Abstract
In vertebrate embryos, cardiac precursor cells of the primary heart field are specified in the lateral mesoderm. These cells converge at the ventral midline to form the linear heart tube, and give rise to the atria and the left ventricle. The right ventricle and the outflow tract are derived from an adjacent population of precursors known as the second heart field. In addition, the cardiac neural crest contributes cells to the septum of the outflow tract to separate the systemic and the pulmonary circulations. The amphibian heart has a single ventricle and an outflow tract with an incomplete spiral septum; however, it is unknown whether the cardiac neural crest is also involved in outflow tract septation, as in amniotes. Using a combination of tissue transplantations and molecular analyses in Xenopus we show that the amphibian outflow tract is derived from a second heart field equivalent to that described in birds and mammals. However, in contrast to what we see in amniotes, it is the second heart field and not the cardiac neural crest that forms the septum of the amphibian outflow tract. In Xenopus, cardiac neural crest cells remain confined to the aortic sac and arch arteries and never populate the outflow tract cushions. This significant difference suggests that cardiac neural crest cell migration into the cardiac cushions is an amniote-specific characteristic, presumably acquired to increase the mass of the outflow tract septum with the evolutionary need for a fully divided circulation.
Keywords: Cardiac cushion, Myocardium, Outflow tract, Spiral septum, Neural crest, Xenopus
INTRODUCTION
The neural crest (NC) is a multipotent population of migratory cells with the remarkable ability to contribute to a broad range of derivatives including the enteric nervous system, pigment cells, craniofacial skeletal elements, thymus, teeth and portions of the cardiovascular system. Because of its contribution to multiple lineages, abnormal development of the NC can result in a wide array of seemingly unrelated clinical manifestations that affect multiple organ systems, as seen in conditions such as Hirschsprung disease (hypopigmentation and aganglionic megacolon) and DiGeorge syndrome (craniofacial and heart defects).
The premigratory NC can be divided into five distinct and partially overlapping functional domains along the anteroposterior axis: the cranial, cardiac, vagal, trunk and sacral NC, each of which forms a specific set of derivatives in the periphery. The cardiac NC is a subdivision of the cranial NC; in amniotes it originates from a region located between the middle of the otic placode and the caudal border of somite 4, corresponding to rhombomeres 6-8 of the hindbrain (for reviews, see Brown and Baldwin, 2006; Snider et al., 2007; Hutson and Kirby, 2007). These cells migrate through pharyngeal arches 3, 4 and 6, where they will contribute to the pharyngeal glands, thymus and parathyroid (Le Lievre and Le Douarin, 1975; Bockman and Kirby, 1984). A subset of NC cells continues migrating into the outflow tract cushions of the heart, where they contribute to the spiral aorticopulmonary septum, which separates the truncus into the aorta and pulmonary arteries (Kirby et al., 1983; Jiang et al., 2000). Cardiac NC also plays an important role in aortic arch artery remodeling (reviewed by Snider et al., 2007). Persistent truncus arteriosus (a failure to separate the aorta and pulmonary artery) occurs after cardiac NC ablation in avian embryos (reviewed by Creazzo et al., 1998; Kirby et al., 1985) and in a number of mouse mutants that lack cardiac NC, as best illustrated by the Pax3 mouse mutant splotch (reviewed by Stoller and Epstein, 2005; Conway et al., 1997).
Zebrafish does not have a separated systemic and pulmonary circulation and lacks an outflow tract septum but still possesses a cardiac NC. Interestingly, cardiac NC cells migrating into the zebrafish heart make a substantial contribution to the myocardial cell lineage in all regions of the developing heart, including outflow tract, atrium, atrioventricular junction and ventricle (Sato and Yost, 2003; Li et al., 2003). In the frog Xenopus laevis, outflow tract septation is incomplete, with a spiral septum directing blood flow to the pulmocutaneous and systemic arteries (de Graaf, 1957; Farmer, 1999; Kolker et al., 2000; Mohun et al., 2000; Lohr and Yost, 2000). This structure is similar to the aorticopulmonary septum of the amniote embryonic heart (Kolker et al., 2000). A number of studies have suggested that the NC cells also contribute to the cardiovascular system in Xenopus (Sadaghiani and Thiébaud, 1987; Martinsen et al., 2004). However, in these studies heart formation was assessed at a stage prior to the initiation of outflow tract cushion formation, which normally occurs around stage 41 in Xenopus (Kolker et al., 2000; Mohun et al., 2000; Lohr and Yost, 2000). Therefore, it is unclear whether NC cells make a significant contribution to the outflow tract septum in Xenopus, as seen in higher vertebrates.
To specifically address this question we combined embryological manipulations and molecular analyses to trace the progeny of the cardiac NC. We show that the cardiac NC in Xenopus remains confined to the aortic sac and arch arteries and never enters the outflow tract cushions. Rather, it is the second heart field that forms the spiral septum of the outflow tract. These results are in contrast to what has been described in birds and mammals, suggesting that the cardiac NC contribution to the outflow tract septum is a later evolutionary novelty, presumably acquired with the need for a more complete separation of systemic and pulmonary circulations.
MATERIALS AND METHODS
Embryos, injections, tissue labeling and transplantation
Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967) and raised in 0.1× NAM (Normal Amphibian Medium) (Slack and Forman, 1980). mRNAs encoding green fluorescent protein (GFP) (Zernicka-Goetz et al., 1996) and monomeric red fluorescent protein (RFP; a gift from Dr Dominique Alfandari, University of Massachusetts Amherst, MA, USA) were synthesized in vitro using the Message Machine Kit (Ambion). Embryos were injected in the animal pole at the two-cell stage with 1 ng RFP mRNA (NC graft) or in the dorsal marginal zone at the four-cell stage with 1 ng GFP mRNA [primary heart field (PHF) and second heart field (SHF) grafts]. NC- and PHF/SHF-labeled explants were dissected at stage 17 and stage 25, respectively, in 1× NAM supplemented with 50 μg/ml gentamycin using a 25-gauge syringe needle. PHF and SHF grafts contain both mesoderm and endoderm layers. Grafts were then transplanted onto the equivalent region of unlabeled stage-matched sibling embryos. Grafted embryos were allowed to heal for 30 minutes in the dissection medium, and were then transferred for long-term culture into 0.1× NAM. Embryos were observed under an epifluorescence microscope (Eclipse E800, Nikon). In some instances, brightfield and fluorescence images were merged to highlight the position of the labeled graft. Each transplantation experiment was performed multiple times on at least three independent batches of embryos.
