Abstract
Gelatin-based microcapsule production using a microfluidic system and the feasibility of the resultant microcapsules for constructing spherical tissues surrounded by heterogeneous cells were studied. The first cell-encapsulation and subsequent cell-enclosing microparticle encapsulation were achieved using a microfluidic flow-focusing droplet production system. A hollow-core structure of about 150 μm in diameter was developed by incubating the resultant microparticles at 37 °C, which induced thermal melting of the enclosed unmodified gelatin microparticles. Mammalian cells filled the hollow-cores after 4 days of incubation. A cell layer on the cell-enclosing microcapsules was developed by simply suspending the microcapsules in medium containing adherent fibroblast cells. This method may prove useful for the generation of gelatin microcapsules using a microfluidic system for formation of artificial tissue constructs.
INTRODUCTION
Cell-enclosing spherical vehicles have been studied as effective tools for the treatment of diseases,1, 2 production of antibodies,3 and drug screening.4 Recently, this type of device has also attracted attention as a novel tool for tissue engineering.5, 6 From a structural viewpoint, the spherical vehicles can be classified into two categories: (1) vehicles with a solid core, designated “microparticles,” and (2) vehicles with a liquid core, designated “microcapsules.” The latter vehicles are more suitable for the interior formation of spherical tissues. Spherical tissues are increasingly recognized as valuable tools in tissue engineering because they appear to mimic the morphology and physiology of the cells in living tissues and organs better than conventional, two-dimensional monolayer cultures.7 One problem that may occur when developing spherical tissues from individual cells in vitro is the formation of necrotic regions inside the tissues resulting from a limited oxygen supply. The maximum allowable size for spherical tissues to avoid formation of necrotic regions is reported to be about 200 μm because of the balance of oxygen diffusion from the surrounding medium and oxygen consumption by cells.8 When considering the growth of spherical tissues within the microcapsules, the size of the hollow-core should be smaller than the maximum critical size. We recently developed this type of microcapsules using a microfluidic system.9, 10, 11 The methodology used in the process first involved enclosing the cells in microparticles of less than 200 μm (denominated as “first microparticle”) and then encapsulating the cell-enclosing microparticles in a second layer of microparticles of around 400 μm (“second microparticle”). These processes were performed using a microfluidic flow-focusing system (Fig. 1). Microfluidic flow-focusing systems attract attention as a useful process for fabricating cell-laden small constructs.12, 13, 14, 15, 16 Finally, the core cell-enclosing microparticles were dissolved under mild conditions suitable for mammalian cells to develop hollow-cores resulting in microcapsules. The first microparticles were prepared by extruding cell-suspending polymer solution from a needle of 300 μm in diameter into water-immiscible liquid flowing in the same direction in a small tubule.17 Another benefit of this microfluidic system is that the viability of the enclosed cells is minimally impacted during the process as shown by a viability of more than 90% for the enclosed cells.17
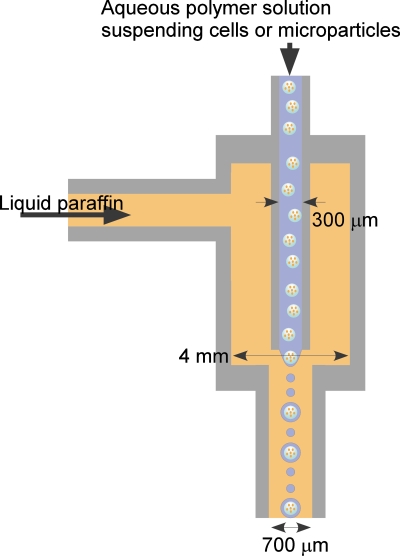
Figure 1.
Schematic illustration of a flow-focusing microdroplet production device.
