal of the present study was to examine MTL activity during trauma encoding in combat veterans using the subsequent memory paradigm. Fifteen combat veterans diagnosed with PTSD and 14 trauma-exposed control participants viewed trauma-related and neutral pictures while undergoing event-related fMRI. Participants returned one week after scanning for a recognition memory test. Region-of-interest (ROI) and voxel-wise whole brain analyses were conducted to examine the neural correlates of successful memory encoding. Patients with PTSD showed greater false alarm rates for novel lures than the trauma-exposed control group, suggesting reliance on gist-based representations in lieu of encoding contextual details. Imaging analyses revealed reduced activity in the amygdala and hippocampus in PTSD patients during successful encoding of trauma-related stimuli. Reduction in left hippocampal activity was associated with high arousal symptoms on the Clinician-Administered PTSD Scale (CAPS). The behavioral false alarm rate for traumatic stimuli co-varied with activity in the bilateral precuneus. These results support neurobiological theories positing reduced hippocampal activity under conditions of high stress and arousal. Reduction in MTL activity for successfully encoded stimuli and increased precuneus activity may underlie reduced stimulus specific encoding and greater gist memory in patients with PTSD, leading to maintenance of the disorder.
Keywords: fMRI, gist, contextual memory, military, subsequent memory paradigm, precuneus
Introduction
Posttraumatic stress disorder (PTSD) has been characterized as a disorder of memory, with key features including intrusive memories of the traumatic event, flashbacks, and nightmares. While the re-experiencing symptoms of traumatic events are often difficult to inhibit, PTSD is paradoxically related to abnormal access to trauma memories and difficulty remembering certain aspects of the trauma (Amir et al., 1998; Foa et al., 1995; Koss et al., 1996). The nature of memory for traumatic events may represent the single most controversial topic in the field of traumatic stress (McNally, 2003) and has garnered great attention due to implications for recovered memories and eyewitness testimony. However, of the few published studies that have examined the neurobiology of negative memory encoding in PTSD (Dickie et al., 2008; Thomaes et al., 2009), none have directly examined memory encoding of trauma-specific information, despite indications that traumatic memories in particular are subject to distortions. In the present study, we provide evidence that altered neural activity for encoding of trauma reminders may have implications for understanding how the disorder is maintained.
A prominent hypothesis of PTSD etiology suggests that inefficient encoding may result in distortions in traumatic memory (Ehlers and Clark, 2000). According to this model, traumatic memories are characterized by confusing sensory impressions that are temporally related to the trauma, but are disconnected from the context in which they were formed. These altered memory traces impede the individual’s ability to discriminate between stimuli that represent real danger and those that serve as relatively harmless reminders of the trauma (e.g., a patient with combat-related PTSD has a flashback at a Fourth of July fireworks display). The clinical implication is maintenance of the disorder through persistent re-experiencing, heightened arousal, and further avoidance of the trauma. There is some evidence that PTSD patients’ memories for emotional events are overly general, or gist-based, rather than detailed (Harvey et al., 1998; Kaspi et al., 1995; McNally et al., 1994). Given that gist-based representations are often subject to misinformation and false alarms (Roediger and McDermott, 1995; Wright and Loftus, 1998), it is possible that encoding of trauma memories that are gist-based and without specific, contextual details is one mechanism associated with memory distortions in PTSD.
Although several researchers have advanced ideas suggesting that encoding abnormalities during the trauma event underlie memory difficulties observed in PTSD (Ehlers and Clark, 2000; Layton and Krikorian, 2002; Nadel and Jacobs, 1998), there is no direct evidence to support these hypotheses. One obvious ethical and practical problem in humans is that it is impossible to study the neurobiology of trauma memory while it is occurring. However, it is possible to examine the maintenance of memory distortions in PTSD by studying memory encoding of trauma reminders. A salient feature of PTSD is that re-experiencing symptoms of the trauma occurs within a safe context, suggesting that patients have difficulty updating their contextual representations of the trauma event. It is possible that maintenance of the disorder may in part be mediated by encoding of trauma reminders that are gist-based and devoid of contextual and specific episodic information.
Drawing from the well-supported animal literature on trauma and stress, involvement of medial temporal lobe (MTL) structures including the hippocampus and amygdala are vital for successful encoding of negative information (McGaugh, 2004). According to the modulation hypothesis, emotional events are generally remembered better than neutral events due to the modulatory effect of the amygdala on other MTL structures including the hippocampus (McGaugh et al., 1996). The hippocampus in particular is necessary to the formation of specific, contextual memories. However, hippocampal function in response to stress may be an inverted U-shape (Nadel and Jacobs, 1998). That is, the hippocampus functions optimally at moderate levels of stress, but suboptimally at very low and very high levels of stress. There is ample evidence that high levels of stress impair hippocampal activity (de Quervain et al., 1998; Foy et al., 1987; Kim and Diamond, 2002) and that, in humans, negative valence increases the likelihood of false memories (Brainerd and Reyna, 2002). Chronic hyperarousal and stress results in elevated secretion of glucocorticoids, catecholamines, and other neuromodulators which affect the hippocampus (Kim and Diamond, 2002). These studies point to a neurotoxic role for corticosteroids that cause atrophy and cell death in hippocampal neurons. Several structural MRI studies have reported smaller hippocampal volume is chronic unremitting PTSD (Bremner et al., 2003a; Gurvits et al., 1996; Stein et al., 1997; Villarreal et al., 2002; Wignall et al., 2004). Magnetic Resonance Spectroscopy (MRS) studies of PTSD have documented decreased N-acetyl aspartate (NAA) in the hippocampus that is suggestive of impaired neuronal integrity (Brown et al., 2003; Schuff et al., 2001; Winter and Irle, 2004). Finally, examination of trauma memory or encoding of trauma-related information provides evidence of diminished hippocampal activation in PTSD (Bremner et al., 1999; Bremner et al., 2003a; Bremner et al., 2003b; Geuze et al., 2008b; Shin et al., 1999). Therefore, suboptimal hippocampal activity during presentation of highly stressful trauma reminders may lead to decontextualized memory traces for these stimuli in patients with PTSD. This hypothesis is consistent with the aforementioned cognitive model of PTSD, which posits that trauma memory traces are characterized by distortion and confused sensory impressions rather than an elaborated memory that can be readily integrated into one’s autobiographical knowledge base.