For DiI labeling of the cardiac NC, a stock solution of DiI (CellTracker CM-DiI, Molecular Probes) was prepared in ethanol (1 mg/ml) and diluted in 0.2 M sucrose immediately before injection (Collazo et al., 1993). The cardiac NC region at stage 17/18 was labeled by multiple injections of a small volume of diluted DiI. Live embryos were observed and photographed under an epifluorescence microscope at various stages of development and then processed at stage 45 for histological analysis to identify the position and the progeny of the labeled cells (see Fig. S1 in the supplementary material).
In situ hybridization
For whole-mount in situ hybridization, embryos were fixed in MEMFA and processed for in situ hybridization as previously described (Harland, 1991). In some instances, the epidermis was manually removed in the cardiac area to allow for a better penetration of the probes. For in situ hybridization on sections and to allow for optimal preservation of the RFP and GFP lineage tracers, embryos were fixed in 4% paraformaldehyde in PBS, embedded in Paraplast+ (McCormick Scientific), sectioned on an Olympus rotary microtome and 12 μm serial sections were hybridized according to the procedure described by Lemaire and Gurdon (Lemaire and Gurdon, 1994). Sections were then briefly counterstained with Eosin. Because RFP and GFP can no longer be visualized following this procedure, sections were first individually photographed and then processed for in situ hybridization. Antisense DIG-labeled probes (Genius Kit, Roche) were synthesized using template cDNA encoding MyoD (Hopwood et al., 1989), Nkx2.5 (Cleaver et al., 1996), Sox8 (O'Donnell et al., 2006), Sox9 (Spokony et al., 2002), Sox10 (Aoki et al., 2003), Tbx5 (Brown et al., 2005) and Tbx20 (Brown et al., 2002).
Immunohistochemistry
Embryos were fixed in Dent's fixative (20% DMSO in methanol) (Dent et al., 1989) overnight at −20°C. Embryos were then rehydrated in 1× phosphate-buffered saline (PBS, Gibco), blocked in PBTS (0.2% bovine serum albumin and 0.1% Triton X-100 in PBS, supplemented with 5% inactivated lamb serum), incubated with MF20 antibody hybridoma supernatant overnight at 4°C (Developmental Studies Hybridoma Bank, University of Iowa; 1:1 dilution), and detected with an anti-mouse IgG FITC-conjugated secondary antibody (Jackson ImmunoResearch; 1:100 dilution) for 1 hour at room temperature. All antibodies were diluted in PBTS. For immunohistochemistry on sections, fixed embryos were embedded in Paraplast+, sectioned on an Olympus rotary microtome and 10 μm serial sections incubated with MF20 antibody as described above.
RESULTS
Defining the position of a putative cardiac neural crest domain in Xenopus
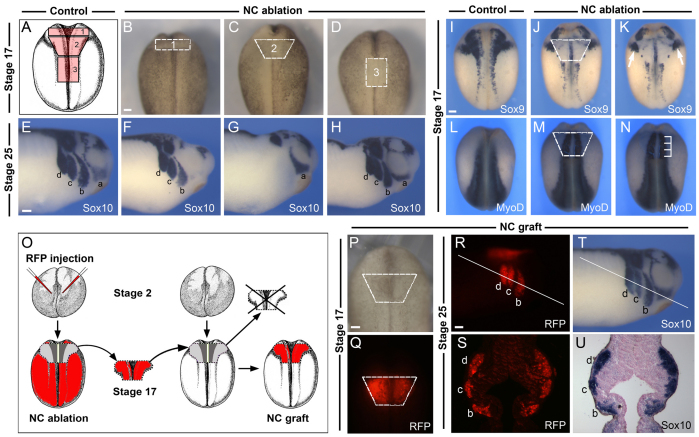
The cardiac NC is a subdivision of the cranial NC (for a review, see Le Douarin and Kalcheim, 1999). In chick and mouse embryos the cardiac NC originates from a region extending from the middle of the presumptive otic placode to the caudal border of the fourth somite, corresponding to the most posterior subdivisions of the hindbrain (Kirby et al., 1985; Jiang et al., 2000). To define a similar domain in Xenopus embryos we excised the premigrating NC from stage 17 embryos at different axial levels (Fig. 1A-D) and analyzed the corresponding embryos at stage 25 with the pan-neural crest marker Sox10 (Fig. 1E-H). In the cranial region, Sox10 is normally expressed in four streams of migrating cranial NC: the mandibular, hyoid, anterior and posterior branchial NC (Aoki et al., 2003). Ablation of the most anterior segment of the NC (#1; Fig. 1A,B) resulted in a specific loss (73.3% of the embryos) or reduction (26.7% of the embryos) of the mandibular NC stream at stage 25 (Fig. 1F; n=15). Ablation of the most posterior segment of the NC (#3; Fig. 1A,D) had no effect on the pattern of migration of any of the four streams of migrating cranial NC (Fig. 1H; 100% of the embryos, n=15). However, ablation of the intermediate segment of the NC (#2; Fig. 1A,C) resulted in a specific loss of the hyoid, anterior and posterior branchial NC streams without affecting the mandibular NC (Fig. 1G; 100% of the embryos, n=20). Based on these initial observations, we propose that the putative cardiac NC is included within the intermediate segment of the NC as described here (#2; Fig. 1A). All subsequent NC transplantation and ablation experiments were performed using this intermediate segment of the NC.
Fig. 1.
Position of the putative cardiac neural crest and experimental design to analyze its contribution to the cardiovascular system. (A) Diagram of a neurula stage Xenopus embryo (stage 17) viewed from the dorsal side (anterior to top), with outline of the segments of the neural crest (NC) ablated along the anteroposterior axis (1-3). (B-D) Stage 17 embryos with the region of the three ablated NC domains indicated. (E-H) In situ hybridization for the NC marker Sox10 in control and NC-ablated embryos at stage 25. (E) In control embryos, Sox10 is expressed within the four streams of the migrating cranial NC: the mandibular (a), hyoid (b), anterior branchial (c) and posterior branchial (d) NC. (F) After ablation of NC domain ‘#1’, the most anterior NC stream (a) is lost. (G) Ablation of NC domain ‘#2’ results in loss of the three most posterior NC streams, without affecting the more anterior mandibular NC stream (a). (H) After ablation of NC domain ‘#3’, all four streams of the migrating cranial NC form normally. (I-N) Position of the ablated/transplanted NC domain at stage 17 with respect to adjacent tissues. The presumptive otic placode (I-K) as revealed by Sox9 expression (arrows) is unaffected after NC ablation. The ablated NC domain spans a region extending from the first to the fourth somite (L-N) as revealed by MyoD expression (brackets). (O) Experimental design to analyze NC contribution to the cardiovascular system. Two-cell stage embryos were injected in the animal pole with mRNA encoding RFP. At stage 17, the RFP-labeled NC is transplanted onto the equivalent region of an unlabeled host embryo (NC graft). (P,Q) RFP-labeled NC graft at stage 17. (R-U) At stage 25, cells derived from the RFP-labeled NC graft are confined to the three most posterior streams of cranial NC (b, c and d), and completely overlap with Sox10 expression as seen in the whole embryo (R,T) and in sections (S,U). The line in R and T indicates the level of the sections shown in S and U, respectively. Scale bars: 100 μm.