The aim of present study was to develop the cell-enclosing microcapsules containing a membrane with high cell adhesiveness and biodegradability in vivo using the microfluidic system, which would result in microcapsules with a hollow-core of less than 200 μm in diameter. High cell adhesiveness of the microcapsule membrane provides an additional cell layer on the microcapsule. Coculture of heterogeneous cells has been reported as an effective method for enhancing functions of a variety of cells.18 Furthermore, it is expected that covering microcapsules with endothelial cells will enhance vascularization between microcapsules after transplantation in vivo. By using microcapsules with a biodegradable membrane, it is also expected that we can develop artificial tissues free of materials that do not normally exist in vivo. In this study, we attempted to develop microcapsules with a gelatin derived membrane containing phenolic hydroxyl groups (Gelatin-Ph). Gelatin-Ph is obtained by incorporating phenolic hydroxyl (Ph) groups using aqueous-phase carbodiimide activation chemistry.19 This gelatin derivative is gellable using conventional thermal gelation and∕or peroxidase-catalyzed cross-linking. The resultant gel cross-linked via the enzymatic reaction does not melt at 37 °C. Furthermore, the gel has high cell adhesiveness and proteolytic biodegradability.19
In the present paper, we report on the production of Gelatin-Ph microcapsules using the microfluidic system, which we have previously used for preparation of polysaccharide-based microcapsules.9, 10, 11 We first studied the possibility of using Gelatin-Ph as a microcapsule membrane by evaluating thermal gelation and melting profiles. Based on the results, we enclosed cells in unmodified gelatin microparticles. Subsequently, we encapsulated the first microparticles in Gelatin-Ph droplets. After cross-linking, the Gelatin-Ph molecules via a horseradish peroxidase (HRP)-catalyzed reaction, a hollow-core structure was developed by incubating the second microparticles at 37 °C. We finally coated the microcapsules with adherent cells.
EXPERIMENTAL
Materials
Gelatin-Phs with different amounts of Ph moieties were synthesized by combining gelatin (type A from porcine skin, 300 Bloom, Sigma, St. Louis, MO) with tyramine hydrochloride (Sigma) via carbodiimide-medicated condensation as reported previously.19 The amount of Ph moieties was determined by measuring the absorbance at 275 nm using an ultraviolet-visible spectrometer. Three derivatives were identified containing 0.6×10−7, 1.1×10−7, and 1.4×10−7 Ph moieties∕mg Gelatin-Ph. These Gelatin derivatives were labeled as Gelatin-LPh, -MPh, and HPh, respectively. HRP was obtained from Wako Pure Chem. (170 units∕mg, Osaka, Japan). Rat adipose-derived stem cells and human hepatocellular carcinoma HepG2 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Sigma) containing 10% (v∕v) fetal bovine serum (FBS), 100 mg∕l penicillin, and 58.8 mg∕l streptomycin. Mouse L929 fibroblast cells were grown in Eagle’s minimum essential medium (MEM, Wako Pure Chem., Osaka, Japan) containing FBS, penicillin, and streptomycin at the same concentration in DMEM. The cells were cultured in a humidified atmosphere at 37 °C under 5% CO2∕95% air.
Thermal gelation and melting properties of Gelatin-Ph
Each Gelatin-Ph was dissolved in distilled water at 3.0% (w∕v) by heating around 70 °C. Unmodified gelatin dissolved in distilled water at 3.0% (w∕v) and 5.0% (w∕v) by heating was used as controls. The solutions (200 μl) were poured into a 300 μl plastic tube and cooled in a refrigerator for 2 h to achieve gelation. Then the tubes were set in a temperature control system (PC808, Astec, Fukuoka, Japan). Melting temperatures of the gels in tubes were determined after 15 min of incubation at the set temperature. Gelation temperatures of the solutions were determined using the same temperature control system by cooling the solutions obtained by heating the gels at 56 °C.
Production of microparticles and microcapsules
An axisymmetric flow-focusing droplet generation device designed in our laboratory was used in this study (Fig. 1). Aqueous polymer solution suspending cells or cell-enclosing microparticles (first microparticles) was extruded from an inner needle of 300 μm i.d. into the flow of liquid paraffin containing lecithin at 3% (w∕w) in a tube of 4 mm i.d. The flow of liquid paraffin was focused by decreasing the diameter of the tube to 700 μm i.d. The position of the tip of the inner small needle was set 1.2 mm above of tip of the tube of 700 μm i.d. The flow rate of the polymer aqueous solution was fixed at 0.05 ml∕min.
