Non-technical summary
Exercise is known to stimulate the production of various exercise factors including the well-described muscle-derived interleukin-6 (IL-6). We show that exercise causes a massive expression of the chemokine CXCL-1 in serum, in skeletal muscle and especially in the liver. Furthermore we find that this exercise-induced liver CXCL-1 expression is regulated by IL-6 and that muscle-derived IL-6 is capable of stimulating liver CXCL-1 expression. Such knowledge of the regulation of exercise factors contributes to the understanding of how the liver and muscle communicate in response to exercise.
Abstract
Abstract
The chemokine CXC ligand-1 (CXCL-1) is a small cytokine that elicits effects by signalling through the chemokine receptor CXCR2. CXCL-1 has neutrophil chemoattractant activity, is involved in the processes of angiogenesis, inflammation and wound healing, and may possess neuroprotective effects. The aim of this study was to unravel the mechanisms whereby CXCL-1 is regulated by exercise in mice. After a single bout of exercise, CXCL-1 protein increased in serum (2.4-fold), and CXCL-1 mRNA in muscle (6.5-fold) and liver (41-fold). These increases in CXCL-1 were preceded by increases in serum interleukin-6 (IL-6) and muscle IL-6 mRNA. In contrast, exercise-induced regulation of liver CXCL-1 mRNA expression was completely blunted in IL-6 knockout mice. Based on these findings, we examined the possible existence of a muscle-to-liver axis by overexpressing IL-6 in muscles. This resulted in increases in serum CXCL-1 (5-fold) and liver CXCL-1 mRNA expression (24-fold) compared with control. Because IL-6 expression and release are known to be augmented during exercise in glycogen-depleted animals, CXCL-1 and IL-6 expression were examined after exercise in overnight-fasted mice. We found that fasting significantly augmented serum CXCL-1, and CXCL-1 expression in liver and muscle. Taken together, these data indicate that liver is the main source of serum CXCL-1 during exercise in mice, and that the CXCL-1 expression in the liver is regulated by muscle-derived IL-6.
Introduction
Exercise induces many beneficial metabolic effects and a link has been widely sought between muscle contraction and exercise-induced metabolic changes in other organs such as the liver and the adipose tissue. Today, it is known that cytokines and other peptides are released from working muscles. They exert either autocrine, endocrine or paracrine effects and are classified as myokines (Pedersen & Febbraio, 2008). Recent proteomic studies suggest that the list of myokines may include more than 600 candidates belonging to distinctly different families (Henningsen et al. 2010).
IL-6 was the first cytokine shown to be released from working muscles. IL-6 increases fat oxidation in muscle (Brandt & Pedersen, 2010), lipolysis in adipose tissue (Trujillo et al. 2004), and is implicated in the regulation of gluconeogenic genes in the liver (Banzet et al. 2009). Moreover, exercise-induced IL-6 mRNA expression and plasma IL-6 have been shown to be more marked when muscle glycogen is low prior to exercise (Keller et al. 2001) and high levels of IL-6 mRNA have been demonstrated to coincide with low muscular glycogen following exercise (Steensberg et al. 2001). This suggests that IL-6 may work as a sensor of carbohydrate availability (Pedersen et al. 2004).
Interestingly, it was recently reported that exercise induces a small increase in skeletal muscle mRNA and serum concentration of the murine chemokine CXC motif ligand 1 (CXCL-1) (Nedachi et al. 2008), suggesting that potentially CXCL-1 acts as a myokine. CXCL-1 belongs to the glutamate–leucine–arginine (ELR)-containing CXC chemokine family and is primarily known as a chemoattractant for neutrophil infiltration (Lira et al. 1994) and as a promoter of tumour growth (Dhawan & Richmond, 2002). It shares the highest sequence homology with human CXCL-1 (90% homology in conserved regions) (Oquendo et al. 1989), but it is often mentioned as the functional homologue to human IL-8 (Rubio & Sanz-Rodriguez, 2007).
The production of different chemokines in the ELR+ CXC family has been shown to be induced by IL-6. IL-6 stimulates CXCL-1 production in thymic epithelial cells in vitro, and this stimulation is dependent on Jak2 activation (Tseng et al. 2010). In line with this, Sheikh et al. (2006) showed that production of the rat homologue to CXCL-1, CINC-1 (cytokine-induced neutrophil chemoattractant-1), is increased upon treatment with IL-6. Likewise, it has been demonstrated that oncostatin M, which belongs to the same cytokine family as IL-6, stimulates CXCL-1 production in HC11 mammary epithelial cells (Bierie et al. 2009). Therefore, the aim of the present study was to test the hypothesis that IL-6 is an important regulator of exercise-induced CXCL-1.
To test our hypothesis, we evaluated CXCL-1 and IL-6 expression in different tissues following an exercise bout in mice. Furthermore, CXCL-1 mRNA expression was investigated in resting and exercising IL-6 knockout (KO) mice and in mice overexpressing IL-6 in skeletal muscle. We were able to demonstrate that the liver is the main source of elevated serum CXCL-1 in response to exercise and that the liver CXCL-1 expression is regulated by muscular IL-6 expression.
Methods
Ethics
All animal experiments were conducted in accordance with the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimentation and after permission from the Danish Animal Experiments Inspectorate.
Animals and tissue preparation
Exercise trial in wild-type mice
Eight- to ten-week-old NMRI mice (own breed) performed 1 h of swimming exercise in 37°C water and were killed immediately, 2 h or 5 h after the exercise bout. The control group did not exercise, but had food and water removed 1 h before they were killed. The exercise started at 9 am, which was during the light period. Three muscles (tibialis cranialis, gastrocnemius, soleus), liver, kidney, heart, and subcutaneous and visceral adipose tissue were dissected immediately after decapitation and frozen in dry ice and absolute alcohol. The exercise trial was repeated with overnight-fasted mice. At the time of killing, blood glucose was measured using a Glucometer Hemocue 201+ system (Hemocue, Sweden).