To further characterize the position of this domain of the NC with regard to surrounding tissues, we analyzed the expression of Sox9 and MyoD in stage 17 embryos immediately after ablation of the NC. At stage 17, Sox9 is normally expressed in the NC and otic placode (Fig. 1I) (Spokony et al., 2002). In NC-ablated embryos, Sox9 expression in the cranial NC was missing, whereas the otic expression of Sox9 was largely unaffected (Fig. 1J,K). MyoD expression is normally restricted to the underlying paraxial mesoderm (Fig. 1L) (Hopwood et al., 1989). MyoD expression in NC-ablated embryos revealed that this NC domain spans a region extending from the first to the fourth somite (Fig. 1M,N).
To evaluate the specific contribution of this segment of the NC to the cardiovascular system, NC grafts isolated from embryos injected with mRNA encoding RFP were transplanted onto the equivalent region of stage-matched unlabeled embryos (Fig. 1O-Q), which were then analyzed at different stages of development. At stage 25, the RFP-labeled NC graft was able to restore a normal pattern of cranial NC migration in the host embryo. Cells of the RFP-labeled NC graft contributed, as expected, to the hyoid, anterior and posterior branchial streams of the NC (Fig. 1R,S), and these cells also expressed Sox10 (Fig. 1T,U), indicating that the transplanted tissue had retained NC characteristics. The transplanted region did not contribute to the mandibular stream of NC, as predicted from our initial ablation experiments (Fig. 1A-D).
The cardiac NC contributes to the aortic sac and aortic arch arteries and is dispensable for outflow tract septation
The development of the cardiac tissue in Xenopus has been described in detail, and valve formation is believed to occur between stages 41 and 44 (Nieuwkoop and Faber, 1967; Kolker et al., 2000; Mohun et al., 2000; Lohr and Yost, 2000; Lee and Saint-Jeannet, 2009). Around stage 41, the cardiac jelly of the outflow tract cushion starts to be populated by mesenchymal cells. As development proceeds, the mesenchymal cells condense in the outflow tract cushions. During further maturation of the heart, the outflow tract cushions undergo extensive remodeling to give rise to the spiral septum.
The progeny of the RFP-labeled NC cells were analyzed at stages 41, 45 and 48 in transplanted embryos. At all stages examined, RFP-labeled NC cells remained confined to the aortic sac and arch arteries (see Table 1). RFP-positive cells were never seen to enter the outflow tract proper (Fig. 2A-D), thus forming a very sharp boundary with the MF20-positive muscle cells in the myocardium of the outflow tract (Fig. 2E-H). A detailed histological analysis of the NC contribution to the large vessels of the heart at stage 48 indicates that cardiac NC cells populate the wall of the bilateral common carotid, systemic and pulmocutaneous arteries (Fig. 2I-L).
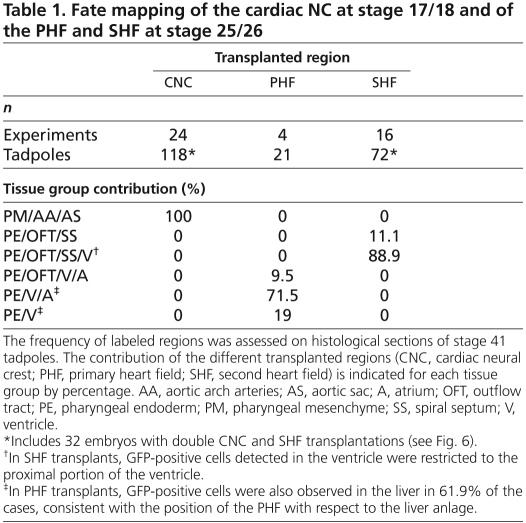
Table 1.
Fate mapping of the cardiac NC at stage 17/18 and of the PHF and SHF at stage 25/26
Fig. 2.
RFP-labeled NC contributes to the aortic sac and arch arteries and is dispensable for outflow tract septation. (A-D) Cells derived from a stage 17 RFP-labeled NC graft populate the wall of the aortic arch arteries at stage 45 and never populate the outflow tract (OFT). B-D are a higher magnification of the boxed area in A. Ventral views, anterior to top. (E-H) Four views of the same Xenopus embryo showing that the RFP-positive cells form a very sharp boundary (arrowheads) and do not overlap with the myocardium muscle cells, as labeled with MF20 antibody. (I-L) At stage 48, RFP-labeled NC-derived cells are confined to the large arteries (LCC, left carotid canal; LPC, left pulmocutaneous canal; RCC, right carotid canal; RPC, right pulmocutaneous canal) as seen in the whole embryo (J) and in sections (K,L). (M-X) Transverse sections of stage 41 embryos showing Nkx2.5 and Sox8 expression in control and manipulated embryos. (M-N) In control embryos, Nkx2.5 is expressed throughout the myocardium [outflow tract and ventricle (V)], whereas Sox8 is confined to the spiral septum (arrows). Sox8 is also detected in the NC-derived head mesenchyme (asterisks). (O-P) NC ablation does not prevent outflow tract septation as revealed by Sox8 expression (arrows). These embryos lack the aortic sac, resulting in a dorsal expansion of Nkx2.5. (Q-T) The RFP-labeled NC-derived cells are detected in the aortic sac and arch arteries (arrowheads) but not in the spiral septum of the outflow tract (arrows). U-X are higher magnification views of Q-T. Scale bars: 100 μm.
At stage 41, the transcription factor Nkx2.5 is expressed throughout the myocardium (Fig. 2M) (Cleaver et al., 1996), whereas Sox8 is restricted to the spiral septum and the NC-derived head mesenchyme (Fig. 2N) (Lee and Saint-Jeannet, 2009). RFP-labeled NC cells in the aortic sac of grafted embryos were seen to abut, but not overlap, with the Nkx2.5 expression domain (Fig. 2Q,S,U,V), and, more importantly, these cells were not detected at anytime in the spiral septum, which expresses Sox8 (Fig. 2R,T,W,X). In all NC-ablated embryos examined (n=130, from 16 independent experiments) the distance between the pharynx and the heart tissue was severely reduced due to the loss of the NC-derived head mesenchyme (Fig. 2O-P). In these embryos, the Nkx2.5 expression domain was expanded dorsally, presumably owing to the loss of the aortic sac and arch arteries (Fig. 2O). Interestingly, whereas Sox8 expression in the head mesenchyme was lost in these embryos, Sox8 expression in the spiral septum was unaffected (Fig. 2P; n=10). These results indicate that cardiac NC cells in Xenopus are dispensable for outflow tract septation: they form the wall of the aortic arch arteries but do not populate the outflow tract cushions.