In order to study the neural underpinnings of encoding of trauma reminders, we employed the subsequent memory paradigm which provides a powerful translational approach in understanding the neural basis of successful memory encoding and retrieval in humans (Paller and Wagner, 2002). In this paradigm, BOLD activity is measured during encoding of stimuli that are probed for memory success after a delay. Differences in encoding activity for successfully remembered or forgotten material is evaluated on a participant-by-participant basis to identify brain regions that mediate the interaction between emotional arousal and successful memory operations. The difference between remembered and forgotten activation is referred to as the ‘difference due to memory effect,’ or Dm effect (Dolcos et al., 2004). As a measure of gist memory and lack of contextual details, novel lures were introduced to the subsequent memory test to examine false alarm rates. Accurate recognition of previously studied items depends on gist and item-specific information, whereas false recognition of related lures depends on remembering gist but not on item-specific information (Budson et al., 2000; Payne et al., 1996; Reyna and Brainerd, 1995b; Schacter et al., 1996; Verfaellie et al., 2002). Due to our strong a priori interest in MTL regions, we used a region-of-interest (ROI) analysis to interrogate activity in the amygdala, hippocampus, and parahippocampul gyrus in response to subsequently remembered and forgotten material. We hypothesized that patients with PTSD would show greater amygdala activity and reduced hippocampal activity during successful encoding of trauma reminders compared with trauma-exposed control participants, and greater false alarms indicating gist-based, rather than specific detail memory representations. To examine the relationship between arousal and hippocampal function, we conducted a correlation analysis between CAPS cluster scores and hippocampal activity with the hypothesis that PTSD hyperarousal symptoms would be negatively correlated with hippocampal activation, in support of the notion that hippocampal activity is disrupted under conditions of high arousal and stress. Finally, as a secondary analysis, we examined the relationship between emotional encoding and memory regions along the longitudinal axis of the MTL. Previous work suggests that MTL memory regions are differentially sensitive to the effects of emotion on successful encoding such that anterior regions of the MTL are more responsive for emotional material while posterior regions of the MTL respond to neutral material (Dolcos et al., 2004). These data support the notion that the amygdala has a modulatory effect on anterior MTL regions. We predicted that trauma exposed controls would show a shift in neural response for emotional and neutral stimuli along the longitudinal axis of the MTL similar to healthy normal subjects, but this MTL relationship might be disrupted in PTSD as a response to the neurotoxic effects of stress on the hippocampus (Kim and Diamond, 2002).
Materials and Methods
Participants
Demographic information of study participants is displayed in Table 1. Twenty-nine veterans who had returned from their deployment in support of recent war operations (e.g., veterans who served in Iraq or Afghanistan) completed the fMRI procedures (average time since return from deployment = 3 years, 9 months). Veterans were recruited from a large registry database for the study of post-deployment mental health. Veterans entering the registry were evaluated for Axis-I disorders using clinician-administered and self-report measures (described more fully in (Hayes et al., 2009). Exclusion criteria for the present study included prior history of psychotic symptoms, past or current substance dependence, or current substance abuse according to the Structured Clinical Interview for DSM-IV-TR disorders (SCID). Veterans reporting Criterion A combat trauma events were contacted by telephone and screened to exclude those with a serious medical illness, head injury resulting in loss of consciousness for more than 20 minutes, and presence of bodily metal incompatible with MRI. Participants provided written informed consent for procedures approved by the Institutional Review Boards at Duke University and the Durham VA Medical Center.
Table 1.
Demographic and clinical characteristics of subject sample
| Characteristic | Patient n = 15 |
Control n = 14 |
t / Chi Square | p |
|---|---|---|---|---|
| Age (years) [S.D.] | 33.93 [8.23] | 34.0 [6.03] | 0.03 | ns |
| Gender, No. (%) of females | 3 (20) | 4 (28.6) | 0.29 | ns |
| Handedness, No. (%) right-handed | 14 (93.3) | 14 (100) | 0.97 | ns |
| Ethnicity, No. (%) of Caucasian subjects | 6 (40) | 7 (50) | 0.29 | ns |
| Education (years) [S.D.] | 14.20 [1.78] | 14.43 [2.28] | 0.30 | ns |
| CAPS Total Score [S.D.] | 75.20 [23.76] | 10 [11.60] | 9.28 | < 0.001 |
| Combat Exposure Scale [S.D.] | 18.73 [9.68] | 11.79 [9.10] | 1.99 | ns |
| Beck Depression Inventory [S.D.] | 27.53 [10.47] | 5.43 [5.29] | 7.10 | < 0.001 |
| AUDIT [S.D.] | 3.00 [3.09] | 4.43 [4.52] | 1.00 | ns |
| Drug Abuse Screening Test [S.D.] | 1.60 [2.32] | 1.14 [1.70] | 0.60 | ns |
CAPS = Clinician-Administered PTSD Scale, AUDIT = Alcohol Use Disorders Identification Test, ns = non-significant
PTSD diagnosis was determined by the Clinician-Administered PTSD Scale (CAPS). Fifteen participants were diagnosed with PTSD and 14 participants composed the trauma-exposed (no PTSD) control group. The representation of women in our sample (20%), matched across groups, is consistent with the gender breakdown of military personnel who served in Iraq and Afghanistan. Control participants did not have current Axis-I disorders according to the SCID. Six PTSD patients and one control participant were taking selective serotonin reuptake inhibitors (SSRIs) at the time of study.
Materials
Stimuli consisted of 60 high-arousing trauma combat photos, 60 neutral photos, and 40 positive photos. Trauma pictures were selected from a set of in-house pictures used previously (Morey et al., 2009; Morey et al., 2008), which represent genuine war photos, the majority taken in Iraq and Afghanistan. Examples included photos of caskets, masked insurgents, and pictures of soldiers and civilians with flesh wounds. Neutral pictures were selected from the International Affective Picture Set (IAPS) (Lang et al., 1997) and supplemented with in-house pictures that had normative data from a separate group of healthy individuals. A smaller set of positive pictures selected from the IAPS database were included to prevent induction of long-lasting negative mood states but were not of interest in analyses. Pictures were pseudorandomly presented so that no more than two trauma pictures were displayed in a row. Trauma and neutral pictures did not differ in luminance, red, blue, or green color (ratings obtained from Adobe Photoshop), or the presence of human bodies.
Procedure
The stimulus set of 160 pictures was divided into 8 encoding runs, 20 pictures in each run. Each trial began with a 3-second presentation of the picture, and participants made a judgment regarding how the picture made them feel on a 4-point scale (1 = highly negative, 4 = highly positive). A fixation cross was presented between pictures and jittered between 12–16 seconds (average = 14 seconds).