The first microparticles, a template of the spherical hollow-cores of microcapsules, were prepared by extruding 5% (w∕v) unmodified gelatin solution [6.4×10−3 Pa s at 37 °C, Krebs Ringer Hepes buffer solution (KRH), pH 7.4] containing rat adipose-derived stem cells or HepG2 cells (4.0×107 cells∕ml) maintained at 37 °C into the flow of the liquid paraffin. The resultant cell-enclosing gelatin droplets were cooled in an ice bath for 6 min to obtain the gelatin microparticles. After several rinses with DMEM, the first microparticles were suspended in 3.0% (w∕v) Gelatin-HPh solution (7.3×10−3 Pa s at 25 °C). Then the suspension was extruded using the same microfluidic system. The Gelatin-HPh solution did not gelate at 25 °C but did at 4 °C. In addition, the first microparticles made from unmodified gelatin did not melt at 25 °C. Therefore, the first microparticles-suspending Gelatin-HPh solution was kept at 25 °C until extruded from the needle. The volume ratio of the first microparticles and Gelatin-HPh solution was 1:10. The resultant emulsion system was cooled in an ice bath for 6 min to achieve thermal gelation of the Gelatin-HPh droplets. Subsequently, KRH containing 50 units∕ml HRP and 1 mM H2O2 was mixed with the emulsion system to develop cross-links between Ph moieties through a HRP-catalyzed reaction. The enzymatically cross-linked Gelatin-HPh gel was denominated as ECGelatin-HPh. After 5 min of mixing at room temperature, the second microparticles were collected by centrifugation to remove liquid paraffin and rinsed several times with DMEM. Liquefaction of the enclosed first microparticles made from unmodified gelatin was accomplished by incubating the resultant second microparticles in DMEM at 37 °C.
Covering microcapsules with cells
A day after the preparation, the microcapsules were placed in a six-well tissue culture dish, and 5 ml of DMEM containing L929 cells at 5.0×105 cells∕well was added and incubated for 1 day at 37 °C.
RESULTS AND DISCUSSION
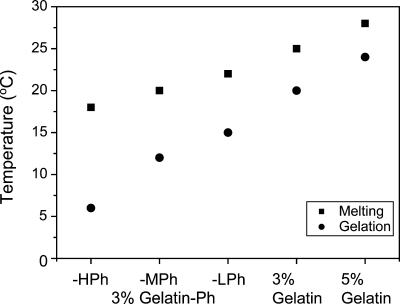
We first investigated the suitability of Gelatin-Ph for microcapsule membrane. The required properties for a Gelatin-Ph solution that is extruded into the coflowing water-immiscible flow after suspending the first microparticles made from unmodified gelatin is that the solution does not gelate without melting the first microparticles at the running temperature. We determined gelation and melting temperatures of unmodified gelatin and Gelatin-Phs with different contents of Ph moieties. Temperatures of solution gelation and gel melting decreased with increasing content of Ph moieties (Fig. 2). The melting and gelation temperatures of 3% (w∕v) Gelatin-HPh gel were 18 and 6 °C, respectively. These were 7 and 14 °C lower than those detected for 3% (w∕v) unmodified gelatin. The lower melting and gelation temperatures of Gelatin-Phs with higher content of Ph moieties were interpreted as a consequence of increasing the degree of hindrance of helix formation and helix growth by the incorporation of Ph moieties onto carboxyl groups. It is well known that the gelation of gelatin solution can occur through the formation of helices from the random coils of the gelatin chains in solution.20 For unmodified gelatin, melting and gelation temperatures increased by 4 and 3 °C, respectively, by increasing the concentration to 5% (w∕v). The nonoverlapped melting temperature of unmodified gelatin gel and gelation temperatures of Gelatin-Phs solutions indicate the possibility of using 3% (w∕v) Gelatin-Ph solutions for suspending unmodified gelatin particles prepared from these solutions without dissolution of the particles. Considering the ease of handling, we decided to use Gelatin-HPh for the microcapsule membrane in the following experiments. We previously reported that Gelatin-Ph was cross-linkable via a HRP-catalyzed reaction and the resultant gels had proteolytic degradability.19
Figure 2.
Thermal gelation and melting temperatures of unmodified gelatin and Gelatin-Phs.
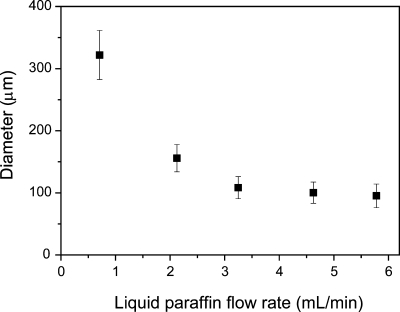
Next, we investigated whether we can prepare Gelatin-HPh microparticles using the microfluidic system. We previously reported that cell-enclosing microparticles of less than 200 μm were successfully prepared from unmodified gelatin solution using the microfluidic system.9 As shown in Fig. 3, we could control the diameter of Gelatin-HPh droplets by changing the flow rate of water-immiscible liquid paraffin to match the unmodified gelatin solution in the range of around 100–300 μm in diameter.10 As was reported for other polymers,17 the flow rate of the Gelatin-HPh solution and the diameter of the inner needle were also effective factors for controlling the diameter of the droplets (data not shown).
Figure 3.