In these experiments we cannot exclude the possibility that unlabeled adjacent NC cells compensate for any damage caused by the ablation/transplantation procedure. To address this, we performed experiments in which cardiac NC cells were labeled by DiI injection at stage 17/18 in otherwise unmanipulated embryos. These embryos were first analyzed at stage 25-27 to confirm, in the whole embryo, labeling of the migrating cardiac NC, and then by histology at stage 45 (see Fig. S1 in the supplementary material). In a small proportion of the injected embryos (11.4%), DiI-positive cells could not be detected at stage 45, whereas in the remainder of the embryos (88.6%; n=88 from eight independent experiments), DiI-positive cells were exclusively restricted to the aortic sac and arch arteries and were never detected in the spiral septum of the outflow tract (see Fig. S1 in the supplementary material), consistent with the results obtained in the transplantation assay. Therefore, using two distinct labeling techniques we demonstrate that cardiac NC cells never enter the outflow tract cushions in Xenopus.
Molecular characterization of the primary and second heart field
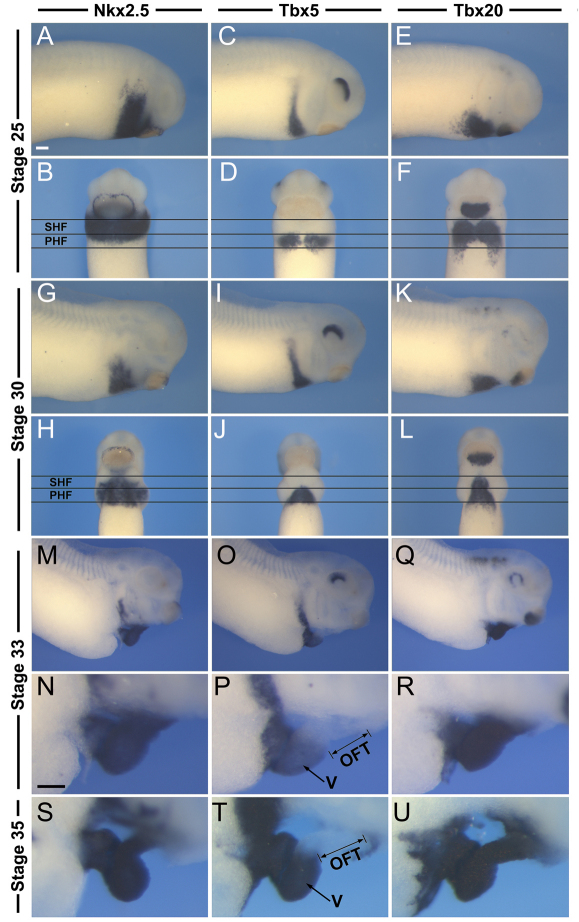
The next aim was to determine the embryonic origin of the spiral septum in Xenopus. A possible candidate is the second heart field (SHF; also referred to as the secondary heart field), which gives rise to the right ventricle and the outflow tract in birds and mammals (reviewed by Buckingham et al., 2005; Dyer and Kirby, 2009; Vincent and Buckingham, 2010). Moreover, a number of recent studies have also suggested the existence of a SHF-like territory in Xenopus (Brade et al., 2007; Gessert and Kühl, 2009). This territory is located anterior to the primary heart field (PHF) and is characterized by the expression of a specific set of transcription factors (Gessert and Kühl, 2009). For example, at stage 25, the presumptive SHF and PHF express Nkx2.5 and Tbx20, whereas Tbx5 expression is restricted to the PHF territory (Fig. 3A-F). At stage 30, this pattern of gene expression is maintained in both territories (Fig. 3G-L). Later, as heart morphogenesis is initiated (stage 33) and heart looping takes place (stage 35), Nkx2.5 and Tbx20 are expressed in all compartments of the heart, whereas Tbx5 is excluded from the developing outflow tract (Fig. 3M-U).
Fig. 3.
Molecular characterization of the primary and second heart fields. (A-U) Developmental expression of Nkx2.5, Tbx5 and Tbx20 at stages 25, 30, 33 and 35 as indicated. The horizontal lines indicate the predicted position of primary heart field (PHF) and second heart field (SHF). All three genes are detected in the PHF, whereas the SHF expresses both Nkx2.5 and Tbx20 but is negative for Tbx5 expression. At later stages, the developing outflow tract (OFT), a derivative of the SHF, remains Tbx5 negative. (A,C,E,G,I,K,M-U) Lateral views, anterior to right. (B,D,F,H,J,L) Ventral views, anterior to top. V, ventricle. Scale bars: 100 μm.
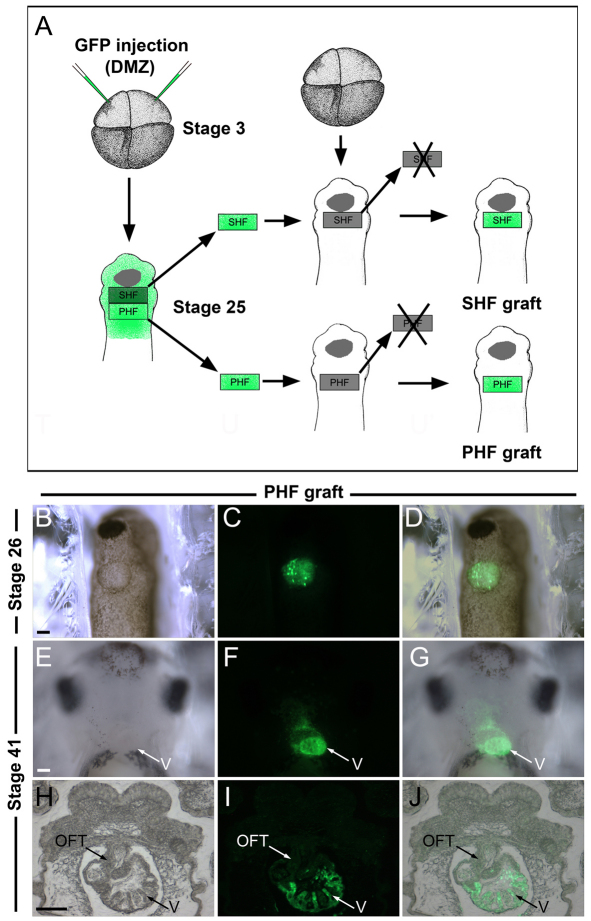
To first confirm the specific contribution of the PHF territory to the developing heart, PHF grafts isolated from embryos injected with mRNA encoding GFP were transplanted onto the equivalent region of stage-matched unlabeled embryos at stage 25 (Fig. 4A-D). Histological analysis of the corresponding embryos at stage 41 indicated that GFP-labeled cells were largely confined to the wall of the ventricle and atrium, with a minimal contribution to the outflow tract (see Table 1; Fig. 4E-J), consistent with the predicted fate of this territory.