Participants returned one week after the encoding session for a surprise memory test (incidental encoding). Participants viewed the original 160 pictures along with 80 novel lures that were selected to depict similar scenes to the original pictures (30 trauma, 30 neutral, 20 positive). The pictures were presented in black and white to increase task difficulty (as based on our previous pilot data). During the recognition memory task, each picture was presented for 2 seconds. Subjects were given 3 seconds to indicate whether the picture was old or new, and then an additional 3 seconds to indicate their confidence level on a 4-point scale. In the behavioral and imaging analyses, hits were defined as items that were subsequently remembered with high confidence (rating of 3 or 4 on a 4-point confidence scale), and compared with all misses. We selected this approach to limit the influence of guesses, which are more likely to occur with low confidence. For behavioral analyses, hit rates were calculated as the number of items remembered correctly with high confidence divided by the total number of items presented at encoding. This approach of using only high confidence hits helped identify a more robust response in fMRI activity associated with item recognition. The FA rate was calculated as the number of falsely remembered lures divided by the total number of lures. .Of the 160 items presented at encoding, high confidence responses were recorded for 74.1% of PTSD participants and 75.3% of TEC participants, whereas low confidence responses were recorded for 25.9% of PTSD participants and 24.7% of TEC participants. We also calculated the response bias (Criterion), using signal detection theory as defined by the formula C = −[Z(Hit) + Z(FA)]/2. Response bias is an index of the tendency to overrespond or underrespond.
MRI Acquisition
Functional images were acquired on a 4-Tesla GE scanner with an inverse spiral pulse sequence to reduce susceptibility artifact (Guo and Song, 2003). A series of 34 interleaved axial functional slices were acquired for full-brain coverage with the following parameters: TR = 2000 ms; TE = 31 ms; flip angle = 60°; FOV = 240 mm2; matrix size = 642; slice thickness = 3.75 × 3.75 × 3.8 mm voxels; 173 image volumes were collected. High-resolution three-dimensional spin-echo co-planar structural images were acquired in 68 axial slices (TR = 12 ms; TE = 5.4 ms; flip angle = 20°; FOV = 240 mm2; matrix size = 2562; slice thickness = 1 × 1 × 1.9 mm voxels).
fMRI Data Analysis
Functional data sets were preprocessed using FSL version 4.1.0. Preprocessing occurred in the following steps: brain extraction for non-brain removal, motion correction using MCFLIRT, spatial smoothing using a Gaussian kernel of FWHM 5 mm, and high-pass filtering (Jenkinson et al., 2002; Smith et al., 2004). Functional images of each subject were co-registered to structural images in native space, and structural images were normalized to MNI space. The same transformation matrices were then used for functional-to-standard space transformations of co-registered functional images. Pre-whitening was estimated and corrected using FMRIB’s Improved Linear Model (Woolrich et al., 2001). Following preprocessing, two main analysis approaches were employed. The first was an ROI based analysis comparing trauma Dm and neutral Dm between patient groups, and the second used a voxelwise whole brain analysis to interrogate voxels correlated with FA rate.
ROI Approach
Structural ROI analyses were conducted to interrogate activity in the bilateral amygdala, hippocampus, and parahippocampal gyrus. The hippocampus and parahippocampal gyrus were further divided into anterior and posterior portions based on evidence for different functions along the longitudinal axis of these structures (Dolcos et al., 2004). ROIs were defined using the Wake Forest Pickatlas version 1.04 (Maldjian et al., 2003). The Dm, difference in brain activity showing greater response for items that were remembered than for items that were forgotten, was calculated for each ROI. This analysis is based on the arithmetic difference in the baseline corrected fMRI signal associated with high confidence hits relative to high confidence misses during the peak image volume for each epoch averaged across trials using custom software tools (Duke-UNC Brain Imaging and Analysis Center). For individual subject analyses, the fMRI signal was selectively averaged in each subject as a function of trial type (trauma hits, trauma misses, neutral hits, neutral misses) and image volume (TR) within the trial epoch (two image volumes preceding epoch onset and eight image volumes following epoch onset). This difference in signal was averaged across voxels within each region of interest (ROI). Data for the difference in fMRI signal was extracted from the overall time course using image volumes representing maximal change relative to the pre-event onset baseline. The maximal change was observed empirically to be 8 s after event onset. Thus, no assumption was made about the shape of the hemodynamic response function. Mean ROI signal from individual subjects was used in higher level analyses (MANOVA) that included group as a between-subject factor (PTSD, TEC), and hemisphere (left, right), ROI (amygdala, anterior hippocampus, posterior hippocampus, anterior parahippocampal gyrus, posterior parahippocampal gyrus), and trial type (trauma, neutral) as within subjects repeated measures. Medication use was included as a covariate of no-interest. As a further check on the role of medication, a repeated measures ANOVA on regional brain activity with a between group factor of antidepressant medication (on meds, off meds), and within subjects factor of picture type (trauma, neutral), region (amygdala, anterior hippocampus, posterior hipocampus, anterior parahippocampal gyrus, posterior parahippocampal gyrus) and hemisphere (left, right) was performed. There was no main effect of medication [F(1,13) = 2.7, P>.1], no interaction of medication*region [F(4,13) = .28, P>.8], and no interaction of medication*region*picture-type [F(4,13) = 1.0, P>.3]. Outliers representing two standard deviations from the mean were excluded from ROI analyses. Dm estimates were correlated with CAPS scores to examine the relationship between memory success and PTSD severity.
Whole-brain voxel wise
Analysis was conducted to identify active voxels outside the MTL. Onset times and the duration of pictures were used to model a signal response containing a regressor for each condition (i.e., high confidence hits and misses for each condition of trauma, neutral, and positive stimuli) and convolved with a gamma function. Low confidence trials were not modeled. Positive stimuli were included in the model to remove related variance from the trauma and neutral parameter estimates but were not considered in contrasts of interest. Parameter estimates from individual fMRI runs were fed into a second-level statistical analysis. Average maps representative of the general population were calculated in a mixed effects higher level analysis using Bayesian estimation techniques, FMRIB Local Analysis of Mixed Effects (FLAME). Employing a Bayesian approach in a multi-level group model reveals that the summary statistics ought to include not just the parameter estimates of model regression from the first-level, but the variance of these parameter estimates as well. Therefore, these summary statistics can be used to make inferences at the group level even when the between subject variance is not known (Beckmann et al., 2003; Woolrich et al., 2004; Woolrich et al., 2009). A cluster mean threshold of Z > 2.3 and a cluster-corrected significance threshold of P < 0.05 was imposed to protect against false positive detection of activation clusters (Forman et al., 1995; Friston et al., 1993; Worsley et al., 1992).