Exercise trial in IL-6 KO mice
C57Bl/6 wild-type (WT; Taconic, Tornbjerggaard, Denmark) and whole-body IL-6 knock-out (KO) 4-month-old male mice (IL-6 breeding pairs were provided by Horst Bluethmann) (Kopf et al. 1994) performed running exercise on a treadmill (model Exer-4 treadmill; Columbus Instruments, OH, USA) for 1 h at a speed of 15.5 m min−1 and a slope of 10%. The mice were killed by cervical dislocation immediately after exercise, or 4 h or 10 h after exercise. In addition, a group of WT and KO mice did not exercise and were killed at the same time points as the exercised mice. Liver tissue and the quadriceps muscle were removed and immediately frozen in liquid nitrogen and stored at −80°C.
IL-6 electrotransfected mice
Overexpression of IL-6 was obtained by electrotransfer of IL-6 into the tibialis cranialis muscle in 8-week-old C57Black/C mice (Taconic), as previously described (Hojman et al. 2007). pCMV-IL6 encoding murine IL-6 under control of a CMV promoter was cloned by Geneart (Regensburg, Germany). DNA preparations were performed using Qiafilter Plasmid Maxiprep kits (Qiagen, Hilden, Germany). In short, anaesthetised mice were injected intramuscularly with 20 μl plasmid solution (0.5 μg μl−1) and electropulsed using 4 mm plate electrodes and electric parameters of one high voltage pulse (100 μs, 800 V cm−1) and one low voltage pulse (400 ms, 100 V cm−1). One week after transfection, the mice were killed and their tibialis cranialis muscle and intact livers were immediately excised.
Blood analysis
Blood glucose
Blood glucose was measured in blood samples using a Glucometer Hemocue 201+ system (Hemocue, Sweden).
Serum cytokines
Five hundred microlitres of blood were drawn immediately after euthanasia and processed to serum. A MesoScaleDiscovery multiplex platform (MesoScaleDiscovery, USA) pre-coated with CXCL-1, IL-6, IL-1 and IL-10 capture antibodies was used according to the manufacturer's protocol. MSD plates were measured on a MSD Sector Imager 2400 plate reader. Raw data were measured as electrochemiluminescence signal (light) detected by photodetectors and analysed using the Discovery Workbench 3.0 software (MSD). A 4-parameter logistic fit curve was generated for each analyte using the standards and concentration of each sample calculated. The sensitivity of each of the analytes was: CXCL-1: 3.3 pg ml−1, IL-6: 4.5 pg ml−1, IL-1: 0.75 pg ml−1 and IL-10: 11 pg ml−1. For each analyte the coefficient of variation was below 10%.
RT-qPCR
Reverse transcription
Total RNA was isolated from frozen tissues by tissue homogenisation and RNA extraction using TRIzol reagent (Invitrogen Life Technologies, Denmark). The purity and concentration of the RNA samples were determined using a Nanodrop spectrophotometer. Reverse transcription was performed using MultiScripe Reverse Transcriptase (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems, UK) and random p(dN)6 primers (Applied Biosystems, UK) following the manufacturer's instructions.
Real-time PCR
All amplifications were carried out in a final volume of 10 μl with 3 μl cDNA using the SYBR Green PCR Master Mix (Applied Biosystems, UK) and sequence-specific primers. The following PCR primers were used: for CXCL-1, forward: 5′-GCTGGGATTCACCTCAAGAA-3′ and reverse: 5′-TGGGGACACCTTTTAGCATC-3′; for IL-6: forward: 5′-CCAGGTAGCTATGGTACTCCAGAA-3′ and reverse: 5′-GCTACCAAACTGATATAATCAGGA-3′. Quantitative PCR and detection were performed using ABI 7900 Sequence Detection System (Applied Biosystems). Quantification was performed using the comparative threshold cycle method. The target genes were normalised to 18S. In IL-6 KO mice, CXCL-1 expression was normalised to β-actin. The efficiency of each individual plate and primer pair were continuously observed by inclusion of standard curves on each plate, in order to ensure a comparable efficiency of 99–101%. Dissociation curve analysis was performed after each PCR to check for unspecific signals.
Western blot analysis
Total protein from muscle and liver was extracted in 500 μl homogenisation buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EGTA, 1 mm EDTA, 0.25% sodium deoxycholate, 1% Triton X-100) containing sodium orthovanadate, phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich) and a complete protease inhibitor cocktail (Roche Basel, Switzerland), followed by homogenisation in pre-cooled racks using a Tissuelyzer (Qiagen, Valencia, CA, USA) for 1 min at 30 Hz followed by 15 min incubation on ice. Homogenates were centrifuged at 16,000 g at 4°C for 25 min. The supernatant protein concentrations were determined using a Bio-Rad DC kit (Bio-Rad, Hercules, CA, USA) and bovine serum albumin as standard. All determinations were done in triplicate. Equal amounts of denatured proteins from muscle and liver tissue homogenates were separated by gel electrophoresis using a NuPage 8% Bis-Tris gel (Invitrogen, Taastrup, Denmark), followed by immunoblotting to polyvinylidene fluoride (PVDF) membranes using Invitrogen iBlot 7 min Dry Blotting System. Membranes were incubated in blocking buffer (TopBlock) for 1 h at room temperature followed by incubation with primary and secondary antibodies for optimised times and concentrations and washed with Tris-buffered saline containing 0.1% Tween-20. The primary antibody used was anti-mCXCL-1/keratinocyte-derived chemokine (R&D systems). The secondary antibody was anti-goat from DakoCytomation (Denmark). Protein bands were detected using Supersignal West Femto (Pierce, Rockford, IL, USA) and quantified using a CCD image sensor (ChemiDocXRS, Bio-Rad) and software (Quantity One, Bio-Rad). Preliminary experiments demonstrated that the amounts of protein loaded were within the dynamic range for the conditions used and the results obtained (data not shown), and immunoreactive bands migrated at expected relative mobilities. Membranes were stained with Reactive Brown (RB) protein and the level of RB was used as a loading and transfer control between samples.
