Abstract
Performance IQ (PIQ) greater than verbal IQ (VIQ) is often observed in studies of the cognitive abilities of autistic individuals. This characteristic is correlated with social and communication impairments, key parts of the autism diagnosis. We present the first genetic analyses of IQ discrepancy (PIQ–VIQ) as an autism-related phenotype. We performed genome-wide joint linkage and segregation analyses on 287 multiplex families, using a Markov chain Monte Carlo approach. Genetic data included a genome-scan of 387 micro-satellite markers in 210 families augmented with additional markers added in a subset of families. Empirical P values were calculated for five interesting regions. Linkage analysis identified five chromosomal regions with substantial regional evidence of linkage; 10p12 [P = 0.001; genome-wide (gw) P = 0.05], 16q23 (P = 0.015; gw P = 0.53), 2p21 (P = 0.03, gw P = 0.78), 6q25 (P = 0.047, gw P = 0.91) and 15q23–25 (P = 0.053, gw P = 0.93). The location of the chromosome 10 linkage signal coincides with a region noted in a much earlier genome-scan for autism, and the chromosome 16 signal coincides exactly with a linkage signal for non-word repetition in specific language impairment. This study provides strong evidence for a QTL influencing IQ discrepancy in families with autistic individuals on chromosome 10, and suggestive evidence for a QTL on chromosome 16. The location of the chromosome 16 signal suggests a candidate gene, CDH13, a T-cadherin expressed in the brain, which has been implicated in previous SNP studies of autism and ADHD.
Introduction
Autism spectrum disorders (ASDs) are a group of developmental disorders that involve qualitative deficits in social interaction and communication, and restricted/repetitive interests and behavior. Included among the ASDs are autistic disorder, with deficits in all three domains observed before the age of 3 years; Asperger’s disorder, where developmental language milestones are achieved within normal limits; and pervasive developmental disorder–not-otherwise-specified (PDD–NOS), which may involve later onset, impairments in only two domains, or lesser severity (APA 2004).
Twin, sibling, and family studies of ASDs demonstrate increased risk of autism and autism-related traits to immediate family members (reviewed by Freitag 2007). Briefly, twin studies (Bailey et al. 1995; Folstein and Rutter 1977; Ritvo et al. 1985; Steffenburg et al. 1989) show MZ twin concordance rates between 36 and 96%, with DZ twin concordance rates ranging between 0 and 30%. Among siblings of autistic individuals, the recurrence risk is estimated at 6%, and increased rates of cognitive and communication abnormalities, social impairments, rituals and repetitive play are noted (Bishop et al. 2006; Bolton et al. 1994; MacLean et al. 1999; Silverman et al. 2002; Spiker et al. 1994). Studies of parents of children with autism show increased rates of various social impairments (Bailey et al. 1995, 1996; Bolton et al. 1994). These studies strongly suggest that genes play an important role in autism susceptibility.
Both rare and common susceptibility alleles have been implicated in the genetics of autism. Recent work indicates a substantial role for rare variants, including chromosomal rearrangements (Szatmari et al. 2007) and copy number variants (Glessner et al. 2009). Genome scans of autism using linkage analysis have been performed in ten independent datasets (Allen-Brady et al. 2009; Auranen et al. 2002; Barrett et al. 1999; Cantor et al. 2005; IMGSAC 1998; Philippe et al. 1999; Risch 1999; Schellenberg et al. 2006; Shao et al. 2002b; Yonan et al. 2003). Some signals have been replicated, including 7q (Barrett et al. 1999; Palferman et al. 2001a; Risch 1999; Schellenberg et al. 2006; Shao et al. 2002b; Yonan et al. 2003), 2q (Buxbaum 2001; Palferman et al. 2001b; Philippe et al. 1999), and 17q (Cantor et al. 2005; Yonan et al. 2003), while other regions have shown interesting results in individual datasets [e.g. 5p (Yonan et al. 2003), 13q (Barrett et al. 1999), 4q (Schellenberg et al. 2006), 19p13 (Liu et al. 2001)]. Failure to replicate linkage signals in some regions is likely due to phenotypic or genotypic heterogeneity and/or different ascertainment schemes.
Attempts to address this heterogeneity fall into three broad classes. The first approach is to identify a subset of affected individuals with distinctive characteristics, and stratify the data accordingly. Families have been classified using gender of autistic individuals (Cantor et al. 2005; Lamb et al. 2005; Schellenberg et al. 2006; Stone et al. 2004), presence of developmental regression (Molloy et al. 2005; Schellenberg et al. 2006), severity of obsessive–compulsive behaviors (Buxbaum et al. 2004), delay in the development of phrase speech (Bradford et al. 2001; Buxbaum 2001; Shao et al. 2002a) and IQ (Liu et al. 2008). Despite the reduction in sample size using this approach, linkage signals on 17q11 and 2q were greatly strengthened by considering male-only sib-pairs (17q11) (Cantor et al. 2005; Stone et al. 2004) and by stratifying on phrase speech delay (2q) (Buxbaum 2001; Shao et al. 2002a). The second approach to reducing heterogeneity is to analyze a related quantitative trait, rather than the qualitative autism diagnosis. This approach may increase power, as analysis based on a quantitative trait may address milder expressions of the phenotype that are obscured by a binary diagnosis, and the dataset need not be stratified. Previously examined quantitative traits include social impairment (Duvall et al. 2007; Liu et al. 2008), non-verbal communication ability (Chen et al. 2006), age at first word, and age at first phrase (Alarcon et al. 2002, 2005), the latter two of which identified a region on chromosome 7q35 (Alarcon et al. 2002, 2005). Finally, genome-wide association studies using very large sample sizes have been carried out. These studies ignore subtleties in the phenotype, in hopes that the large samples used will provide the statistical power necessary to overcome heterogeneity. Results have been disappointing so far: regions have been implicated on 5p14 (Ma et al. 2009; Wang et al. 2009) and 5p15 (Weiss and Arking 2009), but replication of these signals has not been achieved (Anney et al. 2010).
Increased IQ discrepancy (between performance and verbal IQ) is seen in autistic individuals and is correlated with core components of the autism phenotype. IQ discrepancy is a quantitative trait, and is easily measured on parents and unaffected siblings. A review of 23 studies considering the cognitive profile of individuals with autism found that verbal IQ is generally lower than performance IQ (Lincoln et al. 1998), a pattern frequently found in a recent study of over 450 preschoolers with autism (Munson et al. 2008), although not universally observed (e.g. Mayes and Calhoun 2003; Minshew et al. 2005; Siegel et al. 1996; Szatmari et al. 1990). Decreased verbal IQ relative to performance IQ can be thought of as an indirect measure of communication impairment, one of the core components of the autism phenotype. Several studies have documented correlation of IQ discrepancy with autism symptoms in the social and communication domains. Smaller IQ discrepancy is correlated with better language skills (Lincoln et al. 1998), while IQ discrepancy in the direction of higher non-verbal skills is correlated with impaired social skills (Tager-Flusberg and Joseph 2003). Recent studies have found that large IQ discrepancies in either direction were associated with social symptoms of autism (Black et al. 2009), while intellectual disability (as measured by a composite IQ score) was only weakly correlated with severe autistic traits (Hoekstra et al. 2009).
We present the first genetic analysis of IQ discrepancy as a continuous autism-related phenotype. We describe correlations between IQ discrepancy and social and communication impairments, and present evidence for the genetic basis of IQ discrepancy from clustering and segregation analysis. We describe evidence of linkage to five genomic regions based on a genome-scan, including one region that reaches genome-wide significance.
Materials and methods
Ascertainment and phenotyping
Recruited families were identified with two or more children with an ASD, as part of the National Institutes of Health Collaborative Programs of Excellence in Autism. Contributing centers included the University of Washington and the University of Pittsburgh. Exclusion criteria included proband age below 3 years, presence of a known genetic condition, history of a serious head injury or of significant sensory or motor impairment, such as blindness or cerebral palsy. This study was approved by the University of Washington Institutional Review Board, and informed consent was obtained from all participants and/or their parents.