Effect of flow rate of liquid paraffin on diameter of 3% (w∕v) Gelatin-HPh droplets. Bars: mean±SD (n=50). Flow rate of Gelatin-HPh solution was fixed at 0.05 ml∕min.
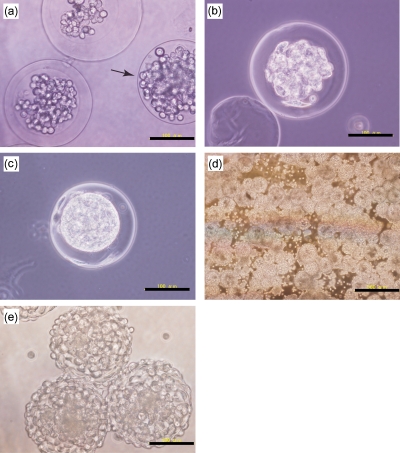
Based on the above results, we prepared second microcapsules from Gelatin-HPh by encapsulating the first microparticles of 168±13 μm in diameter. The second microparticles after undergoing the HRP-catalyzed cross-linking reaction and before incubating at 37 °C are shown in Fig. 4a. About 20% of the resultant second microparticles did not have an intact ECGelatin-HPh membrane [arrow in Fig. 4a]. We thought even these naked microparticles were covered with Gelatin-HPh gel membrane in the initial stage of the first microparticles encapsulation. However, the breakup of the membrane likely occurred during the following stages of enzymatic reaction under mixing, and during the subsequent rinsing step to remove the liquid paraffin;, the naked first microparticles were formed. In fact, we found the second microparticles with a partly broken membrane. Further decrease of the naked first microparticles would be possible by improving individual operations to give less stresses resulting in breakage of the membrane. The naked first microparticles disappeared after incubating at 37 °C because of thermal melting. Only ECGelatin-HPh microcapsules could be collected after 24 h of incubation. This also indicated that hollow-cores were established in the second microparticles with ECGelatin-HPh membrane resulting from melting of the enclosed first microparticles. The enclosed cells existed individually immediately after encapsulation [Fig. 4a]. After 24 h of incubation, the formation of aggregates in the hollow-cores was observed [Fig. 4b]. The aggregates grew and almost occupied the hollow-core at day 4 [Fig. 4c]. From these results, we could not find any adverse effects of using Gelatin-HPh as the microcapsule membrane on the growth of enclosed cells compared with polysaccharide-based microcapsules such as agarose11 and alginate.10 Finally we attempted to add an additional adherent cell layer on the cell-enclosing microcapsules by incubating adherent L929 cells in the medium containing microcapsules. After 24 h of incubation in DMEM, the development of cell layers on the microcapsules was observed and microcapsules were connected by the adhered cells as shown in Figs. 4d, 4e. These results show the feasibility developing the ECGelatin-HPh microcapsules using the microfluidic system and their suitability for engineering tissue constructs composed of spherical aggregates surrounded and connected by a heterogeneous cell layer.
Figure 4.
Microphotographs of rat adipose-derived cells-enclosing ECGelatin-HPh microparticles (a) just after encapsulation of unmodified gelatin microparticles, after (b) 1 and (c) 4 days of incubation, and 1 day after seeding L929 cells on HepG2 cell-enclosing ECGelatin-HPh microcapsules at (d) low magnification and (e) high magnification. The arrow in panel (a) indicates the cell-enclosing unmodified gelatin microparticles without ECGelatin-HPh gel membrane. Bars indicate the scale: either 100 or 500 μm.
CONCLUSIONS
High cell adhesiveness, nontoxicity, and biodegradability are attractive characteristics of gelatin as materials for tissue engineering. In this study, we prepared cell-enclosing microcapsules suitable for fabrication of tissue constructs composed of spherical aggregates surrounded by heterogeneous cells and for enzymatic modification to form cross-linkable gelatin derivatives. The cell-enclosing unmodified gelatin microparticles of less than 200 μm were first fabricated using the microfluidic flow-focusing droplet production system, which generated droplets of aqueous solution in a water-immiscible liquid paraffin flow. Then, the microparticles were coated with Gelatin-HPh using the microfluidic system again followed by HRP-catalyzed cross-linking resulting in an ECGeltain-HPh gel membrane. Enclosed cells grew in the hollow-cores of microcapsules obtained after incubating at 37 °C to melt the enclosed gelatin microparticles. Furthermore, the cell-enclosing ECGelatin-HPh microcapsules were successfully coated with an additional adherent cell layer. These results demonstrate the feasibility of the ECGelatin-Ph microcapsules for forming artificial tissue constructs.