Statistical analysis
One-way ANOVA followed by Tukey's post hoc test was used to evaluate the effect of exercise. Student's t test for independent samples was used to evaluate the effect of IL-6 transfection. Two-way ANOVA was used to test the effect of exercise and genotype, the effect of glucose availability and exercise as well as the effect of exercise on muscle and liver CXCL-1 protein level. If analyses revealed a significant interaction, Bonferroni's post hoc test was used to evaluate intergroup comparisons. The significance level for all comparisons was set at P < 0.05. All data are presented as means ±s.e.m.
Results
Exercise-induced serum CXCL-1 and IL-6
Two hours post exercise serum CXCL-1 increased significantly (127 ± 6.3 pg ml−1) compared with control levels (52 ± 1.9 pg ml−1, P < 0.001). Five hours into the recovery period serum CXCL-1 had declined (73.1 ± 7.7 pg ml−1) but was still significantly enhanced compared with the control group (P < 0.05) (Fig. 1A).
Figure 1. Exercise enhances serum CXCL-1 and IL-6.
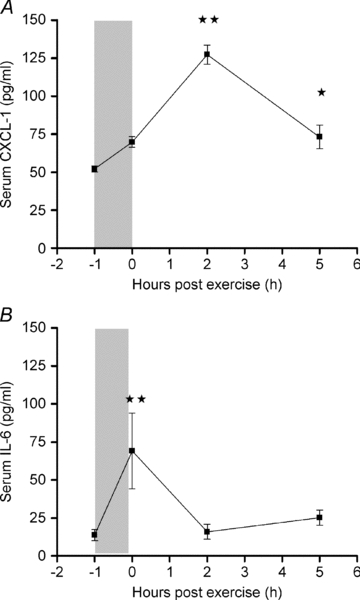
CXCL-1 (A) and IL-6 (B) were measured in serum obtained from mice killed immediately (0 h), 2 h or 5 h after performing a single bout of exercise, and from a non-exercising control group (−1 h). Data represent means ±s.e.m. (n = 8). Statistical analysis was performed using one-way ANOVA (A: P < 0.0001, B: P < 0.0001) and intergroup comparison was tested with Tukey's post hoc test with correction for multiple testing. Significantly different from control (−1 h): *P < 0.05, **P < 0.001.
As expected, serum IL-6 increased following exercise (P < 0.0001). A significant increase in serum IL-6 was found immediately after exercise (69.1 ± 8.8 pg ml−1) compared with control levels (13.8 ± 1.3 pg ml−1, P < 0.001). Two hours post exercise, serum IL-6 had declined to control level (Fig. 1B). An increase in IL-10 immediately after exercise was followed by a rapid decrease in the recovery period, whereas IL-1 did not show any exercise effect (data not shown).
Exercise-induced CXCL-1 mRNA expression in liver and muscle tissue
In order to ascertain the organ source of origin, CXCL-1 mRNA expression was determined in various tissues after a single exercise bout (Fig. 2). The most marked increase in CXCL-1 expression was found in the liver (P < 0.001), peaking 2 h into the recovery period with a 41-fold increase (P < 0.001) (Fig. 2I). It is of note that this increase in liver CXCL-1 mRNA expression coincided with the peak in serum CXCL-1. At 5 h of recovery, liver CXCL-1 mRNA expression had decreased to control level.
Figure 2. Exercise increases CXCL-1 mRNA expression in muscle and liver tissue.
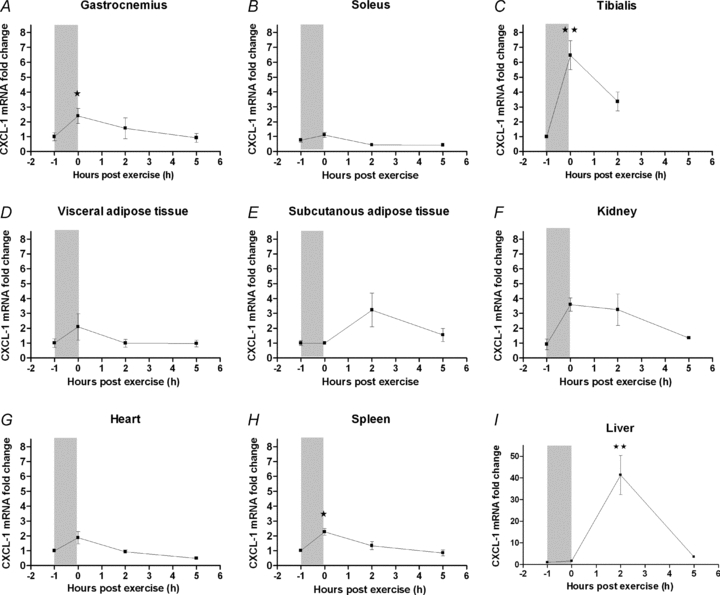
NMRI mice performed a single bout of exercise and were killed immediately (0 h), or at 2 or 5 h after. One group did not exercise (−1 h). The gastrocnemius (A), soleus (B), tibialis cranialis muscles (C), visceral adipose tissue (D), subcutaneous adipose tissue (E), kidney (F), heart (G), spleen (H) and liver (I), were dissected and CXCL-1 mRNA expression was determined. The relative expression of CXCL-1 mRNA is presented as fold change values in proportion to the control (−1 h) expression level. Fold change values are depicted as means ±s.e.m. (n = 8). Statistical analysis was performed using one-way ANOVA followed by intergroup comparison using Tukey's post hoc test. Significantly different from pre-exercise level (−1 h): *P < 0.05, **P < 0.001, post hoc tests.