Children with a reported ASD were directly assessed using the Autism Diagnostic Interview-Revised (Lord et al. 1994) (ADI-R) and Autism Diagnostic Observation Schedule-Generic (Lord et al. 2000) (ADOS-G) by trained clinicians, and assigned a DSM-IV (APA 2004) diagnosis. Individuals designated as affected met DSM-IV criteria for an ASD based on ADI-R, ADOS-G and expert clinical judgment. Affected status in this dataset corresponds to the “broad” autism diagnosis used in a previous study (Schellenberg et al. 2006).
A total of 287 families with two or more affected children were recruited, including 278 families from the University of Washington and 9 from the University of Pittsburgh. Family structures are primarily nuclear (n = 270), with 2–8 children. Other family structures include those with half-siblings (single or multiple; n = 15) and extended families (n = 2). Information regarding race was available for 267 families, 227 of whom self-identified as White, 7 Asian, 4 Black or African-American, 1 Native Hawaiian or Pacific Islander, 1 American Indian, and 27 mixed race.
Cognitive ability was assessed in all willing participants using standardized assessments appropriate to the individual’s age: for ages 2.5–4.5 years, the Mullen Scales of Early Learning (Mullen 1997); for ages 4.5–6 years, the Wechsler Preschool and Primary Scale of Intelligence-Revised (Wechsler 1989) (WPPSI-R); for ages 7–17 years, the Wechsler Intelligence Scale for Children (Wechsler 1991) (WISC-III); and for ages 17 and older, the Wechsler Adult Intelligence Scale (Wechsler 1997) (WAIS-III). The Wechsler short form subtests consisted of Vocabulary, Comprehension, Block Design and Object Assembly. Performance IQ (PIQ), verbal IQ (VIQ) and full-scale IQ (FSIQ) were estimated based on these subtests as recommended (Sattler 2001). From the Mullen scales, we calculated VIQ based on receptive and expressive language scales, and PIQ based on visual reception and fine motor scales. The IQ discrepancy phenotype was defined as PIQ-VIQ and was available for 1,127 of the 1,359 individuals (82%) in these families. Some subtleties in the discrepancy phenotype arise in individuals who receive the lowest possible score (floor), on either or both of the VIQ or PIQ scales. When an individual floored on VIQ alone (n = 102), their discrepancy score was treated as censored from above. Similarly, flooring on PIQ alone (n = 6) represents censoring from below, but due to analytical constraints prohibiting censoring on both ends of the distribution, these scores were taken at nominal value. For individuals who floored on both PIQ and VIQ (n = 58), the IQ discrepancy was considered to be missing. The remaining individuals with missing data were mostly founders.
Genotyping
Genotype data on a genome-scan of micro-satellite markers were available for 1,157 (85%) of 1,359 individuals. The remaining 15% represents unavailable founders. The mean distance between the 387 markers used was 9.29 centi-Morgans (cM), and the largest gap was 22.90 cM. These genotype data, determined with methods previously described (Schellenberg et al. 2006), are henceforth referred to as the ABI dataset.
Supplemental micro-satellite marker data provided 95 new markers on the five chromosomes of interest (28, 20, 19, 14 and 14 markers on chromosomes 2, 6, 10, 15, and 16, respectively). These genotypes were available for up to 857 individuals in the 199 families that were part of a collaboration with the Autism Genome Project (AGP) (Szatmari et al. 2007). An integrated map containing both ABI and supplemental AGP markers was generated for each chromosome, using the information in the Rutgers map (build 35.1) (Kong et al. 2004). The average marker spacing of the integrated maps over the five chromosomes was 4.9 cM, and the largest gap was 15.1 cM. The addition of the AGP dataset does not increase the number of families contributing to the analysis, but rather increases the marker density for those already contributing through the ABI dataset.
A total of 210 families have two or more children who are both phenotyped and genotyped, and therefore contribute to both linkage and trait-modeling analyses. The remaining families have phenotype data alone, and therefore contribute information only to trait-modeling analysis. A subset of these families was used in a previous linkage analysis of the binary autism phenotype (Schellenberg et al. 2006).
Statistical analyses
Correlation and clustering analyses
We tested the null hypotheses that (a) IQ discrepancy is uncorrelated between affected siblings; and (b) high IQ discrepancy does not cluster in families. We considered the 189 families with exactly two affected children for whom IQ discrepancy was measured, and calculated the Pearson correlation coefficient for the continuous IQ discrepancy phenotype. We also defined a qualitative phenotype, “high PIQ”, which is observed when the IQ discrepancy is greater than or equal to 15 points. Each sib-pair was then designated either concordant or discordant for high PIQ status. In each of 10,000 permutations, the individuals with high PIQ were randomly assigned among the 189 families, and the resulting number of concordant sib-pairs was recorded. The P value is the fraction of permutations in which the number of concordant sib-pairs exceeded the number in the observed data.
Joint segregation and linkage analysis
Oligogenic joint segregation and linkage analyses, in addition to segregation analysis alone, were carried out using Loki 2.4.7 (Daw et al. 1999; Heath et al. 1997), a package that models genetic traits using a Bayesian Markov chain Monte Carlo (MCMC) approach. This approach provides computationally tractable simultaneous multipoint analyses of censored continuous traits while incorporating a flexible model that allows for multiple quantitative trait loci (QTLs). The power and reliability of these methods have been described using simulated and real datasets [e.g. HDL variation (Gagnon et al. 2003)], while Wijsman and Yu (2004) provide a detailed review of the attributes and interpretation of the methodology.
The IQ discrepancy trait is modeled as
where yj is the value of PIQ-VIQ for individual j, α the baseline mean, Qij the genotype effect due to QTL i according to individual j’s genotype, and εi is the normally distributed random error. The total number k of QTLs is a random variable estimated in the modeling process. Prior assumptions include (a) the number of QTLs follow a Poisson distribution with mean 2; (b) the genotype effects for each QTL are normally distributed with mean zero and common variance τ = 400, and (c) QTLs are uniformly distributed across the genome.
By use of a Bayesian reversible-jump MCMC sampler, estimates of posterior distributions are obtained conditional on observed data and prior assumptions. Parameters for which the posterior distribution is estimated include the number k of QTLs, the allele frequencies and genotype effects for each, and if marker genotype data were included, QTL locations. Both segregation and linkage analyses were based on 500,000 iterations after 5,000 iterations of burn-in, with values in alternate iterations saved for estimation of the posterior distributions. Single-marker analyses were based on 150,000 iterations.
In analyses where the observed data include marker genotypes, the strength of evidence for linkage to an interval of the genome can be summarized using the Bayes Factor:
where q1 is the posterior probability of a QTL being in the interval of interest, while q0 is the prior probability of a QTL being in that interval, based on the assumption of uniform QTL distributions on the genome. Thus, the BF is the ratio of the posterior odds of linkage to the prior odds of linkage. We use the base-10 logarithm of the BF (calculated in 4 cM intervals across each chromosome) to render the scale similar to that of a traditional LOD score. This is a Bayesian approach, and therefore frequentist P values are not appropriate for interpretation of a single observed BF.
Generation of empirical P values
Region-wide empirical P values were generated by simulation in each of the five regions of interest. We defined a region of 60 cM on each chromosome of interest that included the location of the linkage signal, and used the program SimSuite (Igo and Wijsman 2008) to simulate 1,000 sets of marker data for each. Joint segregation and linkage analyses were performed for each simulated dataset, and the results were summarized by calculation of log(BF) across the region. The P value is the fraction of the simulations for which the observed maximum log(BF) anywhere in the region exceeds that obtained in the original dataset.