Fig. 4.
The PHF contributes to the ventricle. (A) Experimental design to analyze the relative contribution of the PHF and SHF to the developing heart. Four-cell stage Xenopus embryos were injected in the dorsal marginal zone (DMZ) with mRNA encoding GFP. At stage 25, the GFP-labeled PHF or SHF was transplanted onto the equivalent region of an unlabeled host embryo (PHF graft or SHF graft). (B-D) Ventral view (anterior to top) of a host embryo at stage 26 shows the position of GFP-labeled PHF graft. (E-G) At stage 41, the GFP-labeled PHF-derived cells are detected in the developing heart. Ventral view, anterior to top. (H-J) In a transverse section, the GFP-labeled PHF-derived cells are largely confined to the ventricle. OFT, outflow tract; V, ventricle. Scale bars: 100 μm.
The SHF contributes to the outflow tract and forms the spiral septum
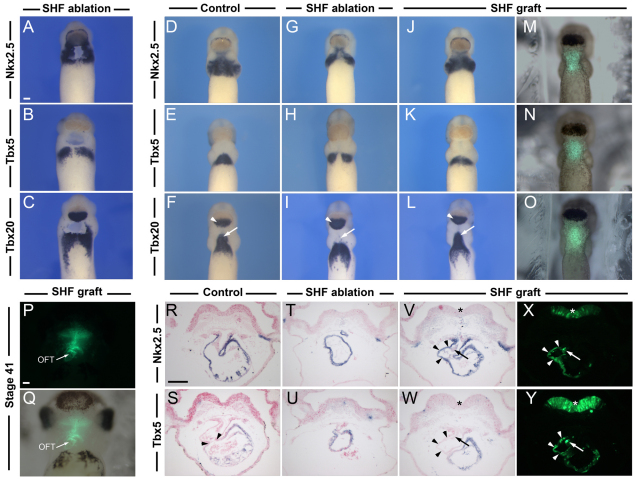
We next analyzed the specific contribution of the SHF territory to the developing heart using the same transplantation strategy (Fig. 4A). At stage 25 the isolated SHF territory overlaps with Nkx2.5 and the most anterior expression domain of Tbx20 (Fig. 5A,B; n=30). This territory is posterior to the cement gland and anterior to the Tbx5 expression domain that predicts the position of the PHF (Fig. 5C; n=15). After ablation of the SHF, all stage 30 embryos showed an overall reduction in Nkx2.5 expression (Fig. 5G) and a loss of the most anterior domain of Tbx20 (Fig. 5I), whereas the Tbx5 expression domain was largely unaffected (Fig. 5H), except for a delay in the fusion of the PHF at the midline (n=36). Upon transplantation of a GFP-labeled SHF, the expression domain of Nkx2.5 (Fig. 5J,M) and the anterior domain of Tbx20 expression (Fig. 5L,O) were fully restored in the host embryos (n=27). The grafted embryos also displayed normal fusion of the PHF at the midline (Fig. 5J-O). These observations demonstrate that we can accurately dissect and transplant PHF and SHF territories without dramatically affecting their molecular signature.
Fig. 5.
The SHF contributes to the outflow tract and forms the spiral septum. (A-C) Xenopus embryos at stage 25 immediately after ablation of the SHF and hybridized with Nkx2.5, Tbx5 and Tbx20 probes indicate that the ablated SHF is anterior to the Tbx5 expression domain. (D-F) Expression of Nkx2.5, Tbx5 and Tbx20 in stage 30 control embryos. (G-I) After ablation of the SHF, embryos show an overall reduction in Nkx2.5 expression and a loss of the most anterior cardiac domain of Tbx20 (arrow). Tbx20 is also expressed in the cement gland (arrowhead). Tbx5 expression is largely unaffected. However, SHF-ablated embryos show a delay in the fusion of the PHF at the midline. (J-O) SHF graft restores the anterior domain of Tbx20 expression at stage 30 (arrow, L), and the fusion at the midline of the PHF (K). (M-O) Brightfield views showing the position of the GFP-labeled SHF graft. (P,Q) Fluorescence and brightfield views of a stage 41 embryo after SHF graft at stage 25 showing GFP-positive cells in the developing heart. (A-Q) Ventral views, anterior to top. (R-Y) Transverse sections of similar stage 41 embryos. (R,S) Expression of Nkx2.5 and Tbx5 in stage 41 control embryos. Whereas Nkx2.5 is expressed throughout the myocardium (outflow tract and ventricle), Tbx5 is restricted to the ventricle, establishing a sharp boundary with the outflow tract (arrowheads). (T,U) After SHF ablation, the overall size of the cardiac tissue is extremely reduced but it expresses both Nkx2.5 and Tbx5, suggesting that it is likely to be PHF derived. (V-Y) GFP-labeled SHF-derived cells are detected in the wall of the outflow tract (arrowheads), part of the ventricle and in the spiral septum (arrows). Labeling of the ventricle suggests that the segregation of the PHF and SHF is likely to be incomplete at the time of transplantation (Gessert and Kühl, 2009). In these embryos, the pharyngeal endoderm is also GFP labeled (asterisks) because the transplanted SHF graft contains both mesoderm and endoderm layers (see Table 1). OFT, outflow tract. Scale bars: 100 μm.
At stage 41, GFP-labeled SHF cells were detected in the outflow tract, the proximal part of the ventricle and in the spiral septum in the majority of the embryos (see Table 1; Fig. 5V-Y). Labeling of the ventricle in these embryos might suggest that the segregation of the PHF and SHF was not fully completed at the time of the transplantation (Gessert and Kühl, 2009). GFP-labeled SHF cells express Nkx2.5 in the outflow tract and the ventricle (Fig. 5V,X). In most species, Tbx5 mRNA and protein are highly enriched in the prospective left ventricle (Bruneau et al., 1999; Takeuchi et al., 2003). In Xenopus, Tbx5 is also expressed throughout the ventricle, extending to the boundary with the outflow tract (Fig. 5S) (Horb and Thomsen, 1999). In SHF-ablated embryos, the overall size of the cardiac tissue was consistently reduced at stage 41 (n=94 from 19 independent experiments) and the remaining tissue expressed both Nkx2.5 and Tbx5 (Fig. 5T,U; n=10), suggesting that it is ventricle in character and likely to be PHF derived. Altogether, these observations suggest that the SHF in Xenopus contributes to the wall of the outflow tract and is directly implicated in outflow tract septation.