Results
Behavioral Results
Valence
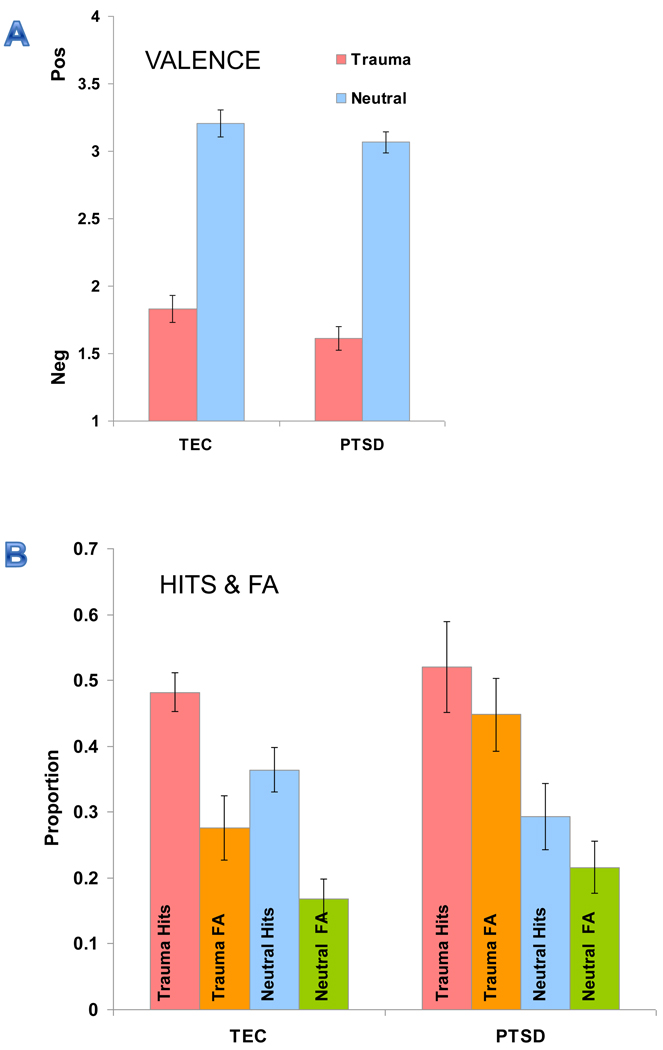
Repeated-measures ANOVA (picture: trauma, neutral) with group as a between-subject variable showed a main effect of picture [F(1,27) = 279.21, p < 0.001] and a trend for a main effect of group [F(1,27) = 3.27, p = 0.08] but no picture*group interaction (see Figure 1a). As expected, participants rated the trauma pictures as more negative than the neutral pictures.
Figure 1.
Valence ratings for PTSD and TEC group. Results show a main effect for picture type (A). Hit & False Alarm rates. PTSD patients showed higher false alarm rates for trauma information than TEC group (B). TEC = Trauma-exposed control group, FA = False Alarm rate, T = Trauma, N = Neutral. Error bars represent the standard error of means.
Memory Performance
D prime, a measure of sensitivity to signal strength, was first calculated using repeated measures ANOVA (picture: trauma, neutral) with group as a between-subjects variable. There were no main effects or significant interactions. To measure item-specific versus gist memory, hit and false alarm rates were examined separately (Figure 1b). Repeated-measures ANOVA for hit rate (trauma, neutral as repeated measures) revealed a main effect for picture type [F(1,27) = 22.54, p < 0.001] but no main effect of group or picture*group interaction. As expected, participants had higher hit rates for the trauma pictures than neutral pictures. Repeated-measures ANOVA for false alarm rate showed a main effect of picture [F(1,27) = 26.65, p < 0.001], group [F(1,27) = 4.19, p = 0.05], and a trend level effect for the picture*group interaction [F(1,27) = 3.51, p = 0.07]. Because we had an a priori interest in the interaction term, we conducted follow up tests to the trend-level omnibus ANOVA results. Follow-up tests confirmed that false alarm rates were highest for the trauma pictures, with the patients having higher false alarm rates than trauma-exposed controls for trauma pictures (p < 0.03) but not for neutral pictures (p > 0.34). These results suggest that although patients with PTSD tend to have less detailed memories across trauma-related and neutral events, the effect was mainly driven by false alarms for trauma-related pictures. Positive response bias was present in both the PTSD and TEC groups, but this bias was not significantly different between the two groups, main effect of group F(1,27) = 1.2, p > 0.2), further reinforcing a reliance on gist memory as the most likely explanation.
Imaging Results
ROI analyses
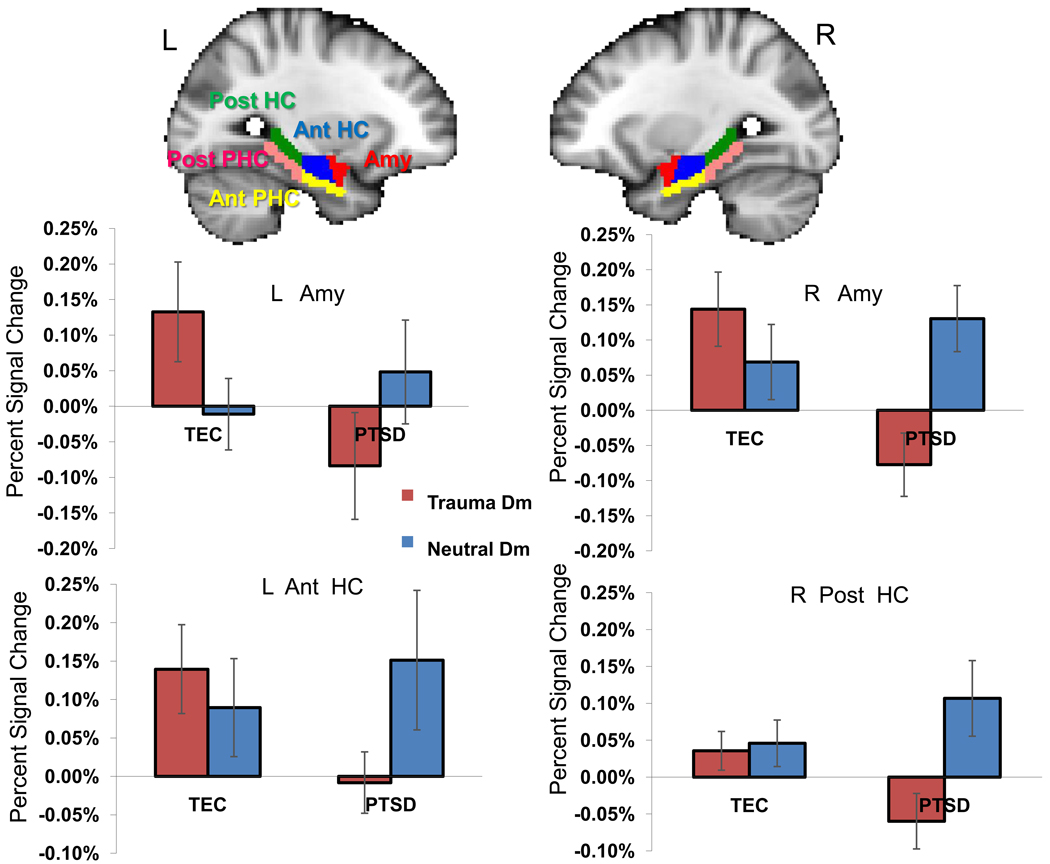
A 2 (picture: trauma, neutral) x 2 (hemisphere: left, right) x 5 (region: amygdala, anterior hippocampus, posterior hippocampus, anterior parahippocampal gyrus, posterior parahippocampal gyrus) repeated-measures MANOVA using group as a between-subjects variable and medication use as a covariate of no-interest revealed a significant picture*hemisphere*region*group interaction [F(4,11) = 3.30, p = 0.05]. Planned comparisons revealed that the PTSD group had lower trauma Dm scores than trauma-exposed controls in the right amygdala (p < 0.004), left amygdala (p < 0.05), left anterior hippocampus (p < 0.05), and right posterior hippocampus (p = 0.05) (Figure 2). There were no group differences in any of the five ROIs for the neutral Dm.