Results
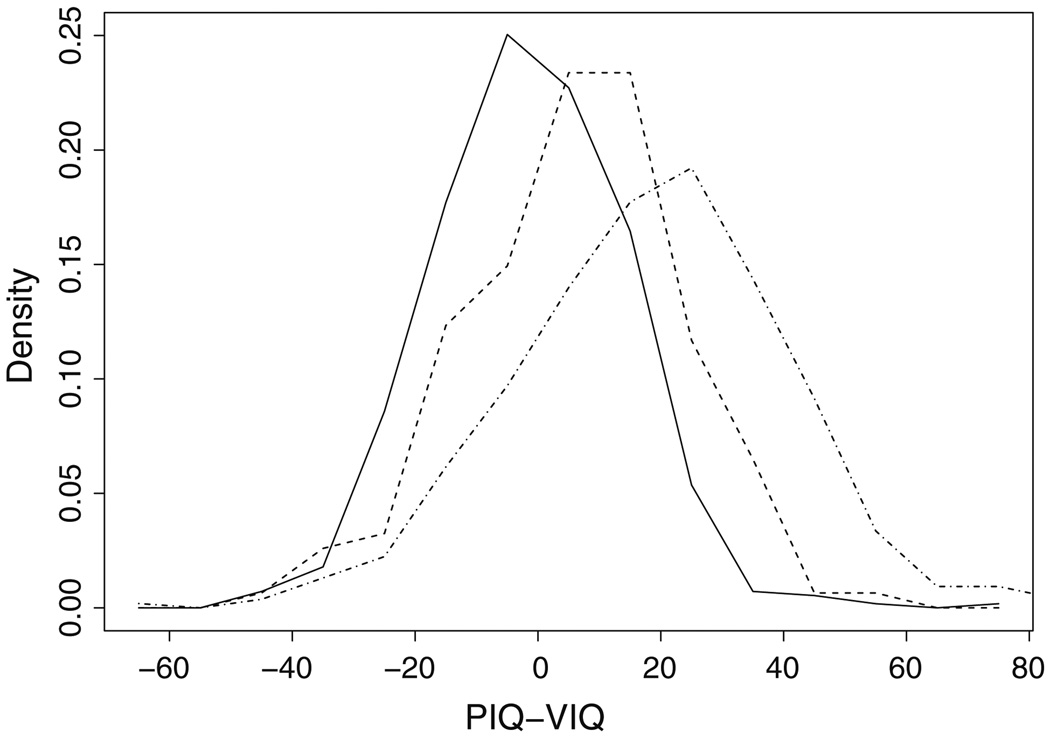
Table 1 shows age and sex demographics along with IQ summary statistics for the 287 families, subdivided into groups of founders (individuals without parents in the pedigree; either parents or grandparents), unaffected children, and affected children. The distributions of the IQ discrepancy score are shown in Fig. 1, and summarized in Table 1. In parents, the IQ discrepancy score is centered near zero (mean = −0.99), with similar proportions of individuals in the high (PIQ–VIQ ≥ 15; 16%) and low (PIQ–VIQ ≤ −15; 19%) tails. In unaffected children, the mean discrepancy score is higher (mean = 6.67) than in parents, and the proportion of individuals scoring in the high tail (33%) is twice that in parents. Finally, the mean discrepancy score in affected children is higher still (mean = 18.69) and a larger proportion of individuals fall in the high tail (58%), than in the low end (6%) of the distribution. These results for children with autism spectrum disorders are in contrast to the distribution of IQ discrepancy scores in the reference population for the WISC-III, where 12.4 and 14.3% of children fell in the low and high tails, respectively (Sattler 2001). In adults assessed with the full WAIS-III, 17.6% of a reference sample fell in the high and low tails combined (Wechsler 1997), as compared to 37% in our sample. It is notable that FSIQ scores in adults in our sample are high, possibly reflecting recruitment bias.
Table 1.
Demographic characteristics and summary statistics for full-scale IQ (FSIQ), verbal IQ (VIQ), performance IQ (PIQ) and IQ discrepancy (PIQ–VIQ) for 287 families
| Founders | Unaffected children | Affected children | |
|---|---|---|---|
| Age (years; months) | |||
| Mean | 39; 8 | 9; 7 | 8; 8 |
| Median | 39; 0 | 8; 9 | 7; 9 |
| SD | 6; 4 | 4; 6 | 4; 2 |
| N | 522 | 115 | 578 |
| Gender | |||
| M | 309 | 78 | 489 |
| F | 294 | 75 | 114 |
| FSIQ | |||
| Mean | 112.4 | 104.6 | 76.2 |
| Median | 114.0 | 106.0 | 73.0 |
| SD | 13.9 | 19.6 | 25.8 |
| N | 520 | 114 | 567 |
| VIQ | |||
| Mean | 112 | 100.7 | 70.2 |
| Median | 113 | 100 | 64 |
| SD | 13.6 | 19.2 | 26.1 |
| N | 501 | 113 | 555 |
| PIQ | |||
| Mean | 110.5 | 107.3 | 86.7 |
| Median | 108 | 108 | 89 |
| SD | 15.1 | 18.0 | 26.9 |
| N | 519 | 114 | 566 |
| PIQ–VIQ | |||
| Mean | −0.99 | 6.67 | 18.69 |
| Median | −1.00 | 8.00 | 20.00 |
| SD | 15.9 | 15.9 | 21.5 |
| N | 500 | 112 | 510 |
| ≤−15 | 96 (19%) | 9 (8%) | 31 (6%) |
| ≥15 | 80 (16%) | 37 (33%) | 298 (58%) |
Founders are unrelated, whereas the two groups of children may include more than one individual from each family
Fig. 1.
Empirical distributions of IQ discrepancy (PIQ–VIQ) in parents (solid line), unaffected siblings (dashed line) and affected individuals (dot-dash)
Phenotypic correlations
Previously identified correlations between IQ discrepancy and core autism symptoms are replicated in our sample. Table 2 summarizes the correlation of IQ discrepancy and raw scores of both social and communication subscales of the ADOS and ADI, with affected individuals grouped according to which module was administered (ADOS) or their verbal/non-verbal status (ADI). ADOS modules correspond to the expressive language level of the person being tested—module 1 is for individuals who are pre-verbal or have single words; module 2 is for individuals with phrase speech. modules 3 and 4 are for children and adults with fluent speech, respectively. Correlation of IQ discrepancy with the ADOS social and communication subscales was significantly different from zero in individuals who were administered module 3. Borderline significance was observed in those given module 4, and for the social subscale in those given module 2. Correlation of IQ discrepancy with ADI social and communication subscales was also significantly different from zero in verbal individuals (see Online Resource 1 for more detail).
Table 2.
Spearman correlation between IQ discrepancy and ADOS and ADI social and communication subscales
| ADOS |
ADI |
|||||
|---|---|---|---|---|---|---|
| Module 1 (n = 87) |
Module 2 (n = 113) |
Module 3 (n = 177) |
Module 4 (n = 26) |
Non-verbal (n = 177) |
Verbal (n = 449) |
|
| Social | −0.07 | 0.17^ | 0.20** | 0.34^ | −0.09 | 0.13** |
| Communication | 0.08 | −0.03 | 0.23** | 0.33^ | −0.05 | 0.19*** |
P < 0.10,
P < 0.05,
P < 0.01,
P < 0.001
Data cleaning
RELPAIR version 2.0 (Epstein et al. 2000) was used to check family structures and identify potential sample swaps, using the assumption of a 1% genotyping error rate. Using the ABI dataset, we identified families with monozygotic twins and apparent non-paternities. Mendelian inconsistencies were identified using Loki (Heath et al. 1997). In the ABI dataset, a total of 1,156 inconsistencies were identified, from a total of 555,573 genotypes, for an overall error rate of 0.2%. These inconsistencies were coded as missing for all individuals in the pedigree in question.
Correlation and clustering analyses
Correlation and clustering analyses indicate that (a) IQ discrepancy is correlated in affected siblings and (b) high IQ discrepancy clusters in families. The correlation coefficient for the quantitative phenotype was 0.27 (P = 0.00014). There is also significant evidence (P = 0.0067) that high PIQ phenotype clusters within families. Of the 378 affected children in the 189 families with exactly two affected children, 219 had high PIQ. Seventy-five sib-pairs were discordant for high PIQ, while 114 were concordant (both had high PIQ in 72 families, both did not in 42 families).
Segregation analysis
MCMC oligogenic segregation analysis demonstrates that there is substantial evidence of familial transmission of IQ discrepancy in this dataset. After approximate Winsorization (Igo et al. 2006) of two potentially influential observations (scores of 71 and −68, Winsorized to 50 and −50, respectively), 3 clear models emerge (see Table 3). Model A, with a broad-sense heritability of 33%, has a minor-allele frequency of 0.25, where the rare homozygote has dramatically increased IQ discrepancy. Model B, with a broad-sense heritability of 18%, has a similar structure, with a minor-allele frequency of 0.36 and a smaller increase in IQ discrepancy in the rare homozygote. In Model C the minor-allele (P = 0.47) conveys risk to the rare homozygote of increased IQ discrepancy in the opposite direction to models A and B (PIQ < VIQ).