The cardiac NC and the SHF contribute to distinct lineages of the cardiovascular system
To fully establish the lineage contribution of the cardiac NC and SHF to the cardiovascular system, we performed double transplantation experiments (n=32; see Table 1). RFP-labeled NC and GFP-labeled SHF grafts were sequentially transplanted onto the same host embryo at stage 17 and stage 25, respectively. Observation of these embryos at different times after transplantation allowed us to document the topographical relationship of these tissues during development (Fig. 6). Histological analysis of these embryos at stage 41 revealed a clear segregation of these two lineages (Fig. 6R-T,V-X), either to the aortic sac and arch arteries (cardiac NC derived) or to the outflow tract, spiral septum and ventricle (SHF derived). Here, the identity of the SHF-derived spiral septum was further confirmed by the expression of Sox8 (Fig. 6Q,U).
Fig. 6.
The NC and SHF contribute to distinct, non-overlapping lineages of the cardiovascular system. (A-X) RFP-labeled NC and GFP-labeled SHF were sequentially transplanted into the same unlabeled host Xenopus embryo at stage 17 and stage 25, respectively. The progeny of the labeled grafts were analyzed at different time points, in the whole embryo at stage 27 (A-H) and stage 35 (I-P) and on sections at stage 41 (Q-X). (A-D,I-L) Lateral views, anterior to right. (E-H,M-P) Ventral views, anterior to top. Sox8 expression at stage 41 is detected in the spiral septum (black arrow), a tissue derived from the GFP-labeled SHF (white arrows). GFP-labeled SHF also contributes to the wall of the outflow tract and to the proximal part of the ventricle. RFP-labeled NC-derived cells are detected in the aortic arch arteries (arrowheads) and in the NC-derived head mesenchyme. Red blood cells in the ventricle show non-specific autofluorescence (asterisks). U-X are higher magnification views of Q-T. Scale bars: 100 μm.
DISCUSSION
Through a series of transplantation experiments, lineage tracing and molecular analyses we provide the first comprehensive description of the cardiac NC in Xenopus. Our results point to remarkable differences in the fate and migration pattern of this cell population across species. In Xenopus embryos, cardiac NC cells migrate into the pharyngeal arches and form the wall of the aortic sac and arch arteries, establishing a very sharp boundary with the myocardial lineage of the outflow tract. These NC cells never enter the outflow tract cushions, which we demonstrate are derived from the SHF. By contrast, cardiac NC cells in chick and mouse migrate into the outflow tract to adopt a mesenchymal fate and populate the cardiac cushions, where they participate in outflow tract septation (for reviews, see Brown and Baldwin, 2006; Snider et al., 2007; Hutson and Kirby, 2007). In zebrafish, the situation is different again: cardiac NC cells migrate even deeper into the cardiac tissue and contribute myocardial muscle cells that populate all segments of the heart, including the outflow tract, atrium, atrioventricular junction and ventricle (Sato and Yost, 2003; Li et al., 2003).
Ablation experiments also support these species differences. Cardiac NC ablation in Xenopus results in a loss of the aortic sac and arteries; however, the outflow tract and the spiral septum develop normally and express the molecular markers specific to these lineages. In chick, cardiac NC ablation affects septation, leading to persistent truncus arteriosus. In addition, the outflow tract fails to elongate resulting in defective cardiac looping (Kirby et al., 1985; Bockman et al., 1987; Yelbuz et al., 2002). By contrast, ablation of cardiac NC in the zebrafish primarily alters the composition of the myocardial wall by decreasing the number of cardiomyocytes (Sato and Yost, 2003; Li et al., 2003).
Because our results indicate that cardiac NC cells do not participate in spiral septum formation in Xenopus, we performed experiments to determine its embryonic origin. In vertebrates, cardiac precursor cells are specified in the lateral plate mesoderm in a region known as the PHF. This territory forms the linear heart tube at the ventral midline, which gives rise to the atria and left ventricle of the multi-chambered heart (Olson, 2006). The right ventricular chamber and outflow tract are later evolutionary innovations, derived from an adjacent population of precursors known as the SHF (Buckingham et al., 2005; Dyer and Kirby, 2009; Vincent and Buckingham, 2010). In chick and mouse, cells from the SHF ingress into the outflow tract from the pharyngeal mesoderm to form a substantial portion of the myocardial precursors of the developing arterial pole of the heart (Kelly et al., 2001; Mjaatvedt et al., 2001; Waldo et al., 2001; Zaffran et al., 2004). SHF-like territories have also been identified in lower vertebrates, including frog (Brade et al., 2007; Gessert and Kühl, 2009), zebrafish (de Pater et al., 2009) and lamprey (Kokubo et al., 2010), as well as in the early chordate Ciona (Stolfi et al., 2010). Consistent with a recent study (Gessert and Kühl, 2009), we found that the SHF forms the wall of the Xenopus outflow tract, similar to what has been reported in chick and mouse embryos. In addition, our experiments establish that the spiral septum in Xenopus is also derived from the SHF. Double transplantations of labeled cardiac NC and SHF further support these findings and demonstrate that these two cell populations contribute to distinct lineages of the cardiovascular system (Fig. 7). In Xenopus, the outflow tract cushion mesenchyme is therefore exclusively generated by epithelial-to-mesenchymal transformation of the endocardial cells lining the cushions (reviewed by Snarr et al., 2008).
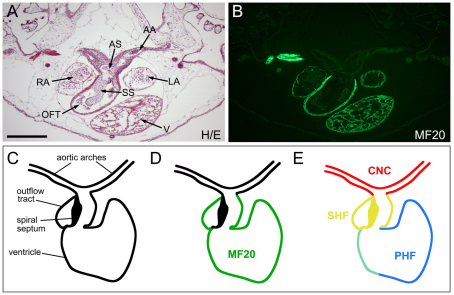
Fig. 7.
The relative contribution of the cardiac NC and SHF to the Xenopus cardiovascular system. (A) Hematoxylin and Eosin (H/E) staining of a transverse section through the heart of a stage 50 embryo. AA, aortic arch arteries; AS, aortic sac; LA, left atrium; OFT, outflow tract; RA, right atrium; SS, spiral septum; V, ventricle. Scale bar: 100 μm. (B) An adjacent section stained with MF20 antibody highlights the very sharp boundary between the muscle cells in the myocardium of the outflow tract and the aortic sac. (C) The Xenopus developing heart. (D) The muscle marker MF20 is expressed in muscle cells of the myocardium, including outflow tract and ventricle, but is excluded from the spiral septum. (E) The structures derived from the cardiac NC (CNC, red), the SHF (yellow) and the PHF (blue) are indicated based on our findings. PHF and SHF overlapping regions that result from the incomplete segregation of the two heart fields at the time of transplantation are in green.