Muscle CXCL-1 mRNA expression increased significantly immediately after the exercise bout with a 2.4-fold increase in the gastrocnemius muscle (P < 0.05) and a 6.5-fold increase in the tibialis cranialis muscle (P < 0.001) (Fig. 2A and C). In both muscles, CXCL-1 expression had decreased to control level 2 h into the recovery period. The actual CXCL-1 mRNA expression levels showed that in resting conditions CXCL-1 was expressed at similar levels in liver and muscle. However, after exercise the increase in CXCL-1 mRNA expression in the liver (Ct = 22.7 ± 0.4) (Ct: threshold cycle) far exceeded that of muscle tissue (Ct = 27.2 ± 0.2) (see Supplemental Table 1, available online only).
In continuation, we determined the CXCL-1 protein expression in liver and muscle. At control levels, CXCL-1 protein was not significantly higher in the liver than in the muscle (P = 0.085). After 1 h of swimming, CXCL-1 protein in the liver increased markedly, and was 3-fold up-regulated compared to muscle CXCL-1 protein levels at the same time point. This increase was significant immediately after exercise (0H) and 2 h after exercise (2H) (Fig. 3). Taken together, these data suggest that both liver and muscles contribute to serum CXCL-1 with liver as the main source.
Figure 3. Exercise increases CXCL-1 protein in liver but not muscle.
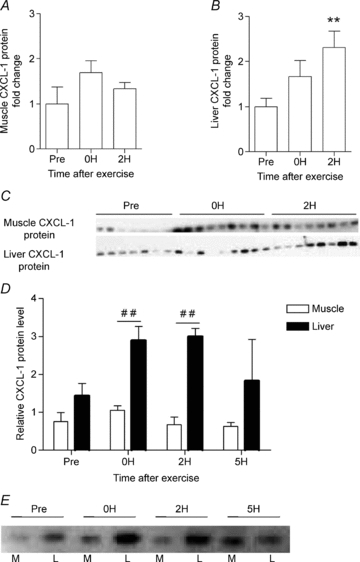
NMRI mice performed a single bout of exercise and were killed immediately (0 h), or at 2 (2H) or 5 h (5H). One group did not exercise (Pre) 5H only shown in panels D and E. CXCL-1 protein expression (Western blotting) was determined separately in muscle (A) and liver (B) tissue (n = 8). Statistical analysis of CXCL-1 protein expression in each tissue was performed using one-way ANOVA followed by intergroup comparison by Tukey's post hoc test with correction for multiple testing. ** indicates significance at P < 0.01 from pre-exercise level (Pre). The two corresponding CXCL-1 protein blots for liver and the muscle are showed in (C). Comparison of the relative level of CXCL-1 protein expression in muscle and liver at the different time points before and after exercise are illustrated in (D), with the representative protein blot shown in E (n = 3). Statistical analysis was performed by two-way ANOVA with intergroup comparison by Bonferroni's post hoc test. ## indicates statistical significance between muscle and liver tissue at the respective time points. All CXCL-1 protein levels are normalised to the corresponding level of Reactive Brown. The relative protein expression is presented as fold change values in proportion to the control (Pre) expression level. Fold change values are depicted as means ±s.e.m.
Exercise-induced liver CXCL-1 expression is abolished in IL-6 knockout mice
To investigate the link between IL-6 and the increase in liver CXCL-1 expression in response to exercise, liver CXCL-1 induction was measured in IL-6 KO mice after acute exercise. CXCL-1 mRNA expression in non-exercised mice was not different between wild-type (WT) and IL-6 KO mice (Fig. 4A). One hour of treadmill running induced a 2-fold increase in liver CXCL-1 mRNA expression in WT animals immediately after exercise. This increase was completely blunted in the IL-6 KO mice (P < 0.05), which did not show an increase in CXCL-1 expression at any time points in response to exercise (Fig. 4B). In muscle, the treadmill run induced a 3-fold increase in CXCL-1 mRNA expression in WT animals immediately after exercise (P = 0.111). The exercise-induced increase in CXCL-1 expression was blunted in the IL-6 KO mice, which did not show an increase in CXCL-1 expression at any time point (P < 0.05) (see online Supplemental Fig. 1).
Figure 4. Exercise-induced CXCL-1 mRNA expression is inhibited in IL-6 knockout mice.
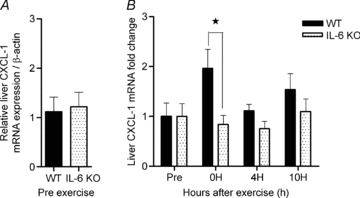
A, the relative expression of liver CXCL-1 mRNA was measured in WT and IL-6 KO non-exercising mice. B, immediately (0 h), 4 h (4H) or 10 h (10H) after a single bout of exercise, liver tissue was dissected and the relative CXCL-1 mRNA expression was measured and presented as fold change values proportional to a non-exercising control group. Data are depicted as means ±s.e.m. (n = 12). Statistical significance was tested by Student's t test (A) and two-way ANOVA with Bonferroni's post hoc test (B). *Significance at P < 0.05 in the post hoc tests.