Figure 2.
Region-of-interest analysis results indicate reduced Dm effect for trauma pictures in PTSD group in bilateral amygdala, left anterior hippocampus and right posterior hippocampus. L = left, R = right, Dm = Difference due to memory effect, TEC = Trauma-exposed control group, Amy = amygdala, Ant = Anterior, HC = Hippocampus, PHC = Parahippocampal cortex, Post = Posterior. Error bars represent the standard error of means.
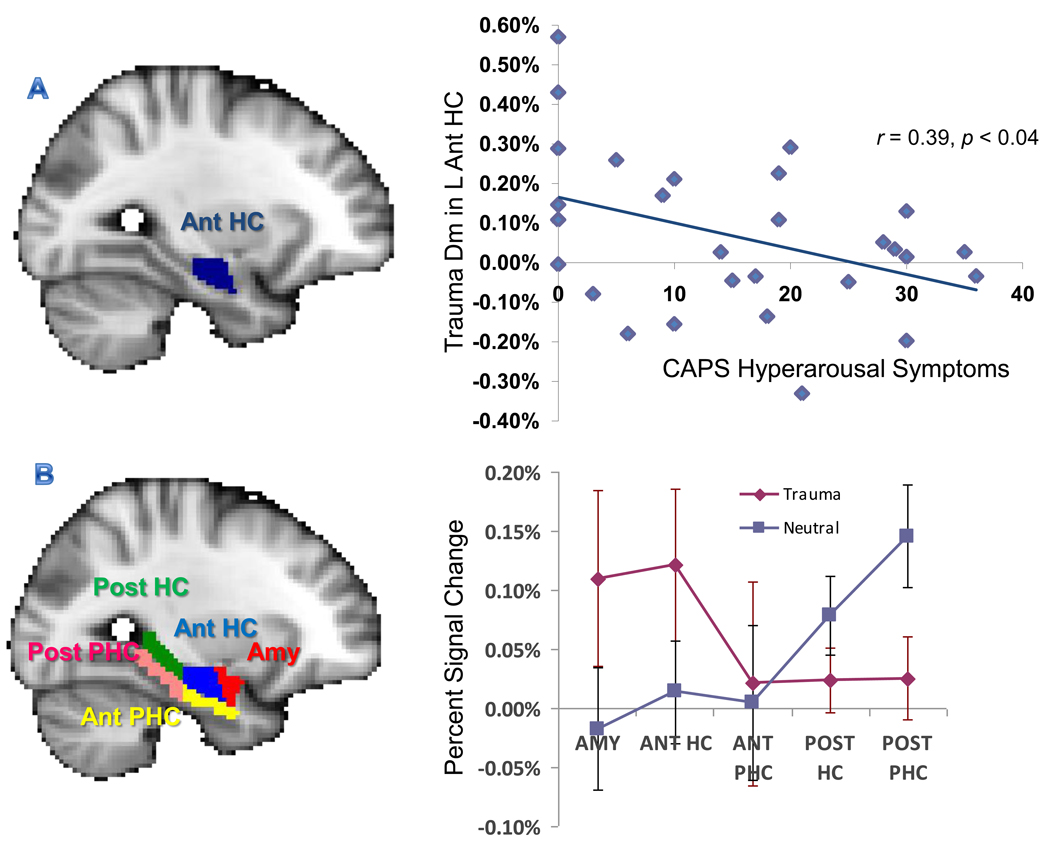
Correlation analyses between Dm and symptom clusters on the CAPS revealed that the trauma Dm in the left anterior hippocampus was negatively correlated with symptom cluster D (hyperarousal) on the CAPS (r = −0.39, p < 0.04; uncorrected) (Figure 3a). This finding suggests that veterans with greater levels of trauma-related arousal showed lower activity in the anterior hippocampus for trauma memory encoding. There were no significant correlations between neutral Dm and any of the CAPS symptom clusters.
Figure 3.
Negative correlation between the left anterior hippocampus Trauma Dm and hyperarousal symptoms on the CAPS (A). Activity shift for trauma and neutral Dm along the anterior-posterior medial temporal lobe axis in the TEC group (B). Dm = Difference due to memory effect, Amy = amygdala, ANT = anterior, POST = posterior, HC = hippocampus, PHC = parahippocampal gyrus. Error bars represent the standard error of means.
To test a secondary hypothesis that healthy individuals show a shift in activation for emotional and neutral stimuli along the longitudinal axis of the MTL, we examined activity for trauma and neutral stimuli in the trauma-exposed control and PTSD groups separately. Repeated-measures ANOVA in the trauma-exposed control group revealed a significant picture*region interaction in the left hemisphere [F(4,40) = 2.78, p < 0.04]. Consistent with our previous findings, follow-up tests indicated that there was an anterior-posterior shift in MTL activity, with greater activity for trauma Dm in anterior regions of the MTL, and greater activity for neutral Dm in posterior MTL regions (Figure 3b). This pattern was not replicated in the PTSD group.
Voxel-Wise Whole Brain Analysis
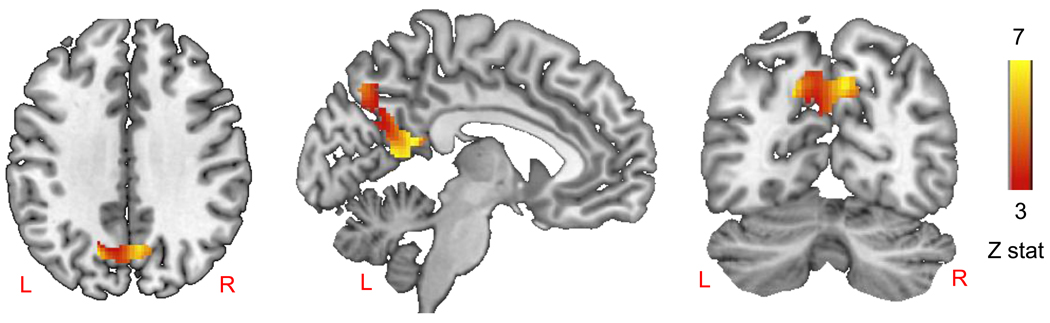
Given that PTSD patients showed greater false alarm (FA) rates on the behavioral task, we examined activity for traumatic stimuli (across hits and misses) as a function of individual differences in FA rate for the trauma pictures. Using FA rate as a covariate of interest in the voxel-wise whole brain analysis for the entire group, the analysis revealed that FA rate for trauma pictures co-varied with trauma-related activity (high confidence trauma hits > baseline) in the bilateral precuneus only (Figure 4). This finding suggests that individuals who produced more behavioral false alarms for traumatic pictures showed greater activity in bilateral precuneus during viewing of traumatic pictures.