Table 3.
Models resulting from MCMC segregation analyses of IQ discrepancy, allowing for censoring from above
| Model | pA | μAA | μAB | μBB | ||||
|---|---|---|---|---|---|---|---|---|
| A | 0.75 | 1.43 | −9.82 | 46.10 | 170.14 | 0.33 | ||
| B | 0.64 | 5.46 | −9.68 | 17.17 | 93.60 | 0.18 | ||
| C | 0.47 | −12.51 | 9.99 | −7.88 | 101.73 | 0.20 |
pA is the frequency of allele A, μAA, μAB and μBB are the means for genotypes AA, AB and BB respectively, is the genetic variance, and is the total variance
Joint segregation and linkage analysis
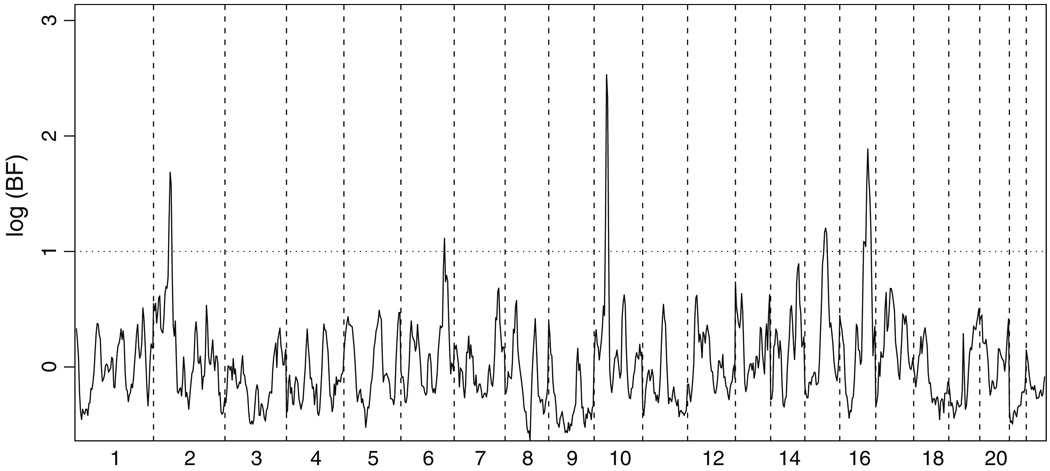
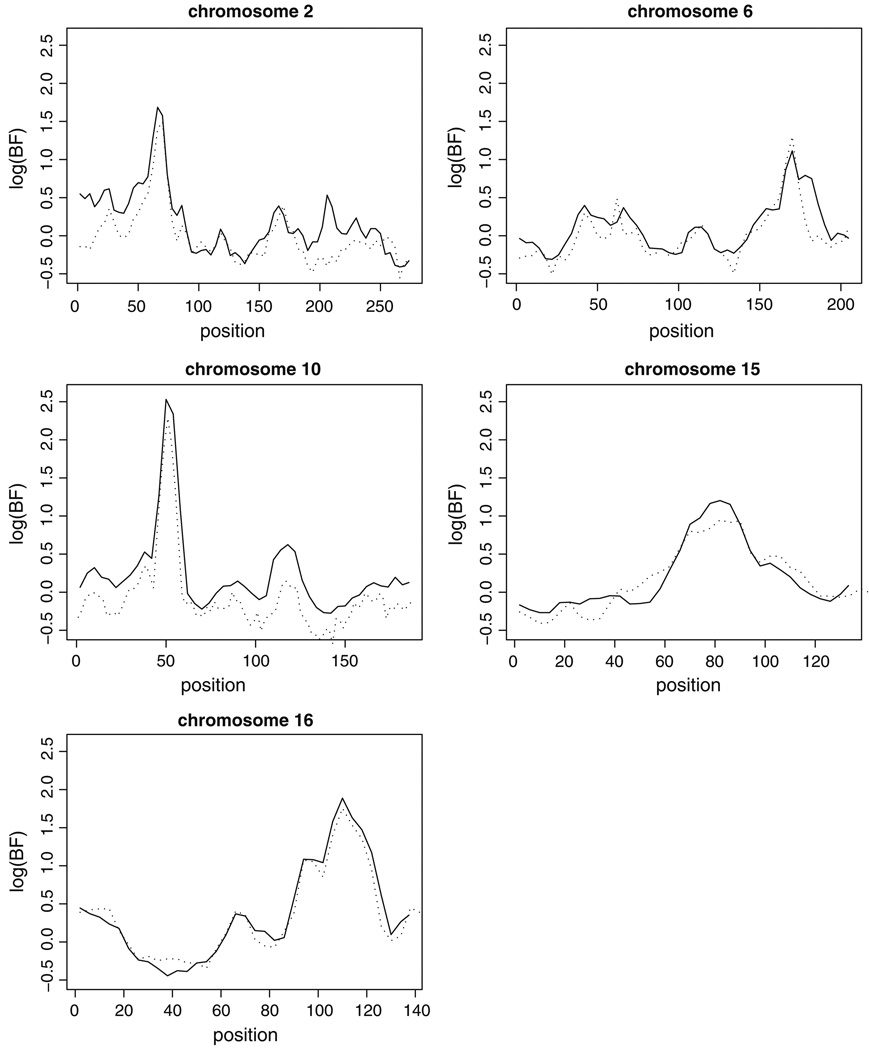
Genome-scan analyses identified five chromosomal regions with substantial evidence of linkage to IQ discrepancy. Figure 2 shows the log(BF) across the genome. A log(BF) of 1 as a lower threshold for notable linkage signals identified five regions. By far the strongest signal genome-wide is on chromosome 10, where log(BF) achieves a maximum value [logmax(BF)] of 2.53 at D10S197, and log(BF) > 1 over a 12 cM region. Chromosomes 2 and 16 yielded moderate signals with logmax(BF) values of 1.69 (near D2S2259) and 1.89 (near D16S3091), respectively. The region of interest covers 12 cM on chromosome 2, and 32 cM on chromosome 16. The weakest signals are on chromosomes 6 and 15, where logmax(BF) is 1.11 and 1.21, respectively. On chromosome 6, log(BF) > 1 in only one 4 cM interval (between D6S441 and D6S1581), although log(BF) is inflated over background over an interval of 14 cM. The interval on chromosome 15 is larger, with log(BF) > 1 over 12 cM (between D15S131 and D15S205). Single-marker analyses in all five chromosomal regions (see Online Resource 2) confirm that each signal is due to multiple markers, and support the multipoint results. The addition of the AGP markers on the chromosomes of interest results in only subtle changes in the interpretation of the results. Figure 3 shows log(BF) on chromosomes 2, 6, 10, 15 and 16, using both the ABI marker set alone, and the combined ABI and AGP markers. Differences are small and likely reflect stochastic variation among MCMC runs.
Fig. 2.
Genome-scan results using the ABI dataset. The base-10 logarithm of the Bayes Factor, calculated in 4 cM intervals, is plotted against the genetic location along each chromosome using the Haldane map. Chromosomes are labeled across the bottom of the figure
Fig. 3.
Whole-chromosome scan results for each of the five chromosomes of interest, using both the ABI dataset (solid line) and the combined ABI and AGP datasets (dot-dash line). The base-10 logarithm of the Bayes Factor is plotted against the genetic location (cM) along the chromosome
Empirical P values confirm that the linkage signals on chromosomes 10 and 16 showed strongest significance, with the chromosome 10 signal achieving genomewide significance (Table 4). On chromosome 10, logmax(BF) = 2.65 had a region-wide empirical P value of 0.001, which corresponds to a genome-wide P value of 0.05 (after a conservative Bonferroni-style correction based on the total genome length assumed in the analysis). On chromosome 16, logmax(BF) = 1.67 had a region-wide empirical P value of 0.015 (genome-wide P = 0.53), and signals on chromosomes 2, 6 and 15 had region-wide P values of 0.03, 0.047 and 0.053 (Bonferroni corrected genome-wide P values 0.78, 0.91 and 0.93), respectively.