Overexpression of IL-6 in muscle increases CXCL-1 expression in the liver
To further investigate if muscle-derived IL-6 is responsible for CXCL-1 expression in the liver, we overexpressed IL-6 in tibialis cranialis muscles by electrotransfer. One week after IL-6 electrotransfer, serum CXCL-1 (5-fold) and serum IL-6 (14-fold) were increased relative to control (P < 0.0001) (Fig. 5A and B). IL-6 electrotransfer resulted in a significant 24-fold liver CXCL-1 mRNA increase compared with control animals (P < 0.001) (Fig. 5C). No increase in liver IL-6 expression was detected, indicating that muscle-derived IL-6 signals to the liver and promotes CXCL-1 expression. Of note, muscle IL-6 electrotransfer also increases the local muscle CXCL-1 expression by 5-fold (P < 0.01) (Fig. 5E and F).
Figure 5. Overexpression of IL-6 in the tibialis cranialis muscle increases liver CXCL-1 expression.
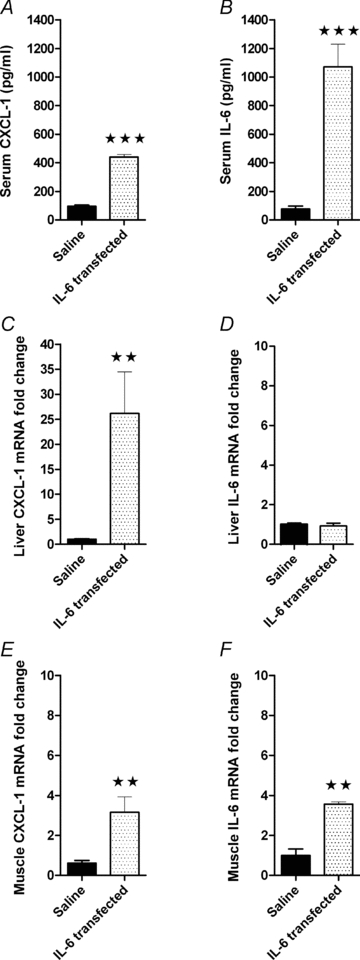
Mice were electrotransfected with IL-6 into the tibialis cranialis muscle and killed after 1 week. Serum CXCL-1 (A) and IL-6 (B), as well as liver CXCL-1 (C) and IL-6 (D), and muscle CXCL-1 (E) and IL-6 (F) mRNA expression were determined. The relative expression of CXCL-1 and IL-6 is presented as fold change values proportional to the control group (Saline). Data are presented as means ±s.e.m. (n = 8). Statistical analysis was performed by Student's t test. **Significantly different from saline, P < 0.001. ***significance at P < 0.001.
Overnight fasting increases exercise-induced IL-6 and CXCL-1 expression
It is well documented that exercise-induced muscular IL-6 mRNA expression correlates with intramuscular glycogen levels. Thus, to further investigate the role of IL-6 in regulating exercise-induced CXCL-1, we performed an exercise study in which half of the mice were fasted overnight in order for them to deplete their glycogen deposits. The overnight fast markedly decreased control blood glucose levels (8.1 ± 0.2 mmol l−1 in fed vs. 6.1 ± 0.8 mmol l−1 in fasted mice, P < 0.001). Blood glucose was also significantly lower in fasted than in fed mice at the end of the exercise bout (0H) (3.9 ± 0.3 mmol l−1 in fasted vs. 8.0 ± 0.3 mmol l−1 in fed mice, P < 0.001) (Fig. 6A).
Figure 6. Fasted/fed state influences IL-6 and CXCL-1 mRNA expression in liver and tibialis cranialis muscle.
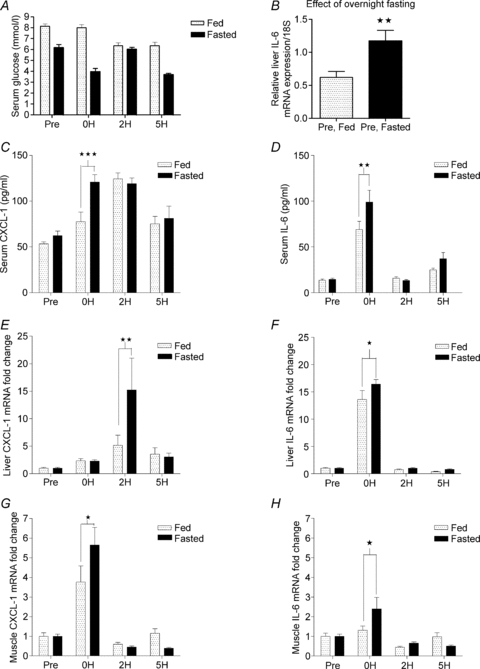
Overnight fasted and normal fed mice performed a single bout of exercise and were killed immediately, 2 h or 5 h after exercise. In addition, fasted and normal fed mice that did not exercise served as control groups. Immediately after the mice were killed, blood glucose (A), and serum CXCL-1 and IL-6 were measured (C and D), liver CXCL-1 and IL-6 mRNA expressions (B, E and F) were determined, as were muscle CXCL-1 (G) and IL-6 (H) mRNA expressions. The relative mRNA expression is depicted as fold changes proportional to the control expression level. Data are presented as means ±s.e.m. (n = 8). Statistical significance was tested by Student's t test (B) and two-way ANOVA with Bonferroni's post hoc test (C–F). *Significance at P < 0.05; **significance at P < 0.01, ***significance at P < 0.001, in the post hoc tests.