Figure 4.
Precuneus activity associated with false alarm rate in all participants (peak voxel −2, −56, 10) shown in three orthogonal views.
Peak activations for the main effects of picture type and memory are reported in supplementary tables S1 and S2.
Discussion
The present study used fMRI to examine the neural basis of traumatic memory encoding in recent war veterans with PTSD and a comparison group of trauma-exposed controls. Neuroimaging results suggest that there may be at least two neural pathways involved in gist-based memory; a MTL pathway with reduced response during successful trauma memory encoding, and a pathway involving the precuneus where activation is associated with greater false alarms. Prior studies have shown that increased hippocampal activity is associated with successful encoding and retrieval of item-specific and detailed memories (Eichenbaum et al., 2007). In the present study, reduction in hippocampal activity for successfully encoded memories in the PTSD group may reflect inefficient encoding of item-specific, contextual information for trauma stimuli. Interestingly, the negative correlation between anterior hippocampal Dm activity and reported arousal symptoms on the CAPS suggests that participants with high levels of arousal had lower hippocampal activity for memory success.This finding may be evidence for the inverted U-shaped profile in the hippocampus as a function of stress (Nadel and Jacobs, 1998). That is, high levels of arousal and stress may impair hippocampal activity in patients with PTSD, leading to poorer memory encoding. Activation associated with trauma memories or encoding of trauma-related information shows evidence of diminished hippocampal activation (Bremner et al., 1999; Bremner et al., 2003a; Bremner et al., 2003b; Shin et al., 1999). Left posterior hippocampal activity associated with non-trauma related words measured at retrieval was also reduced in PTSD patients (Geuze et al., 2008b).
The behavioral data showed a trend consistent with the literature that patients with PTSD have distorted trauma memories. Memory distortion has been studied using a powerful behavioral paradigm that enables measurement of semantically related and unrelated intrusions as well as a similar type of memory distortion known as false recognition that occurs when individuals incorrectly report to have previously encountered a novel item related to a previously studied item. False recognition has been studied more extensively than recall intrusions (Schacter et al., 2000) and therefore provides insights into memory distortions in patients that would be hard to obtain from experiments of intrusion errors. Studies using a paradigm originally developed by (Deese, 1959) and subsequently modified by (McDermott, 1996) have clearly established false recognition in healthy adults as well as in memory disordered subjects (Budson et al., 2000). After studying lists of semantically connected words (forest, vine, leaves, shade, bark, canopy, etc) that are linked to an unstudied “related lure” (e.g. tree), healthy participants experience frequent intrusions of the related lure on free recall tests (Deese, 1959) and made high levels of false alarms to these words on recognition tests at a rate comparable to the hit rate (Roediger and McDermott, 2004). The act of recall actually enhanced later remembering of both studied and nonstudied material.
One interpretation of these results is that true and false recognition depend on memory for two different kinds of information, specific details of a prior encounter with a particular item (item specific recollection) and the general meaning, idea, or gist conveyed by a collection of items. As the study list is presented in the Deese-style paradigm, a gist representation is developed, which may result in an experience familiarity when either a studied item or a related lure is presented on a later recognition test. Thus in the Deese paradigm, accurate recognition of previously studied items depends on item-specific and gist information, whereas false recognition of related lure words depends on remembering gist but not on item-specific information (Budson et al., 2000; Payne et al., 1996; Reyna and Brainerd, 1995b; Schacter et al., 1996; Verfaellie et al., 2002). An alternative interpretation is that implicit semantic associations are formed during the Deese paradigm. However, when pictorial stimuli are used, gist-based explanations of false recognition are favored because novel picture lures would not likely be spontaneously generated by participants (Koustaal & Schacter, 1997; Simons et al., 2005).
In the present study, the similarity of content across trauma scenes can lead to a reliance on gist memory that results in false recognition, as evidenced by elevated false alarms for novel lures in the PTSD group compared to the trauma exposed control (TEC) group. This behavior is consistent with reports of gist based memories in PTSD that lack specific information (Rubin et al., 2008), and is consistent with the interpretation of prior reports on false recognition tasks using pictorial material (Koutstaal et al., 2001; Simons et al., 2005). While it is also possible that higher false alarm rates may indicate a response bias in the PTSD patients, this effect would extend also to the hit rate, which was not the case in our study. Importantly, this effect was driven by higher false alarms for trauma-related information. Although the behavioral effect was modest, the data may suggest that gist-based memory for negative information is not limited to autobiographical memories (Kaspi et al., 1995), but also to newly encoded trauma information. The clinical implication of this finding would be that maintenance of the disorder is associated with gist-based rather than detailed memories for trauma reminders.
Neurobiological theories posit that the combination of increased release of corticosteroids and modulation from a hyperactive amygdala during extreme levels of stress may be responsible for inhibiting hippocampal activity (Kim and Diamond, 2002). The putative function of the amygdala is to rapidly detect emotionally salient information in the environment (Larson et al., 2006; LeDoux, 2000) and has been shown to have a tight coupling with the hippocampus during encoding of negative information (Dolcos et al., 2004). Contrary to our expectations, the PTSD group did not show greater amygdala activity during the task than trauma-exposed control participants. Although most studies examining the neural circuitry of PTSD have found greater amygdala activity in patients with PTSD, our findings add to the small literature of studies showing no change or reduction in amygdala activity (Britton et al., 2005; Lanius et al., 2001; Phan et al., 2006). However, it is noteworthy that in the present study, PTSD patients had relatively greater activity for forgotten items than remembered items across the left and right amygdala, reflected in a negative trauma Dm (see Figure 2). It is possible that amygdala hyperactivity led to later forgetting of those items. Taken together, the results from the present study suggest that amygdala and hippocampal signal reduction for trauma-specific reminders in PTSD patients may underlie encoding of gist-based representations in lieu of specific and detailed contextual memories.