Table 4.
Region-wide empirical P values based on 1,000 simulated datasets for each chromosome
| Chromosome | Interval (cM) | Max log(BF) (position, cM) |
Empirical P value |
Analysis time (days) |
|---|---|---|---|---|
| 2 | 30–90 | 1.49 (67.9) | 0.030 | 33 |
| 6 | 130–190 | 1.28 (170.1) | 0.047 | 27 |
| 10 | 20–80 | 2.65 (49.7) | 0.001 | 38 |
| 15 | 60–120 | 1.31 (77.5) | 0.053 | 29 |
| 16 | 80–140 | 1.67 (109.4) | 0.015 | 29 |
The three models identified by the segregation analysis reappear in the joint segregation and linkage analysis, with results supporting the specific localization of each model. The QTLs with locations on chromosomes 10 and 16 represent models A and B, respectively, while those on chromosome 15 overwhelmingly represent model C. The models are less clearly defined on chromosomes 2 and 6: QTLs with chromosome 2 locations represent both models B and C, while those on chromosome 6 represent models A and C.
Discussion
The work presented here demonstrates that IQ discrepancy is a useful autism-related phenotype, and may also be of interest in other disorders that involve deficits in language and/or communication. In the current study, increased performance IQ relative to verbal IQ was observed in siblings of autistic individuals, and to an even greater degree in the probands themselves. Such an observation is important, and can only be demonstrated in a dataset such as ours where the phenotype has been measured in additional relatives. IQ discrepancy in the same direction as observed here is also observed in specific language impairment [where children have language below age-expectations but normal or higher non-verbal intelligence (Rice et al. 2005)], learning-disabilities including dyslexia (e.g. Alm and Kaufman 2002), and in some samples of children with ADHD (Wechsler 1991). The genes that affect IQ discrepancy in autism may also be relevant to these other disorders—but only further study can answer this question.
The use of IQ discrepancy as a phenotype for genetic analysis, while novel, is supported by results in cognitively normal subjects from linkage studies of component domains of FSIQ, and the observation of consistent discrepancy in autism samples (reviewed in “Introduction”). Previous genome-scan linkage analyses (Dick et al. 2006; Luciano et al. 2006; Posthuma et al. 2005) of FSIQ, PIQ, and VIQ have demonstrated that the different domains of IQ (i.e. PIQ and VIQ) may map to different locations in the genome. Potential limitations with the use of IQ discrepancy include (a) greater relative variability due to the use of a difference measure, and (b) the possibility that PIQ and VIQ may assess slightly differing aspects of intelligence at different points in development (Sattler 2001). Despite these challenges, our results clearly demonstrate that strong signals of a genetic basis can persist. Replication of our signals in other datasets will require careful selection of a phenotype that is the same or very similar to that measured here, and may also require availability of the phenotype in parents and siblings to achieve sufficient power. For example, identical tests may be necessary because different tests measuring the same domain can yield different results, and even within the same test, subtle differences in subtest composition can be important.
Analysis of IQ discrepancy as an autism-related phenotype in these multiplex families demonstrated that this trait segregates in a manner consistent with the presence of several major genes. Joint segregation and linkage analysis highlighted five regions with evidence for linkage, the strongest on chromosomes 10p12 and 16q23. Regions implicated by other groups for linkage to the binary autism phenotype on 2q, 7q and 17q were not replicated in the current analysis, suggesting that IQ discrepancy addresses a novel aspect of the autism phenotype that has not been explored before. From an analytical standpoint, IQ discrepancy has two particular strengths. First, as a continuous measure, it maximizes power to detect linkage. Second, it is directly measurable in both adults and children, with the availability of parental data contributing information about the transmission of the trait from parent to child.
This is the first genome-scan in which chromosome 10 yielded the strongest signal for an autism phenotype, although the region has been previously implicated. The first analysis of the International Molecular Genetic Study of Autism Consortium (IMGSAC 1998) implicated exactly the same marker (D10S197). Analyses of two distinct subsets of the Autism Genetic Resource Exchange database found weak evidence of linkage in male-only affected sib-pairs (Cantor et al. 2005) and in sib-pairs with obsessive–compulsive traits (Buxbaum et al. 2004), at locations about 15 and 25 cM p-ter to D10S197, respectively. Further evidence implicating this region includes a report of a child with PDD-NOS and a complex chromosomal rearrangement involving breakpoints in both 10p11 and 10p12 (Zwaigenbaum et al. 2005). Together these results indicate that further consideration of this region of chromosome 10 is warranted.
The chromosome 16 signal, while statistically less significant, is particularly interesting because the maximum evidence of linkage (at D16S3091) falls in the same location as a strong linkage signal for a non-word repetition (NWR) phenotype in a sample of families with specific language impairment (SLI) (Newbury et al. 2004). Studies to date of NWR in both autism and SLI are reviewed in Whitehouse et al. (2008). SLI is often thought to result from deficits in phonological short-term memory (Gathercole and Baddeley 1990), quantified using tests based on NWR, and poor performance on such tests is a defining feature of SLI (Tager-Flusberg and Cooper 1999). Significantly, poor performance on NWR tasks has also been observed in a subset of autistic individuals with language impairments (Kjelgaard and Tager-Flusberg 2001). This striking finding of deficits in NWR tasks in both SLI and autism has been interpreted in different ways. Some argue that there is a common underlying etiology for both SLI and the language impairment aspects of autism (Tager-Flusberg 2006). Alternatively, others argue that other aspects of language impairment differ in the two populations, and in fact the types of error made in the NWR tasks are also different (Whitehouse et al. 2008). Allelic heterogeneity provides a plausible explanation for both the coincident linkage findings and the observation of differing errors on NWR tests in the SLI and autistic populations. Allelic heterogeneity is a well known phenomenon, and includes the classic case of Duchenne and Becker muscular dystrophies, both of which involve mutations in the dystrophin (OMIM #300377) gene, but with very different presentation and disease course (Bushby 1992). The coincidence of our linkage signal for IQ discrepancy in autism with a strong linkage signal for NWR in SLI supports the idea of a common etiology for aspects of these disorders, and provides an exciting new avenue of study.
The region of interest on chromosome 16 contains an obvious candidate gene: CDH13. D16S3091, which is at the location with the strongest evidence for linkage, is in the gene for CDH13, a T-cadherin. CDH13 is expressed in adult cerebral cortex and medulla, and may play a role in neural cell proliferation (Takeuchi et al. 2000). There is a high degree of amino acid conservation between mouse and human T-cadherin sequence, suggesting that the gene is of particular importance (Takeuchi et al. 2000). Thus, CDH13 is a plausible candidate gene for autism and related phenotypes. In a recent genome-wide association study, a SNP in CDH13 was significantly associated with autism (P = 0.000845) in combined samples from the Autism Genetics Resource Exchange and the Autism Case Control cohort (Wang et al. 2009). Furthermore, CDH13 has been implicated in three genome-wide association studies of ADHD (Franke et al. 2009). Children with ADHD and ASD share impairments in executive functioning and have overlapping behavioral concerns, and involvement of common neurological pathways and neuro-anatomical structures (Corbett et al. 2009). It is plausible that mutations in CDH13 contribute to ADHD, SLI and autism—a parsimonious hypothesis that would relate three complex developmental phenotypes through a gene expressed in the brain. Confirmation of this hypothesis awaits replication of the linkage signal in other datasets, and close examination of the region with dense SNP panels or DNA sequencing.
Supplementary Material
Acknowledgments
Funding was provided by the Autism Centers of Excellence (HD055782). Some genotyping services were provided by the Center for Inherited Disease Research (CIDR), or supported by Autism Speaks, through collaboration with the Autism Genome Project. CIDR is funded through a federal contract from the NIH to The Johns Hopkins University, contract number HHSN268200782096C. We also gratefully acknowledge the contributions of the families who participated in this study, the contributions of the Clinical and Statistical Cores of the UW ACE for diagnostic and databasing support. The authors thank the anonymous reviewers for helpful comments that greatly improved this paper.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00439-010-0899-z) contains supplementary material, which is available to authorized users.