Interestingly, the overnight fast itself induced a 2-fold increase in control (non-exercised) level of liver IL-6 mRNA (Fig. 6B). Control levels of tibialis IL-6 and CXCL-1 and liver CXCL-1 mRNA expression did not change between fed and fasted mice (data not shown).
As expected, exercise resulted in a higher level of serum IL-6 in fasted mice (99.2 ± 12.6 pg ml−1) than in fed mice immediately after exercise (69.1 ± 8.8 pg ml−1, P < 0.01). CXCL-1 was also higher in fasted (120.9 ± 7.9 pg ml−1) than in fed mice (69.7 ± 3.6 pg ml−1, P < 0.001) immediately after exercise, while the increase was similar at 2 h of recovery (Fig. 6C and D).
In the liver, CXCL-1 mRNA expression peaked around 2 h after exercise and was also significantly higher in fasted mice (15-fold) than in fed mice (5-fold, P < 0.01). Liver IL-6 was also significantly higher in fasted mice (16-fold) compared with fed mice (14-fold, P < 0.05). In the tibialis cranialis muscle, CXCL-1 mRNA increased by 6-fold in fasted mice, which was significantly (P < 0.05) more than the increase detected in fed mice (4-fold). As expected, the exercise-induced IL-6 mRNA expression in the tibialis cranialis muscle increased significantly more in fasted mice than in fed mice immediately after exercise (2-fold in fasted vs. 1.1-fold in fed, P < 0.05) (Fig. 6E–H).
Discussion
In this study we demonstrate that acute exercise increases the level of circulating CXCL-1 especially during the recovery period. The increase correlates with large increases in liver CXCL-1 mRNA and protein expression, suggesting that the liver is the main source of circulating CXCL-1 after exercise. Moreover, we provide evidence that IL-6 released from muscle tissue regulates liver CXCL-1 expression, suggesting the existence of a muscle-to-liver axis in response to exercise.
A recent study by Nedachi and colleagues (Nedachi et al. 2008) detected an immediate increase in muscle expression and serum levels of CXCL-1 in mice after a bout of exercise. In accordance with this, we found an increase in serum CXCL-1 following acute exercise, peaking 2 h into the recovery period (Fig. 1). The source of exercise-induced serum CXCL-1 has not previously been fully evaluated. In agreement with the previous study (Nedachi et al. 2008), we found that muscle CXCL-1 mRNA expression increased immediately after exercise (Fig. 2), but this CXCL-1 expression declined rapidly towards control level during the recovery period. Importantly, in the present study the induction of CXCL-1 expression was by far the highest in the liver (Fig. 2). It is of note that the liver CXCL-1 mRNA and protein expression peaked 2 h into the recovery period, at the same time as serum CXCL-1, indicating that the liver is the main source of the exercise-induced increase in serum CXCL-1. In line with this, the subsequent decline in liver CXCL-1 expression was accompanied by a proportional decrease in serum CXCL-1.
The stimulus for exercise-induced liver CXCL-1 expression is not known. Several studies point to a role of IL-6 in regulating CXCL-1 expression (Sheikh et al. 2006; Bierie et al. 2009; Tseng et al. 2010). To address whether the IL-6 stimulus is essential for exercise-induced liver CXCL-1 expression, CXCL-1 mRNA expression was determined in IL-6 KO mice in response to exercise. The present observation that the exercise-induced CXCL-1 mRNA induction in the liver was completely blunted in IL-6 KO mice strongly indicates that the IL-6 stimulus is essential for exercise-induced liver CXCL-1 expression (Fig. 4). It is of note that the mice in the IL-6 KO running exercise trial were analysed immediately after exercise and 4 h into the recovery period. Our earlier finding showed that liver CXCL-1 mRNA expression peaked around 2 h into the recovery period in swimming NMRI mice. Despite this difference in strain and exercise protocol, we were able to detect the same levels of CXCL-1 induction in the two trials immediately after the exercise bout. Importantly, no difference in liver CXCL-1 mRNA expression was found between non-exercised WT and non-exercised IL-6 KO mice, indicating that only the exercise-induced liver CXCL-1 expression and not the basal liver CXCL-1 expression is dependent on IL-6 signalling (Fig. 4A).
In agreement with previous exercise studies, we found that serum IL-6 increased immediately after exercise (Fig. 1B). The earlier finding that skeletal muscle is the major source of exercise-induced plasma IL-6 (Pedersen & Febbraio, 2008) raises the intriguing possibility that IL-6 released from the working muscle induces liver CXCL-1 expression. The existence of such a muscle-to-liver axis with muscle-derived IL-6 stimulating liver CXCL-1 expression was further supported in the present study by a marked increase in liver CXCL-1 upon overexpression of IL-6 in the tibialis cranialis muscle (Fig. 5). Importantly, no increase in liver IL-6 expression was detected after the transfection, indicating that the increase in liver CXCL-1 expression was not caused by a local effect of IL-6 within the liver, but was rather an effect of IL-6 released from the transfected muscle into the circulation. In agreement with this, serum IL-6 increased markedly in response to the transfection. In further support of this, Castell et al. (1988) have shown that radiolabelled IL-6 injected into rats localises mainly at the surface of liver parenchyma cell, demonstrating that IL-6 homes to hepatocytes in vivo. Additionally, autocrine IL-6 regulation of CXCL-1 has been shown to be dependent on Jak2 signalling (Tseng et al. 2010). Differential gene expression analysis has shown that exercise, among others, induces Jak–Stat signalling in livers (Hoene et al. 2010). Hence, IL-6 binds to hepatocytes and signals within this organ and the present findings suggest that such a mechanism exists in response to exercise.