References
- Murua A., Portero A., Orive G., Hernandez R. M., de Castro M., and Pedraz J. L., J. Controlled Release 132, 76 (2008). 10.1016/j.jconrel.2008.08.010 [DOI] [PubMed] [Google Scholar]
- Chang T. M., Nat. Rev. Drug Discovery 4, 221 (2005). 10.1038/nrd1659 [DOI] [PubMed] [Google Scholar]
- Goosen M. F. A., King G. A., McKnight C. A., and Marcotte N., J. Membr. Sci. 41, 323 (1989). 10.1016/S0376-7388(00)82412-6 [DOI] [Google Scholar]
- Zhang X., Wang W., Yu W., Xie Y., Zhang X., Zhang Y., and Ma X., Biotechnol. Prog. 21, 1289 (2005). 10.1021/bp050003l [DOI] [PubMed] [Google Scholar]
- Ando T., Yamazoe H., Moriyasu K., Ueda Y., and Iwata H., Tissue Eng. 13, 2539 (2007). 10.1089/ten.2007.0045 [DOI] [PubMed] [Google Scholar]
- Pound J. C., Green D. W., Chaudhuri J. B., Mann S., Roach H. I., and Oreffo R. O., Tissue Eng. 12, 2789 (2006). 10.1089/ten.2006.12.2789 [DOI] [PubMed] [Google Scholar]
- Koide N., Shinji T., Tanabe T., Asano K., Kawaguchi M., Sakaguchi K., Koide Y., Mori M., and Tsuji T., Biochem. Biophys. Res. Commun. 161, 385 (1989). 10.1016/0006-291X(89)91609-4 [DOI] [PubMed] [Google Scholar]
- Lin R. Z. and Chang H. Y., Biotechnol. J. 3, 1172 (2008). 10.1002/biot.200700228 [DOI] [PubMed] [Google Scholar]
- Sakai S., Ito S., and Kawakami K., Acta Biomater. 6, 3132 (2010). 10.1016/j.actbio.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Sakai S., Ito S., Ogushi Y., Hashimoto I., Hosoda N., Sawae Y., and Kawakami K., Biomaterials 30, 5937 (2009). 10.1016/j.biomaterials.2009.07.031 [DOI] [PubMed] [Google Scholar]
- Sakai S., Hashimoto I., and Kawakami K., Biotechnol. Bioeng. 99, 235 (2008). 10.1002/bit.21624 [DOI] [PubMed] [Google Scholar]
- Arumuganathar S., Irvine S., McEwan J. R., and Jayasinghe S. N., Biomed. Mater. 2, 158 (2007). 10.1088/1748-6041/2/2/015 [DOI] [PubMed] [Google Scholar]
- Arumuganathar S., Irvine S., McEwan J. R., and Jayasinghe S. N., J. Appl. Polym. Sci. 107, 1215 (2008). 10.1002/app.27190 [DOI] [Google Scholar]
- Köster S., Angile F. E., Duan H., Agresti J. J., Wintner A., Schmitz C., Rowat A. C., Merten C. A., Pisignano D., Griffiths A. D., and Weitz D. A., Lab Chip 8, 1110 (2008). 10.1039/b802941e [DOI] [PubMed] [Google Scholar]
- Hong J., deMello A. J., and Jayasinghe S. N., Biomed. Mater. 5, 021001 (2010). 10.1088/1748-6041/5/2/021001 [DOI] [PubMed] [Google Scholar]
- Chabert M. and Viovy J. L., Proc. Natl. Acad. Sci. U.S.A. 105, 3191 (2008). 10.1073/pnas.0708321105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S., Kawabata K., Ono T., Ijima H., and Kawakami K., Biotechnol. Bioeng. 86, 168 (2004). 10.1002/bit.20006 [DOI] [PubMed] [Google Scholar]
- Yamauchi N., Yamada O., Takahashi T., Imai K., Sato T., Ito A., and Hashizume K., Placenta 24, 258 (2003). 10.1053/plac.2002.0901 [DOI] [PubMed] [Google Scholar]
- Sakai S., Hirose K., Taguchi K., Ogushi Y., and Kawakami K., Biomaterials 30, 3371 (2009). 10.1016/j.biomaterials.2009.03.030 [DOI] [PubMed] [Google Scholar]
- Zandi M., Mirzadeh H., and Mayer C., Eur. Polym. J. 43, 1480 (2007). 10.1016/j.eurpolymj.2007.01.011 [DOI] [Google Scholar]