The precuneus cortex may be a second region that underlies gist memory in PTSD. Robust bilateral precuneus activity was positively correlated with tendency to produce false alarms for trauma information. Theoretical accounts of distortions in memory postulate that falsely remembered items may be driven by internally generated material at the time of encoding that increases familiarity for the item (Reyna and Brainerd, 1995a) or the ease and fluency by which the stimulus is processed (Jacoby, 1991) rather than memory for specific item and contextual details (Eichenbaum et al., 2007). Neuroimaging studies have supported these ideas. For instance, (Gonsalves et al., 2004) found greater precuneus and inferior parietal cortex activity when individuals reported having seen visual stimuli that they had actually only imagined. By contrast, encoding of neutral (non-trauma) items showed decreased precuneus activity was associated PTSD severity (Geuze et al., 2008a). Although speculative, it is possible that patients with PTSD may imagine their related personal stressors when confronted with trauma reminders, interfering with the encoding of stimulus-specific details. Reliving trauma may impair one’s ability to distinguish between previously seen trauma reminders and novel trauma lures, which in turn contributes to the production of greater false alarm rates. Falsely remembered items, in turn, may be incorporated into one’s prior memories for trauma and lead to gist-based representations in memory and the maintenance of PTSD.
Psychosocial therapies for PTSD that aim to provide a corrective memory experience have shown great success. For example, in cognitive processing therapy, patients write a trauma narrative that is subsequently challenged to provide better recall and contextualization of trauma memories (Monson et al., 2006; Resick and Schnicke, 1993). A study by Sutherland and Bryant suggested that PTSD patients were able to retrieve more specific details of memories after engaging in cognitive therapy (Sutherland and Bryant, 2007). These data are compelling evidence that memory distortions for one’s trauma are related to maintenance of the disorder. A natural future direction of this work would examine whether successful psychosocial intervention leads to an increase in MTL activity for trauma reminders.
The present results also extend findings from the cognitive neuroscience literature showing dissociation in function along the longitudinal axis of the MTL. Dolcos and colleagues demonstrated that the posterior parahippocampal gyrus was more active in response to neutral stimuli whereas the anterior parahippocampal gyrus was activated in response to emotional stimuli (Dolcos et al., 2004). We observed a similar pattern of results in the left MTL in the trauma-exposed control group only, in which the amygdala and anterior hippocampus showed greater response for negative pictures whereas the posterior hippocampus and posterior parahippocampal gyrus were more responsive to neutral stimuli. These results suggest that the trauma-exposed control group generally showed similar patterns of activity to healthy individuals, providing evidence that some neural processes that remain consistent regardless of trauma exposure (Dolcos et al., 2004).
Limitations of the present study deserve mention. First, we employed the false alarm rate as a measure of gist memory based on the assumption that high false alarms rates reflect poorer encoding of contextual details. However, false alarm rates may also be influenced by group differences in monitoring memory output at retrieval. Two additional design elements can address this limitation in future studies, including directly querying participants about stimulus-specific details to glean a more direct measure of contextual memory encoding, and scanning at retrieval to examine potential group differences in metamemorial monitoring. Second, our patient sample included nine veterans (60%) who were diagnosed with current co-morbid major depressive disorder (MDD) on the SCID interview. We did not exclude these individuals given the well-established evidence that a PTSD diagnosis carries a strikingly high risk of co-morbid depressive symptomatology. For instance, 77% of the PTSD patients in one large community sample were diagnosed with co-morbid MDD (Brown et al., 2001). In war veterans specifically, there is about 50% comorbidity rate of PTSD and MDD (Bleich et al., 1997; Dedert et al., 2009). Despite this perceived limitation, a strength of the present study is that these results can be generalized to the population of recent war veterans, who report both PTSD and depressive symptoms after their traumatic event (Dedert et al., 2009). Third, the negative correlation of the CAPS hyperarousal cluster with left anterior hippocampal activation was not corrected for multiple comparisons given that we tested hyperarousal in anterior and posterior subregions of the hippocampus in left and right hemispheres. Finally, while the representation of women in our sample (20%) is consistent with the gender breakdown of military personnel who served in Iraq and/or Afghanistan, the present findings may not generalize to individuals from other backgrounds and trauma experiences.
Conclusions
Results from the present study offer a neural account of distorted trauma memory representations in PTSD. We conclude that reduced activity in the amygdala and hippocampus during successful encoding of trauma memories may reflect encoding of gist-based trauma representations in lieu of detailed trauma memories. The overall clinical implication of these findings is that incomplete or gist-based representations of trauma memory may maintain the memory distortions observed in PTSD.
Supplementary Material
Acknowledgments
This work was supported by the Department of Veterans Affairs, Mental Illness Research Education and Clinical Center Grant for Post-Deployment Mental Health, National Institute of Mental Health Grants K23 MH073091 and K23 MH084013, and U.S. National Institutes of Health grant 2 P01 NS041328.
The VISN 6 Mid-Atlantic Mental Illness Research, Education, and Clinical Center workgroup (MIRECC) members include Jean C. Beckham, Patrick S. Calhoun, Rita M. Davison, A. Meade Eggleston, John A. Fairbank, Kimberly T. Green, Angela C. Kirby, Jeffrey M. Hoerle, Christine E. Marx, Scott D. Moore, Victoria Payne, Mary C. Pender, Christopher Petty, Jennifer L. Strauss, Kristy A. Straits-Troster, and Richard D. Weiner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir N, Stafford J, Freshman M, Foa E. Relationship between trauma narratives and trauma pathology. Journal of Traumatic Stress. 1998;11:385–392. doi: 10.1023/A:1024415523495. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bleich A, Koslowsky M, Dolev A, Lerer B. Post-traumatic stress disorder and depression. An analysis of comorbidity. The British Journal of Psychiatry. 1997;170:479. doi: 10.1192/bjp.170.5.479. [DOI] [PubMed] [Google Scholar]
- Brainerd C, Reyna V. Fuzzy-trace theory and false memory. Current Directions in Psychological Science. 2002;11:164. [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry. 2003a;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, Charney DS. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological Psychiatry. 2003b;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Brown S, Freeman T, Kimbrell T, Cardwell D, Komoroski R. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of former prisoners of war with and without posttraumatic stress disorder. Journal of Neuropsychiatry & Clinical Neurosciences. 2003;15:367–370. doi: 10.1176/jnp.15.3.367. [DOI] [PubMed] [Google Scholar]