Although several studies have started to address the role of the NC in Xenopus heart development, none has directly evaluated the contribution of the NC to the outflow tract cushions (Sadaghiani and Thiébaud, 1987; Martinsen et al., 2004). In transplantation experiments using Xenopus borealis-Xenopus laevis chimeras, the rhombencephalic NC was seen to contribute a few cells to the wall of the truncus arteriosus (Sadaghiani and Thiébaud, 1987). More recently, ablation of NC progenitors from the entire cranial and trunk regions resulted in a broad range of cardiac defects, including an elongated heart and enlarged pericardial cavity (Martinsen et al., 2004), suggesting a role for the NC in cardiac development. Our NC ablation experiments did not result in any major cardiac anomalies. This difference could be due to the size of the ablated NC domain, as ablation of a large segment of the NC (Martinsen et al., 2004) may have damaging secondary consequences on embryonic development.
Anuran amphibians have a complete atrial septum but lack septal division in the ventricle; however, the alignment of myocytes provides a fairly good functional separation of pulmocutaneous and systemic blood, with an incomplete spiral septum that helps separate circulation at the arterial pole (de Graaf, 1957; Farmer, 1999). This is in contrast to chick and mouse, in which the two circulations are fully separated at the level of the truncus arteriosus. We propose that the cardiac NC was recruited into the amniote outflow tract to increase the mass of the septum in order to complete the separation of the systemic and pulmonary circulations at the arterial pole. Therefore, cardiac NC contribution to outflow tract septation is an acquired characteristic of higher vertebrates that is associated with the evolutionary need for a fully divided circulation.
It is remarkable to see the extent to which the fate and the migration pattern of this cell population have been altered during evolution. One can speculate that signals present in zebrafish to promote cardiac NC migration deep into the heart tube might have been lost in amphibians but subsequently partially restored in amniotes, allowing these cells to migrate into the outflow tract and participate in its septation. Interestingly, during that process, the fate of these cells has been redirected from a myocardial to a mesenchymal phenotype. Alternatively, we can also envision a scenario in which amphibians have evolved a specific mechanism to block cardiac NC migration into the heart, a mechanism that is further refined in amniotes to allow the migration of a subset of these cells into the outflow tract to mediate septation. It would be of particular interest to define in these organisms the signals and molecular mechanisms that regulate the differential migration of cardiac NC cells after they enter the pharyngeal arches.
Supplementary Material
Acknowledgements
We are extremely grateful to Drs Patricia Labosky and Alvin Chin for discussions and comments on the manuscript. We thank Drs Dominique Alfandari, Helene Cousin, Frank Conlon and Paul Krieg for plasmids. This work was supported by Bridge Funds from the University of Pennsylvania and the School of Veterinary Medicine and by a grant from the National Institutes of Health to J.-P.S.-J. (RO1-DE014212). Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.061614/-/DC1
References
- Aoki Y., Saint-Germain N., Gyda M., Magner-Fink E., Lee Y.-H., Credidio C., Saint-Jeannet J.-P. (2003). Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev. Biol. 259, 19-33 [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Kirby M. L. (1984). Dependence of thymus development on derivatives of the neural crest. Science 223, 498-500 [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Redmond M. E., Waldo K., Davis H., Kirby M. L. (1987). Effect of neural crest ablation on development of the heart and arch arteries in the chick. Am. J. Anat. 180, 332-341 [DOI] [PubMed] [Google Scholar]
- Brade T., Gessert S., Kühl M., Pandur P. (2007). The amphibian second heart field: Xenopus islet-1 is required for cardiovascular development. Dev. Biol. 311, 297-310 [DOI] [PubMed] [Google Scholar]
- Brown C. B., Baldwin H. S. (2006). Neural crest contribution to the cardiovascular system. Adv. Exp. Med. Biol. 589, 134-154 [DOI] [PubMed] [Google Scholar]
- Brown D. D., Binder O., Pagratis M., Parr B. A., Conlon F. L. (2002). Developmental expression of the Xenopus laevis Tbx20 orthologue. Dev. Genes Evol. 212, 604-697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Martz S. N., Binder O., Goetz S. C., Price B. M., Smith J. C., Conlon F. L. (2005). Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development 132, 553-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau B. G., Logan M., Davis N., Levi T., Tabin C. J., Seidman J. G., Seidman C. E. (1999). Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev. Biol. 211, 100-108 [DOI] [PubMed] [Google Scholar]
- Buckingham M. E., Meilhac S., Zaffran S. (2005). Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 6, 826-835 [DOI] [PubMed] [Google Scholar]
- Cleaver O. B., Patterson K. D., Krieg P. A. (1996). Overexpression of the tinman-related genes XNkx-2.5 and XNkx-2.3 in Xenopus embryos results in myocardial hyperplasia. Development 122, 3549-3556 [DOI] [PubMed] [Google Scholar]
- Collazo A., Bronner-Fraser M., Fraser S. E. (1993). Vital dye labeling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development 118, 363-376 [DOI] [PubMed] [Google Scholar]
- Conway S. J., Henderson D. J., Copp A. J. (1997). Pax3 is required for cardiac neural crest migration in the mouse: evidence from Splotch (Sp2H) mutant. Development 124, 505-514 [DOI] [PubMed] [Google Scholar]
- Creazzo T. L., Godt R. E., Leatherbury L., Conway S., Kirby M. L. (1998). Role of cardiac neural crest cells in cardiovascular development. Annu. Rev. Physiol. 60, 267-286 [DOI] [PubMed] [Google Scholar]
- de Graaf A. R. (1957). Investigations into the distribution of blood in the heart and aortic arches of Xenopus laevis. J. Exp. Biol. 34, 143-172 [Google Scholar]
- de Pater E., Clijsters L., Marques S. R., Lin Y. F., Garavito-Aguilar Z. V., Yelon D., Bakkers J. (2009). Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 136, 1633-1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J. A., Polson A. G., Klymkowsky M. W. (1989). A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development 105, 61-74 [DOI] [PubMed] [Google Scholar]
- Dyer L. A., Kirby M. L. (2009). The role of secondary heart field in cardiac development. Dev. Biol. 336, 137-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer C. G. (1999). Evolution of the vertebrate cardio-pulmonary system. Annu. Rev. Physiol. 61, 573-592 [DOI] [PubMed] [Google Scholar]