Conflict of interest All authors report no conflicts of interest. Experiments conducted comply with the current laws of the country in which they were performed.
Contributor Information
Nicola H. Chapman, Department of Medicine, University of Washington, Seattle, WA, USA
Annette Estes, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, USA.
Jeff Munson, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, USA.
Raphael Bernier, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, USA.
Sara J. Webb, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, USA
Joseph H. Rothstein, Department of Medicine, University of Washington, Seattle, WA, USA
Nancy J. Minshew, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA Department of Neurology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Geraldine Dawson, Autism Speaks, University of North Carolina Chapel Hill, Chapel Hill, NC, USA; Department of Psychiatry, University of North Carolina Chapel Hill, Chapel Hill, NC, USA.
Gerard D. Schellenberg, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Ellen M. Wijsman, Email: wijsman@u.washington.edu, Department of Medicine, University of Washington, Seattle, WA, USA; Department of Biostatistics, University of Washington, Seattle, WA, USA; Department of Genome Sciences, University of Washington, Seattle, WA, USA; Statistical Genetics Lab, T15, 4333 Brooklyn Ave NE, Seattle, WA 98195-9460, USA.
References
- Alarcon M, Cantor RM, Liu J, Gilliam TC, Geschwind DH Consortium AGRE. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Am J Hum Genet. 2002;70:60–71. doi: 10.1086/338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Yonan AL, Gilliam TC, Cantor RM, Geschwind DH. Quantitative genome scan and ordered-subsets analysis of autism endophenotypes support language QTLs. Mol Psychiatry. 2005;10:747–757. doi: 10.1038/sj.mp.4001666. [DOI] [PubMed] [Google Scholar]
- Allen-Brady K, Robison R, Cannon D, Varvil T, Villalobos ME, Pingree C, Leppert M, Miller J, McMahon WM, Coon H. Genome-wide linkage in Utah autism pedigrees. Mol Psychiatry. 2009;15:1006–1015. doi: 10.1038/mp.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm J, Kaufman AS. The Swedish WAIS-R factor structure and cognitive profiles for adults with dyslexia. J Learn Disabil. 2002;35:321–333. doi: 10.1177/00222194020350040301. [DOI] [PubMed] [Google Scholar]
- Anney R, Klei L, Pinto D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Sykes N, Pagnamenta AT, Almeida J, Bacchelli E, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Carson AR, Casallo G, Casey J, Chu SH, Cochrane L, Corsello C, Crawford EL, Crossett A, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Melhem NM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Piven J, Posey DJ, Poustka A, Poustka F. A genome-wide scan for common alleles affecting risk for autism. Hum Mol Genet. 2010;19:4072–4082. doi: 10.1093/hmg/ddq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th edn. Washington, DC: American Psychiatric Association; 2004. [Google Scholar]
- Auranen M, Vanhala R, Varilo T, Ayers K, Kempas E, Ylisaukko-oja T, Sinsheimer JS, Peltonen L, Jarvela I. A genomewide screen for autism-spectrum disorders: evidence for a major susceptibility locus on chromosome 3q25–27. Am J Hum Genet. 2002;71:777–790. doi: 10.1086/342720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Lecouteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder—evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, Rutter M. Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Barrett S, Beck JC, Bernier R, Bisson E, Braun TA, Casavant TL, Childress D, Folstein SE, Garcia M, Gardiner MB, Gilman S, Haines JL, Hopkins K, Landa R, Meyer NH, Mullane JA, Nishimura DY, Palmer P, Piven J, Purdy J, Santangelo SL, Searby C, Sheffield V, Singleton J, Slager S, Struchen T, Svenson S, Vieland V, Wang K, Winklosky B Autism CLS. An autosomal genomic screen for autism. Am J Med Genet. 1999;88:609–615. doi: 10.1002/(sici)1096-8628(19991215)88:6<609::aid-ajmg7>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Maybery M, Wong D, Maley A, Hallmayer J. Characteristics of the broader phenotype in autism: a study of siblings using the Children’s Communication Checklist-2. Am J Med Genet Part B Neuropsychiatr Genet. 2006;141B:117–122. doi: 10.1002/ajmg.b.30267. [DOI] [PubMed] [Google Scholar]
- Black DO, Wallace GL, Sokoloff JL, Kenworthy L. IQ split predicts social symptoms and communication abilities in high-functioning children with autism spectrum disorders. J Autism Dev Disord. 2009;39:1613–1619. doi: 10.1007/s10803-009-0795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, Bailey A, Rutter M. A case–control family history study of autism. J Child Psychol Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Bradford Y, Haines J, Hutcheson H, Gardiner M, Braun T, Sheffield V, Cassavant T, Huang W, Wang K, Vieland V, Folstein S, Santangelo S, Piven J Autism CLS. Incorporating language phenotypes strengthens evidence of linkage to autism. Am J Med Genet. 2001;105:539–547. [PubMed] [Google Scholar]
- Bushby KMD. Genetic and clinical correlations of Xp21 muscular-dystrophy. J Inherit Metab Dis. 1992;15:551–564. doi: 10.1007/BF01799614. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, Smith CH, Kilifarski M, Reichert J, Hollander E, Lawlor BA, Fitzgerald M, Greenberg DA, Davis KL. Evidence for a susceptibility gene for autism on chromosome 2 and for genetic heterogeneity. Am J Hum Genet. 2001;68:1514–1520. doi: 10.1086/320588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman J, Keddache M, Smith CJ, Hollander E, Ramoz N, Reichert JG. Linkage analysis for autism in a subset families with obsessive-compulsive behaviors: evidence for an autism susceptibility gene on chromosome 1 and further support for susceptibility genes on chromosome 6 and 19. Mol Psychiatry. 2004;9:144–150. doi: 10.1038/sj.mp.4001465. [DOI] [PubMed] [Google Scholar]
- Cantor RM, Kono N, Duvall JA, Alvarez-Retuerto A, Stone JL, Alarcon M, Nelson SF, Geschwind DH. Replication of autism linkage: fine-mapping peak at 17q21. Am J Hum Genet. 2005;76:1050–1056. doi: 10.1086/430278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GK, Kono N, Geschwind DH, Cantor RM. Quantitative trait locus analysis of nonverbal communication in autism spectrum disorder. Mol Psychiatry. 2006;11:214–220. doi: 10.1038/sj.mp.4001753. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166:210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw EW, Heath SC, Wijsman EM. Multipoint oligogenic analysis of age-at-onset data with applications to Alzheimer’s disease pedigrees. Am J Hum Genet. 1999;64:839–851. doi: 10.1086/302276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Bierut L, Goate A, Rice JP, Hinrichs A, Bertelsen S, Wang JC, Dunn G, Kuperman S, Schuckit M, Nurneberger J, Jr, Porjesz B, Begleiter H, Kramer J, Hesselbrock V. Linkage analysis of IQ in the collaborative study on the genetics of alcoholism (COGA) sample. Behav Genet. 2006;36:77–86. doi: 10.1007/s10519-005-9009-8. [DOI] [PubMed] [Google Scholar]
- Duvall JA, Lu A, Cantor RM, Todd RD, Constantino JN, Geschwind DH. A quantitative trait locus analysis of social responsiveness in multiplex autism families. Am J Psychiatry. 2007;164:656–662. doi: 10.1176/ajp.2007.164.4.656. [DOI] [PubMed] [Google Scholar]