Exercise-induced muscle IL-6 expression is augmented when intramuscular glycogen content is low (Steensberg et al. 2001). Furthermore, plasma IL-6 is not only affected by exercise, but is also inversely related to plasma glucose level (Nehlsen-Cannarella et al. 1997). Therefore it is thought that IL-6 works as a sensor of carbohydrate availability, and it is consistently suggested that IL-6 is released from working muscle in order to signal to liver or adipose tissue to increase glycogenolysis and lipolysis (Pedersen et al. 2003; van Hall et al. 2003; Banzet et al. 2009). As expected, we found that exercise-induced muscle IL-6 mRNA expression and serum IL-6 increased in response to overnight fasting (Fig. 6D and H). Considering our present finding that IL-6 stimulates liver CXCL-1 expression (Figs 4 and 5), the increase in exercise-induced muscle-derived IL-6 in response to overnight fasting would be expected to cause a larger increase in exercise-induced liver CXCL-1 mRNA expression. Accordingly, liver CXCL-1 expression in the exercised, overnight-fasted mice (Fig. 6E) significantly preceded that of fed, exercised mice. As shown in the present study (Fig. 2), liver CXCL-1 mRNA expression peaks 2 h into the recovery period in the fed mice. Interestingly, however, in the fasted mice the serum CXCL-1 increased already immediately after exercise and this level persisted 2 h into the recovery period. This suggests that overnight fasting hastened the exercise-induced release of CXCL-1 to the circulation (Fig. 6C). Since overnight fasting by itself increased control (non-exercised) liver IL-6 expression (Fig. 6B) it is tempting to speculate that the immediate rise in serum CXCL-1 could be explained by an additional fasting-induced increase in liver CXCL-1 expression prior to exercise. However, we did not detect any effect on liver CXCL-1 expression (data not shown). Hence, the larger immediate exercise-induced increase in serum CXCL-1 in overnight fasted mice could be a result of the hastened increase in exercise-induced muscle CXCL-1 expression after overnight fasting (Fig. 6G). The fasting-induced augmentation in exercise-induced muscle CXCL-1 may be promoted by the augmentation of muscle IL-6. This is substantiated by the observation that overexpression of IL-6 in the muscle increases muscle CXCL-1 expression (Fig. 5E and F).
Exercise-induced myokines are most probably involved in mediating some of the beneficial effects of exercise (Pedersen & Fischer, 2007). However, the effect of exercise-induced CXCL-1 remains to be investigated. The CXC chemokines are mostly recognised as inflammatory mediators of neutrophil attraction. Nevertheless, it has become clear that the function of the CXC chemokines extends well beyond chemotaxis. They are hypothesised to be involved in different homeostatic processes, and the list of their functional attributes is expanded to include organ development, angiogenesis, proliferation, cell adhesion and cytokine secretion (Bacon & Harrison, 2000; Le et al. 2004). CXCL-1 binds to the CXCR2 receptor through which it plays important roles, e.g. in mediating angiogenesis during ischaemia (Mohsenin et al. 2007). IL-8, the human functional equivalent to murine CXCL-1, is identified as a myokine and human studies have shown that IL-8 is regulated in muscles by exercise, but no release to the circulation has been demonstrated (Akerstrom et al. 2005). Thus IL-8 is believed to play a paracrine role in muscles; in contrast, we find that CXCL-1 is released to the circulation. IL-8 is suggested to induce angiogenesis locally in the muscle in response to exercise (Addison et al. 2000; Frydelund-Larsen et al. 2007) and we might speculate that the exercise-induced increase in CXCL-1 contributes to similar exercise-mediated angiogenesis that occurs within the active muscle in an autocrine fashion.
Although speculative, the finding of a marked increase in CXCL-1 in the blood following exercise suggests that CXCL-1 may also contribute to the mediation of some of the systemic effects observed with regular exercise. CXCL-1 is involved in inflammation and wound healing and evidence exists in mice that CXCL-1 decreases the severity of multiple sclerosis and may offer a neuroprotective effect (Omari et al. 2009).
In summary this study suggests that the liver is an important source of serum CXCL-1 in response to exercise, with muscular IL-6 as an essential stimulator of the exercise-induced liver CXCL-1 expression. Overexpression of IL-6 in skeletal muscle induced liver CXCL-1 mRNA expression without increasing IL-6 production within the liver, which points to muscle-derived IL-6 as the regulator of exercise-induced liver CXCL-1 expression. Taken together, our findings strongly support the existence of a muscle-to-liver axis, which is an important observation; as a major challenge in understanding how our body responds to exercise is the mapping of the muscle–liver communication.
Acknowledgments
Ruth Rousing and Hanne Villumsen are acknowledged for their technical assistance. The Centre of Inflammation and Metabolism (CIM) is supported by a grant from the Danish National Research Foundation (no. 02-512-55). This study was further supported by the Danish Medical Research Council, the Commission of the European Communities (Grant Agreement no. 223576 – MYOAGE), the Novo Nordisk Foundation and by a grant from the Novo Scholarship Program 2010. CIM is part of the UNIK Project: Food, Fitness & Pharma for Health and Disease, supported by the Danish Ministry of Science, Technology and Innovation. CIM is a member of DD2 – the Danish Centre for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant nos 09-067009 and 09-075724). The Copenhagen Muscle Research Centre is supported by a grant from the Capital Region of Denmark. The authors have no conflicts of interest to disclose.