- Gessert S., Kühl M. (2009). Comparative gene expression analysis and fate mapping studies suggest an early segregation of cardiogenic lineages in Xenopus laevis. Dev. Biol. 334, 395-408 [DOI] [PubMed] [Google Scholar]
- Harland R. M. (1991). In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 36, 685-695 [DOI] [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon JB. (1989). MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 8, 3409-3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horb M. E., Thomsen G. H. (1999). Tbx5 is essential for heart development. Development 126, 1739-1751 [DOI] [PubMed] [Google Scholar]
- Hutson M. R., Kirby M. L. (2007). Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin. Cell Dev. Biol. 18, 101-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Rowitch D. H., Soriano P., McMahon A. P., Sucov H. M. (2000). Fate of the mammalian cardiac neural crest. Development 127, 1607-1616 [DOI] [PubMed] [Google Scholar]
- Kelly R. G., Brown N. A., Buckingham M. E. (2001). The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. Cell 1, 435-440 [DOI] [PubMed] [Google Scholar]
- Kirby M. L., Gale T. F., Stewart D. E. (1983). Neural crest cells contribute to normal aorticopulmonary septation. Science 220, 1059-1061 [DOI] [PubMed] [Google Scholar]
- Kirby M. L., Turnage K. L., Hays B. M. (1985). Characterization of conotruncal malformations following ablation of ‘cardiac’ neural crest. Anat. Rec. 213, 87-93 [DOI] [PubMed] [Google Scholar]
- Kokubo N., Matsura M., Onimaru K., Tiecke E., Kuraku S., Kuratani S., Tanaka M. (2010). Mechanisms of heart development in the Japanese lamprey, Lethenteron japonicum. Evol. Dev. 12, 34-44 [DOI] [PubMed] [Google Scholar]
- Kolker S. J., Tajchman U., Weeks D. L. (2000). Confocal imaging of early heart development in Xenopus laevis. Dev. Biol. 218, 64-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Kalcheim C. (1999). The Neural Crest (2nd edn). Cambridge: Cambridge University Press; [Google Scholar]
- Le Lievre C. S., Le Douarin N. M. (1975). Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J. Embryol. Exp. Morphol. 34, 125-154 [PubMed] [Google Scholar]
- Lee Y.-H., Saint-Jeannet J.-P. (2009). Characterization of molecular markers to assess cardiac cushions formation in Xenopus. Dev. Dyn. 238, 3257-3265 [DOI] [PubMed] [Google Scholar]
- Lemaire P., Gurdon J. B. (1994). A role for cytoplasmic determinants in mesoderm patterning: cell-autonomous activation of the goosecoid and Xwnt-8 genes along the dorsoventral axis of early Xenopus embryos. Development 120, 1191-1199 [DOI] [PubMed] [Google Scholar]
- Li Y. X., Zdanowicz M., Young L., Kumiski D., Leatherbury L., Kirby M. L. (2003). Cardiac neural crest in zebrafish embryos contributes to myocardial cell lineage and early heart function. Dev. Dyn. 226, 540-550 [DOI] [PubMed] [Google Scholar]
- Lohr J. L., Yost H. J. (2000). Vertebrate model systems in the study of early heart development: Xenopus and zebrafish. Am. J. Med. Genet. 97, 248-257 [DOI] [PubMed] [Google Scholar]
- Martinsen B. J., Frasier A. J., Baker C. V., Lohr J. L. (2004). Cardiac neural crest ablation alters Id2 gene expression in the developing heart. Dev. Biol. 272, 176-190 [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C. H., Nakaoka T., Moreno-Rodriguez R., Norris R. A., Kern M. J., Eisenberg C. A., Turner D., Markwald R. R. (2001). The outflow tract of the heart is recruited from a novel heart-forming field. Dev. Biol. 238, 97-109 [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Leong L. M., Weninger W. J., Sparrow D. B. (2000). The morphology of heart development in Xenopus laevis. Dev. Biol. 218, 74-88 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1967). Normal Table of Xenopus laevis (Daudin). Amsterdam: North Holland Publishing Company; [Google Scholar]
- O'Donnell M., Hong C.-S., Huang X., Delnicki R. J., Saint-Jeannet J.-P. (2006). Functional analysis of Sox8 during neural crest development in Xenopus. Development 133, 3817-3826 [DOI] [PubMed] [Google Scholar]
- Olson E. N. (2006). Gene regulatory networks in the evolution and development of the heart. Science 313, 1922-1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani B., Thiebaud C. H. (1987). Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Dev. Biol. 124, 91-110 [DOI] [PubMed] [Google Scholar]
- Sato M., Yost H. J. (2003). Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Dev. Biol. 257, 127-139 [DOI] [PubMed] [Google Scholar]
- Slack J. M., Forman D. (1980). An interaction between dorsal and ventral regions of the marginal zone in early amphibian embryos. J. Embryol. Exp. Morphol. 56, 283-299 [PubMed] [Google Scholar]
- Snarr B. S., Kern C. B., Wessels A. (2008). Origin and fate of cardiac mesenchyme. Dev. Dyn. 237, 2804-2819 [DOI] [PubMed] [Google Scholar]
- Snider P., Olaopa M., Firulli A. B., Conway S. J. (2007). Cardiovascular development and the colonizing cardiac neural crest lineage. ScientificWorldJournal 7, 1090-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokony R. F., Aoki Y., Saint-Germain N., Magner-Fink E. K., Saint-Jeannet J.-P. (2002). The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development 129, 421-432 [DOI] [PubMed] [Google Scholar]
- Stolfi A., Gainous T. B., Young J. J., Mori A., Levine M., Christiaen L. (2010). Early chordate origins of the vertebrate second heart field. Science 239, 565-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller J. Z., Epstein J. A. (2005). Cardiac neural crest. Semin. Cell Dev. Biol. 16, 704-715 [DOI] [PubMed] [Google Scholar]
- Takeuchi J. K., Ohgi M., Koshiba-Takeuchi K., Shiratori H., Sakaki I., Ogura K., Saijoh Y., Ogura T. (2003). Tbx5 specifies the left/right ventricles and ventricular septum position during cardiogenesis. Development 130, 5953-5964 [DOI] [PubMed] [Google Scholar]
- Vincent S. D., Buckingham M. E. (2010). How to make a heart: the origin and regulation of cardiac progenitor cells. Curr. Top. Dev. Biol. 90, 1-41 [DOI] [PubMed] [Google Scholar]
- Waldo K. L., Kumiski D. H., Wallis K. T., Stadt H. A., Hutson M. R., Platt D. H., Kirby M. L. (2001). Conotruncal myocardium arises from a secondary heart field. Development 128, 3179-3188 [DOI] [PubMed] [Google Scholar]
- Yelbuz T. M., Waldo K. L., Kumiski D. H., Stadt H. A., Wolfe R. R., Leatherbury L., Kirby M. L. (2002). Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation 106, 504-510 [DOI] [PubMed] [Google Scholar]
- Zaffran S., Kelly R. G., Meilhac S. M., Buckingham M. E., Brown N. A. (2004). Right ventricular myocardium derives from the anterior heart field. Circ. Res. 95, 261-268 [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M., Pines J., Ryan K., Siemering K. R., Haseloff J., Evans M. J., Gurdon J. B. (1996). An indelible lineage marker for Xenopus using a mutated green fluorescent protein. Development 122, 3719-3724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.