- Epstein MP, Duren WL, Boehnke M. Improved inference of relationship for pairs of individuals. Am J Hum Genet. 2000;67:1219–1231. doi: 10.1016/s0002-9297(07)62952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile-autism—genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Franke B, Neale BM, Faraone S. Genome-wide association studies in ADHD. Hum Genet. 2009;126:13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- Gagnon F, Jarvik G, Motulsky A, Deeb S, Brunzell J, Wijsman E. Evidence of linkage of HDL level variation to APOC3 in two samples with different ascertainment. Hum Genet. 2003;113:522–533. doi: 10.1007/s00439-003-1006-5. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. Phonological memory deficits in language disordered children—is there a causal connection. J Mem Lang. 1990;29:336–360. [Google Scholar]
- Glessner JT, Wang K, Cai GQ, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath SC, Snow GL, Thompson EA, Tseng C, Wijsman EM. MCMC segregation and linkage analysis. Genet Epidemiol. 1997;14:1011–1016. doi: 10.1002/(SICI)1098-2272(1997)14:6<1011::AID-GEPI75>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Happe F, Baron-Cohen S, Ronald A. Association between extreme autistic traits and intellectual disability: insights from a general population twin study. Br J Psychiatry. 2009;195:531–536. doi: 10.1192/bjp.bp.108.060889. [DOI] [PubMed] [Google Scholar]
- Igo RP, Wijsman EM. Empirical significance values for linkage analysis: trait simulation using posterior model distributions from MCMC oligogenic segregation analysis. Genet Epidemiol. 2008;32:119–131. doi: 10.1002/gepi.20267. [DOI] [PubMed] [Google Scholar]
- Igo RP, Chapman NH, Wijsman EM. Segregation analysis of a complex quantitative trait: approaches for identifying influential data points. Hum Hered. 2006;61:80–86. doi: 10.1159/000093085. [DOI] [PubMed] [Google Scholar]
- IMGSAC. A full genome screen for autism with evidence for linkage to a region on chromosome 7q. Hum Mol Genet. 1998;7:571–578. doi: 10.1093/hmg/7.3.571. [DOI] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: implications for genetic subgroups. Lang Cogn Process. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Murphy K, Raj T, He C, White PS, Matise TC. A combined linkage-physical map of the human genome. Am J Hum Genet. 2004;75:1143–1148. doi: 10.1086/426405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JA, Barnby G, Bonora E, Sykes N, Bacchelli E, Blasi F, Maestrini E, Broxholme J, Tzenova J, Weeks D, Bailey AJ, Monaco AP IMGSAC. Analysis of IMGSAC autism susceptibility loci: evidence for sex limited and parent of origin specific effects. J Med Genet. 2005;42:132–137. doi: 10.1136/jmg.2004.025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln A, Courchesne E, Allen M, Hanson E, Ene M. Neurobiology of Asperger syndrome: seven case studies and quantitative magnetic resonance imaging findings. In: Schopler E, Mesibov G, Kunce LJ, editors. Asperger syndrome or high-functioning autism? New York: Kluwer Academic Press; 1998. pp. 145–166. [Google Scholar]
- Liu J, Nyholt DR, Magnussen P, Parano E, Pavone P, Geschwind D, Lord C, Iversen P, Hoh J, Ott J, Gilliam TC AGRE. A genomewide screen for autism susceptibility loci. Am J Hum Genet. 2001;69:327–340. doi: 10.1086/321980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-Q, Paterson AD, Szatmari P Consortium TAGP. Genome-wide linkage analyses of quantitative and categorical autism subphenotypes. Biol Psychiatry. 2008;64:561–570. doi: 10.1016/j.biopsych.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Lecouteur A. Autism diagnostic interview-revised—a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Luciano M, Wright MJ, Duffy DL, Wainwright MA, Zhu G, Evans DM, Geffen GM, Montgomery GW, Martin NG. Genome-wide scan of IQ finds significant linkage to a quantitative trait locus on 2q. Behav Genet. 2006;36:45–55. doi: 10.1007/s10519-005-9003-1. [DOI] [PubMed] [Google Scholar]
- Ma DQ, Salyakina D, Jaworski JM, Konidari I, Whitehead PL, Andersen AN, Hoffman JD, Slifer SH, Hedges DJ, Cukier HN, Griswold AJ, McCauley JL, Beecham GW, Wright HH, Abramson RK, Martin ER, Hussman JP, Gilbert JR, Cuccaro ML, Haines JL, Pericak-Vance MA. A genome-wide association study of autism reveals a common novel risk locus at 5p14.1. Ann Hum Genet. 2009;73:263–273. doi: 10.1111/j.1469-1809.2009.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JE, Szatmari P, Jones MB, Bryson SE, Mahoney WJ, Bartolucci G, Tuff L. Familial factors influence level of functioning in pervasive developmental disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:746–753. doi: 10.1097/00004583-199906000-00023. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Ability profiles in children with autism—influence of age and IQ. Autism. 2003;7:65–80. doi: 10.1177/1362361303007001006. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Turner CA, Goldstein G. The application of short forms of the Wechsler intelligence scales in adults and children with high functioning autism. J Autism Dev Disord. 2005;35:45–52. doi: 10.1007/s10803-004-1030-x. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Keddache M, Martin LJ. Evidence for linkage on 21q and 7q in a subset of autism characterized by developmental regression. Mol Psychiatry. 2005;10:741–746. doi: 10.1038/sj.mp.4001691. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Los Angeles: Western Psychological Services; 1997. [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Koehler E, Lord C, Rogers S, Sigman M, Estes A, Abbott R. Evidence for latent classes of IQ in young children with autism spectrum disorder. Am J Mental Retard. 2008;113:439–452. doi: 10.1352/2008.113:439-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Cleak JD, Banfield E, Marlow AJ, Fisher SE, Monaco AP, Stott CM, Merricks MJ, Goodyer IM, Bolton PF, Slonims V, Baird G, Everitt A, Hennessy E, Main M, Helms P, Kindley AD, Hodson A, Watson J, O’Hare A, Cohen W, Cowie H, Steel J, MacLean A, Seckl J, Nasir J, Bishop DVM, Simkin Z, Conti-Ramsden G, Pickles A Consortium S. Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am J Hum Genet. 2004;74:1225–1238. doi: 10.1086/421529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palferman S, Matthews N, Turner H, Moore J, Hervas A, Aubin A, Wallace S, Michelotti J, Wainhouse C, Paul A, Thompson E, Murin M, Gupta R, Garner C, Pickles A, Rutter M, Bailey A, Lamb JA, Marlow A, Scudder P, Barnby G, Monaco AP, Baird G, Cox A, Docherty Z, Warburton P, Green EP, Abbs SJ, Le Couteur A, McConachie HR, Berney T, Kelly TP, De Vries PJ, Bolton P, Green J, Gilchrist A, Whittacker J, Bolton B, Packer R, Maestrini E, Van Engeland H, De Jonge MV, Kemner C, Klauck SM, Beyer KS, Epp S, Poustka A, Benner A, Poustka F, Ruhl D, Schmotzer G, Bolte S, Feineis-Matthews S, Fombonne E, Roge B, Fremolle-Kruck J, Pienkowski C, Tauber MT, Pedersen L, Brondum-Nielsen K, Eriksen G, Haracopos D, Cotterill RMJ, Tsiantis J, Papanikolaou K, Lord C, Corsello C, Guter S, Leventhal B, Cook E, Smalley S, Bailey J, Liu A, Dedricks M, Chrzanowski L, Levitt J, Pauls D, Volkmar F, Weeks DE Autis IMGS. Further characterization of the autism susceptibility locus AUTS1 on chromosome 7q. Hum Mol Genet. 2001a;10:973–982. doi: 10.1093/hmg/10.9.973. [DOI] [PubMed] [Google Scholar]