Glossary
Abbreviations
- CXCL-1
chemokine ligand 1
- IL
interleukin
- KO
knockout
- WT
wild-type
Author contributions
L. Pedersen drafted the manuscript, conceived the experiments and performed the collection, analysis and interpretation of data. J. Hansen, C. Brandt, H. Adser and J. Hidalgo contributed to collection of data. J. Olesen contributed to collection and analysis of data. H. Pilegaard and B. K. Pedersen revised the article. P. Hojman conceived the study design, undertook interpretation of data and revised the article. All authors approved the final version of the manuscript. The animal experiments were carried out at the Department of Oncology, Herlev Hospital, Denmark, while the molecular analyses were performed at the Centre of Inflammation and Metabolism, Copenhagen University Hospital, Denmark.
Supplementary material
Supplemental Figure 1.
Supplemental table 1.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- Akerstrom T, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK. Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol. 2005;563:507–516. doi: 10.1113/jphysiol.2004.077610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bacon KB, Harrison JK. Chemokines and their receptors in neurobiology: perspectives in physiology and homeostasis. J Neuroimmunol. 2000;104:92–97. doi: 10.1016/s0165-5728(99)00266-0. [DOI] [PubMed] [Google Scholar]
- Banzet S, Koulmann N, Simler N, Sanchez H, Chapot R, Serrurier B, Peinnequin A, Bigard X. Control of gluconeogenic genes during intense/prolonged exercise: hormone-independent effect of muscle-derived IL-6 on hepatic tissue and PEPCK mRNA. J Appl Physiol. 2009;107:1830–1839. doi: 10.1152/japplphysiol.00739.2009. [DOI] [PubMed] [Google Scholar]
- Bierie B, Chung CH, Parker JS, Stover DG, Cheng N, Chytil A, Aakre M, Shyr Y, Moses HL. Abrogation of TGF-β signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119:1571–1582. doi: 10.1172/JCI37480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010:520258. doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell JV, Geiger T, Gross V, Andus T, Walter E, Hirano T, Kishimoto T, Heinrich PC. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem. 1988;177:357–361. doi: 10.1111/j.1432-1033.1988.tb14384.x. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72:9–18. [PMC free article] [PubMed] [Google Scholar]
- Frydelund-Larsen L, Penkowa M, Akerstrom T, Zankari A, Nielsen S, Pedersen BK. Exercise induces interleukin-8 receptor(CXCR2) expression in human skeletal muscle. Exp Physiol. 2007;92:233–240. doi: 10.1113/expphysiol.2006.034769. [DOI] [PubMed] [Google Scholar]
- Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9:2482–2496. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoene M, Franken H, Fritsche L, Lehmann R, Pohl AK, Haring HU, Zell A, Schleicher ED, Weigert C. Activation of the mitogen-activated protein kinase (MAPK) signalling pathway in the liver of mice is related to plasma glucose levels after acute exercise. Diabetologia. 2010;53:1131–1141. doi: 10.1007/s00125-010-1666-3. [DOI] [PubMed] [Google Scholar]
- Hojman P, Gissel H, Gehl J. Sensitive and precise regulation of haemoglobin after gene transfer of erythropoietin to muscle tissue using electroporation. Gene Ther. 2007;14:950–959. doi: 10.1038/sj.gt.3302951. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- Lira SA, Zalamea P, Heinrich JN, Fuentes ME, Carrasco D, Lewin AC, Barton DS, Durham S, Bravo R. Expression of the chemokine N51/KC in the thymus and epidermis of transgenic mice results in marked infiltration of a single class of inflammatory cells. J Exp Med. 1994;180:2039–2048. doi: 10.1084/jem.180.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsenin A, Burdick MD, Molina JG, Keane MP, Blackburn MR. Enhanced CXCL1 production and angiogenesis in adenosine-mediated lung disease. FASEB J. 2007;21:1026–1036. doi: 10.1096/fj.06-7301com. [DOI] [PubMed] [Google Scholar]
- Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E1191–E1204. doi: 10.1152/ajpendo.90280.2008. [DOI] [PubMed] [Google Scholar]
- Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM. Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol. 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- Omari KM, Lutz SE, Santambrogio L, Lira SA, Raine CS. Neuroprotection and remyelination after autoimmune demyelination in mice that inducibly overexpress CXCL1. Am J Pathol. 2009;174:164–176. doi: 10.2353/ajpath.2009.080350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo P, Alberta J, Wen DZ, Graycar JL, Derynck R, Stiles CD. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet α-granule proteins. J Biol Chem. 1989;264:4133–4137. [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Fischer CP. Beneficial health effects of exercise – the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28:152–156. doi: 10.1016/j.tips.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24:113–119. doi: 10.1023/a:1026070911202. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Wolsk-Petersen E, Febbraio M. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proc Nutr Soc. 2004;63:263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- Rubio N, Sanz-Rodriguez F. Induction of the CXCL1 (KC) chemokine in mouse astrocytes by infection with the murine encephalomyelitis virus of Theiler. Virology. 2007;358:98–108. doi: 10.1016/j.virol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Sheikh N, Tron K, Dudas J, Ramadori G. Cytokine-induced neutrophil chemoattractant-1 is released by the noninjured liver in a rat acute-phase model. Lab Invest. 2006;86:800–814. doi: 10.1038/labinvest.3700435. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J Clin Endocrinol Metab. 2004;89:5577–5582. doi: 10.1210/jc.2004-0603. [DOI] [PubMed] [Google Scholar]
- Tseng YL, Wu MH, Yang HC, Wang CY, Lin CF. Autocrine IL-6 regulates GRO-α production in thymic epithelial cells. Cytokine. 2010;51:195–201. doi: 10.1016/j.cyto.2010.05.002. [DOI] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Møller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