- Brown T, Campbell L, Lehman C, Grisham J, Mancill R. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: gist-based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- de Quervain D, Roozendaal B, McGaugh J. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Dedert E, Green K, Calhoun P, Yoash-Gantz R, Taber K, Mumford M, Tupler L, Morey R. Association of trauma exposure with psychiatric morbidity in military veterans who have served since September 11, 2001. Journal of Psychiatric Research. 2009;43:830–836. doi: 10.1016/j.jpsychires.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- Dickie E, Brunet A, Akerib V, Armony J. An fMRI investigation of memory encoding in PTSD: Influence of symptom severity. Neuropsychologia. 2008;46:1522–1531. doi: 10.1016/j.neuropsychologia.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas A, Ranganath C. The Medial Temporal Lobe and Recognition Memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Molnar C, Cashman L. Change in rape narratives during exposure therapy for posttraumatic stress disorder. Journal of Traumatic Stress. 1995;8:675–690. doi: 10.1007/BF02102894. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Foy M, Stanton M, Levine S, Thompson R. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behavioral and Neural Biology. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1993;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, de Kloet CS, Westenberg HGM. Precuneal activity during encoding in veterans with posttraumatic stress disorder. Progress in Brain Research. 2008a;167:293–297. doi: 10.1016/S0079-6123(07)67026-5. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Ruf M, de Kloet CS, Westenberg HGM. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. Journal of Psychiatric Research. 2008b;42:659–669. doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Gonsalves B, Reber P, Gitelman D, Parrish T, Mesulam M, Paller K. Neural evidence that vivid imagining can lead to false remembering. Psychological Science. 2004;15:655. doi: 10.1111/j.0956-7976.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Guo H, Song AW. Spiral-in-and-out functional image acquisition with embedded z-shimming for susceptibility signal recovery. Journal of Magnetic Resonance Imaging. 2003;18:389–395. doi: 10.1002/jmri.10355. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biological Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A, Bryant R, Dang S. Autobiographical memory in acute stress disorder. Journal of Consulting and Clinical Psychology. 1998;66:500–506. doi: 10.1037//0022-006x.66.3.500. [DOI] [PubMed] [Google Scholar]
- Hayes J, LaBar K, Petty C, McCarthy G, Morey R. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry research Neuroimaging. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby L. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kaspi SP, McNally RJ, Amir N. Cognitive processing of emotional information in posttraumatic stress disorder. Cognitive Therapy & Research. 1995;19:433–444. [Google Scholar]
- Kim J, Diamond D. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Koss M, Figueredo A, Bell I, Tharan M, Tromp S. Traumatic memory characteristics: A cross-validated mediational model of response to rape among employed women. Journal of Abnormal Psychology. 1996;105:421–432. doi: 10.1037//0021-843x.105.3.421. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Schacter DL, Brenner C. Dual task demands and gist-based false recognition of pictures in younger and older adults. Journal of Memory and Language. 2001;44:399–426. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System. Gainesville: NIMH Center for the Study of Emotion and Attention; 1997. [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Larson C, Schaefer H, Siegle G, Jackson C, Anderle M, Davidson R. Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60:410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Layton B, Krikorian R. Memory mechanisms in posttraumatic stress disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:254–261. doi: 10.1176/jnp.14.3.254. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McDermott KB. The persistence of false memories in list recall. Journal of Memory and Language. 1996;35:212–230. [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: Interaction with other brain systems. PNAS: Proc Natl Acad Sci. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally R. Progress and controversy in the study of posttraumatic stress disorder. Annual Review of Psychology. 2003;54:229–252. doi: 10.1146/annurev.psych.54.101601.145112. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Litz BT, Prassas A, Shin LM, et al. Emotional priming of autobiographical memory in post-traumatic stress disorder. Cognition & Emotion. 1994;8:351–367. [Google Scholar]
- Monson C, Schnurr P, Resick P, Friedman M, Young-Xu Y, Stevens S. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 2006;74:898–907. doi: 10.1037/0022-006X.74.5.898. [DOI] [PubMed] [Google Scholar]
- Morey R, Dolcos F, Petty C, Cooper D, Hayes J, LaBar K, McCarthy G. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. Journal of Psychiatric Research. 2009;43:809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Cooper DA, LaBar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Research: Neuroimaging. 2008;162:59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Jacobs WJ. Traumatic Memory is Special. Curr Dir Psychol Sci. 1998;7:154–157. [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Payne DG, Elie CJ, Blackwell JM, Neuschatz JS. Memory illusions: Recalling, recognizing, and recollecting events that never occurred. Journal of Memory and Language. 1996;35:261–285. [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Resick P, Schnicke M. Cognitive processing therapy for rape victims: A treatment manual (Sage Pubns) 1993 [Google Scholar]
- Reyna V, Brainerd C. Fuzzy-trace theory: An interim synthesis. Learning and Individual Differences. 1995a;7:1–75. [Google Scholar]
- Reyna VF, Brainerd CJ. Fuzzy-trace theory: An interim synthesis. Learning and Individual Differences. 1995b;7:1–75. [Google Scholar]
- Roediger H, McDermott K. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology-learning memory and cognition. 1995;21:803–814. [Google Scholar]
- Roediger HL, III, McDermott KB. Creating False Memories: Remembering Words Not Presented in Lists. New York, NY US: Psychology Press; 2004. [Google Scholar]
- Rubin DC, Berntsen D, Bohni MK. A memory-based model of posttraumatic stress disorder: evaluating basic assumptions underlying the PTSD diagnosis. Psychological Review. 2008;115:985–1011. doi: 10.1037/a0013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. In: Bjorklund DF, editor. False-memory creation in children and adults: Theory, research, and implications. Mahwah, NJ US: Lawrence Erlbaum Associates Publishers; 2000. pp. 129–168. [Google Scholar]
- Schacter DL, Verfaellie M, Pradere D. The neuropsychology of memory illusions: False recall and recognition in amnesic patients. Journal of Memory and Language. 1996;35:319–334. [Google Scholar]
- Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biological Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. American Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Simons JS, Verfaellie M, Hodges JR, Lee ACH, Graham KS, Koutstaal W, Schacter DL, Budson AE. Failing to get the gist: reduced false recognition of semantic associates in semantic dementia. Neuropsychology. 2005;19:353–361. doi: 10.1037/0894-4105.19.3.353. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Sutherland K, Bryant R. Autobiographical memory in posttraumatic stress disorder before and after treatment. Behaviour Research and Therapy. 2007;45:2915–2923. doi: 10.1016/j.brat.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter M, Elzinga B, van Balkom A, Smoor P, Smit J, Veltman D. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: A pilot study. Psychiatry Research: Neuroimaging. 2009;171:44–53. doi: 10.1016/j.pscychresns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Schacter DL, Cook SP. The effects of retrieval instructions on false recognition: Exploring the nature of the gist memory impairment in amnesia. Neuropsychologia. 2002;40:2360–2368. doi: 10.1016/s0028-3932(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biological Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- Wignall EL, Dickson JM, Vaughan P, Farrow TFD, Wilkinson ID, Hunter MD, Woodruff PWR. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biological Psychiatry. 2004;56:832–836. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. American Journal of Psychiatry. 2004;161:2194–2200. doi: 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Wright D, Loftus E. How misinformation alters memories. Journal of Experimental Child Psychology. 1998;71:155–164. doi: 10.1006/jecp.1998.2467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.