- Palferman S, Matthews N, Turner M, Moore J, Hervas A, Aubin A, Wallace S, Michelotti J, Wainhouse C, Paul A, Thompson E, Gupta R, Garner C, Murin M, Freitag C, Ryder N, Cottington E, Parr J, Pickles A, Rutter M, Bailey A, Barnby G, Lamb JA, Marlow A, Scudder P, Monaco AP, Baird G, Cox A, Docherty Z, Warburton P, Green EP, Abbs SJ, Le Couteur A, McConachie HR, Berney T, Kelly TP, De Vries PJ, Bolton PF, Green J, Gilchrist A, Whittacker J, Bolton B, Packer R, Maestrini E, Blasi F, Van Engeland H, De Jonge MV, Kemner C, Klauck SM, Beyer KS, Epp S, Poustka A, Benner A, Goethe JW, Poustka F, Ruhl D, Schmotzer G, Boolte S, Feineis-Matthews S, Fombonne E, Rogee B, Fremolle-Kruck J, Pienkowski C, Tauber MT, Pedersen L, Nielsen KB, Eriksen G, Haracopos D, Cotterill RMJ, Tsiantis J, Papanikolaou K, Lord C, Corsello C, Guter S, Leventhal B, Cook E, Smalley SL, Bailey J, McGough J, Levitt J, Pauls D, Volkmar F, Weeks DE Cons IMGSA. A genomewide screen for autism: strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet. 2001b;69:570–581. doi: 10.1086/323264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe A, Martinez M, Guilloud-Bataille M, Gillberg C, Rastam M, Sponheim E, Coleman M, Zappella M, Aschauer H, Van Maldergem L, Penet C, Feingold J, Brice A, Leboyer M Study PARIS. Genome-wide scan for autism susceptibility genes. Hum Mol Genet. 1999;8:805–812. doi: 10.1093/hmg/8.5.805. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Luciano M, de Geus EJC, Wright MJ, Slagboom PE, Montgomery GW, Boomsma DI, Martin NG. A genome-wide scan for intelligence identifies quantitative trait loci on 2q and 6p. Am J Hum Genet. 2005;77:318–326. doi: 10.1086/432647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Warren SF, Betz SK. Language symptoms of developmental language disorders: an overview of autism, Down syndrome, fragile X, specific language impairment, and Williams syndrome. Appl Psycholinguist. 2005;26:7–27. [Google Scholar]
- Risch N. A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet. 1999;65:493–507. doi: 10.1086/302497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo ER, Spence MA, Freeman BJ, Masonbrothers A, Mo A, Marazita ML. Evidence for autosomal recessive inheritance in 46 families with multiple incidences of autism. Am J Psychiatry. 1985;142:187–192. doi: 10.1176/ajp.142.2.187. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children. 4th edn. San Diego: Jerome M. Sattler; 2001. [Google Scholar]
- Schellenberg GD, Dawson G, Sung YJ, Estes A, Munson J, Rosenthal E, Rothstein J, Flodman P, Smith M, Coon H, Leong L, Yu CE, Stodgell C, Rodier PM, Spence MA, Minshew N, McMahon WM, Wijsman EM. Evidence for multiple loci from a genome scan of autism kindreds. Mol Psychiatry. 2006;11:1049–1060. doi: 10.1038/sj.mp.4001874. [DOI] [PubMed] [Google Scholar]
- Shao YJ, Raiford KL, Wolpert CM, Cope HA, Ravan SA, Ashley-Koch AA, Abramson RK, Wright HH, DeLong RG, Gilbert JR, Cuccaro ML, Pericak-Vance MA. Phenotypic homogeneity provides increased support for linkage on chromosome 2 in autistic disorder. Am J Hum Genet. 2002a;70:1058–1061. doi: 10.1086/339765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao YJ, Wolpert CM, Raiford KL, Menold MM, Donnelly SL, Ravan SA, Bass MP, McClain C, von Wendt L, Vance JM, Abramson RH, Wright HH, Ashley-Koch A, Gilbert JR, DeLong RG, Cuccaro ML, Pericak-Vance MA. Genomic screen and follow-up analysis for autistic disorder. Am J Med Genet. 2002b;114:99–105. doi: 10.1002/ajmg.10153. [DOI] [PubMed] [Google Scholar]
- Siegel DJ, Minshew NJ, Goldstein G. Wechsler IQ profiles in diagnosis of high functioning autism. J Autism Dev Disord. 1996;26:389–406. doi: 10.1007/BF02172825. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Smith CJ, Schmeidler J, Hollander E, Lawlor BA, Fitzgerald M, Buxbaum JD, Delaney K, Galvin P Consort AGRE. Symptom domains in autism and related conditions: evidence for familiality. Am J Med Genet. 2002;114:64–73. doi: 10.1002/ajmg.10048. [DOI] [PubMed] [Google Scholar]
- Spiker D, Lotspeich L, Kraemer HC, Hallmayer J, Mcmahon W, Petersen PB, Nicholas P, Pingree C, Wieseslater S, Chiotti C, Wong DL, Dimicelli S, Ritvo E, Cavallisforza LL, Ciaranello RD. Genetics of autism—characteristics of affected and unaffected children from 37 multiplex families. Am J Med Genet. 1994;54:27–35. doi: 10.1002/ajmg.1320540107. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Stone JL, Merriman B, Cantor RM, Yonan AL, Gilliam TC, Geschwind DH, Nelson SF. Evidence for sex-specific risk alleles in autism spectrum disorder. Am J Hum Genet. 2004;75:1117–1123. doi: 10.1086/426034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Tuff L, Finlayson MA, Bartolucci G. Asperger’s syndrome and autism: neurocognitive aspects. J Am Acad Child Adolesc Psychiatry. 1990;29:130–136. doi: 10.1097/00004583-199001000-00021. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R, Segre A, Pericak-Vance MA, Cuccaro ML, Gilbert JR, Wright HH, Abramson RK, Betancur C, Bourgeron T, Gillberg C, Leboyer M, Buxbaum JD, Davis KL, Hollander E, Silverman JM, Hallmayer J, Lotspeich L, Sutcliffe JS, Haines JL, Folstein SE, Piven J, Wassink TH, Sheffield V, Geschwind DH, Bucan M, Brown WT, Cantor RM, Constantino JN, Gilliam TC, Herbert M, LaJonchere C, Ledbetter DH, Lese-Martin C, Miller J, Nelson S, Samango-Sprouse CA, Spence S, State M, Tanzi RE, Coon H, Dawson G, Devlin B, Estes A, Flodman P, Klei L, McMahon WM, Minshew N, Munson J, Korvatska E, Rodier PM, Schellenberg GD, Smith M, Spence MA, Stodgell C, Tepper PG, Wijsman EM, Yu CE, Roge B, Mantoulan C, Wittemeyer K, Poustka A, Felder B, Klauck SM, Schuster C, Poustka F, Bolte S, Feineis-Matthews S, Herbrecht E, Schmotzer G, Tsiantis J, Papanikolaou K, Maestrini E, Bacchelli E, Blasi F, Carone S, Toma C, Van Engeland H, de Jonge M, Kemner C, Koop F, Langemeijer M. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. Defining language phenotypes in autism. Clin Neurosci Res. 2006;6:219–224. [Google Scholar]
- Tager-Flusberg H, Cooper J. Present and future possibilities for defining a phenotype for specific language impairment. J Speech Lang Hearing Res. 1999;42:1275–1278. doi: 10.1044/jslhr.4205.1275. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph R. Identifying neurocognitive phenotypes in autism. Philos Trans Roy Soc B Biol Sci. 2003;358:303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Misaki A, Liang SB, Tachibana A, Hayashi N, Sonobe H, Ohtsuki Y. Expression of T-cadherin (CDH13, H-cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J Neurochem. 2000;74:1489–1497. doi: 10.1046/j.1471-4159.2000.0741489.x. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman PM, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler preschool and primary scale of intelligence-revised. San Antonio: The Psychological Corporation; 1989. [Google Scholar]
- Wechsler D. Manual for the Wechsler intelligence scale for children. 3rd edn. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. 3rd edn. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Weiss LA, Arking DE The Gene Discovery Project of Johns Hopkins, The Autism Consortium. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–808. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJO, Barry JG, Bishop DVM. Further defining the language impairment of autism: is there a specific language impairment subtype? J Commun Disord. 2008;41:319–336. doi: 10.1016/j.jcomdis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Wijsman EM, Yu D. Joint oligogenic segregation and linkage analysis using Bayesian Markov chain Monte Carlo methods. Mol Biotechnol. 2004;28:205–226. doi: 10.1385/MB:28:3:205. [DOI] [PubMed] [Google Scholar]
- Yonan AL, Alarcon M, Cheng R, Magnusson PKE, Spence SJ, Palmer AA, Grunn A, Juo SHH, Terwilliger JD, Liu JJ, Cantor RM, Geschwind DH, Gilliam TC. A genomewide screen of 345 families for autism-susceptibility loci. Am J Hum Genet. 2003;73:886–897. doi: 10.1086/378778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Sonnenberg LK, Heshka T, Eastwood S, Xu J. A girl with pervasive developmental disorder and complex chromosome rearrangement involving 8p and 10p. J Autism Dev Disord. 2005;35:393–399. doi: 10.1007/s10803-005-3307-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.