Summary
Mammalian fertilization is dependent upon a series of bicarbonate-induced, cAMP-dependent processes sperm undergo as they “capacitate,” i.e., acquire the ability to fertilize eggs. Male mice lacking the bicarbonate- and calcium-responsive soluble adenylyl cyclase (sAC), the predominant source of cAMP in male germ cells, are infertile, as the sperm are immotile. Membrane-permeable cAMP analogs are reported to rescue the motility defect, but we now show that these “rescued” null sperm were not hyperactive, displayed flagellar angulation, and remained unable to fertilize eggs in vitro. These deficits uncover a requirement for sAC during spermatogenesis and/or epididymal maturation and reveal limitations inherent in studying sAC function using knockout mice. To circumvent this restriction, we identified a specific sAC inhibitor that allowed temporal control over sAC activity. This inhibitor revealed that capacitation is defined by separable events: induction of protein tyrosine phosphorylation and motility are sAC dependent while acrosomal exocytosis is not dependent on sAC.
Introduction
The second messenger cyclic AMP (cAMP) is critical for mammalian spermatogenesis, for maturation of sperm in the epididymis, and for capacitation, a maturational process that sperm undergo prior to fertilization. Sperm must successfully complete all of these events to become fertilization competent. Transcriptional regulation during spermatogenesis is mediated, in part, by cAMP-responsive nuclear factors (Blendy et al., 1996; Nantel et al., 1996). The end product of spermatogenesis, the transcriptionally inactive spermatozoon, is a highly compartmentalized, polarized cell that has but one function, i.e., to fertilize an egg. These testicular sperm continue maturation in the epididymis, where the sperm acquire forward progressive motility, an event also modulated by cAMP (Tash and Means, 1988).
Even after becoming motile, the morphologically mature epididymal sperm do not have the “capacity” to fertilize an egg (Yanagimachi, 1994). They acquire fertilization competence during ejaculation and transit through the female reproductive tract. This critical process involves many changes in the sperm, including alterations in plasma membrane fluidity, protein tyrosine phosphorylation, and protein tyrosine nitration, as well as the acquisition of a hyperactive motility pattern and the ability to undergo acrosomal exocytosis (AE). These changes are collectively grouped under a single term, “capacitation.” Although the molecular basis for capacitation is not well understood, it can be recapitulated in vitro under defined conditions. Such experiments reveal the crucial role played by the various fluids bathing the sperm; chief among these is the difference in bicarbonate concentrations between the male and female reproductive tracts. Sperm remain in a dormant state within the epididymis where the bicarbonate concentration is actively maintained at ~5 mM. Upon ejaculation, and in the female reproductive tract, sperm are exposed to higher bicarbonate levels (~25 mM) (Johnson, 1998; Levine and Marsh, 1971; Pitts, 1974). This bicarbonate elevation is one of the key initiators of capacitation.
Extensive evidence links cAMP to many aspects of sperm capacitation (Fraser, 1981; Visconti et al., 1995b). Among the first definable events in capacitation are elevations in the intracellular concentrations of calcium and bicarbonate followed by an increase in cAMP. Subsequent activation of protein kinase A leads to an increase in the tyrosine phosphorylation of a subset of sperm proteins; this prototypical pattern of protein tyrosine phosphorylation represents the best molecularly defined hallmark of capacitation (Osheroff et al., 1999; Visconti et al., 1995a, 1999). Membrane-permeable cAMP analogs also induce other events of capacitation, including acquisition of hyperactive motility and AE (Lefievre et al., 2002b; White and Aitken, 1989), but the dependence of these events on each other and their relationships to the tyrosine phosphorylation of proteins are not understood.
Cyclic AMP concentrations are regulated by modulating its synthesis by adenylyl cyclases (ACs) and/or its degradation by phosphodiesterases (PDEs). Although a number of PDE isoforms have been detected in mammalian sperm using type-specific PDE inhibitors (Fisch et al., 1998), their specific contributions to capacitation are not well defined (Galantino-Homer et al., 2004; Lefievre et al., 2002a). There are two classes of adenylyl cyclases in mammalian cells: G protein-regulated transmembrane adenylyl cyclases (tmACs) and bicarbonate- and calcium-regulated soluble adenylyl cyclase (sAC). Multiple members of the family of hormonally responsive tmACs are expressed in germ cells and sperm (Baxendale and Fraser, 2003; Defer et al., 1998; Gautier-Courteille et al., 1998; Leclerc et al., 1996), but these do not appear to be involved in the bicarbonate-induced, cAMP-dependent capacitation for a number of reasons. Chief among these, tmACs are not regulated by bicarbonate. Additionally, with the possible exception of AE, specific stimulation of tmACs by the nonphysiological activator, forskolin, does not induce capacitation-related events (Leclerc et al., 1996).
A nontransmembrane-associated AC activity was described almost 30 years ago in cytosolic extracts of mammalian testis and the particulate fraction of sperm homogenates (Braun and Dods, 1975). The gene encoding this “soluble” AC (sAC) generates a protein of Mr ~ 187,000 with low specific activity (Buck et al., 1999) and a high specific activity, alternatively spliced isoform of Mr ~50,000 (Buck et al., 1999; Jaiswal and Conti, 2001). Both full-length (sACfl) and truncated (sACt) isoforms possess two domains that are most similar to the catalytic regions of ACs from cyanobacteria and myxobacteria, revealing an evolutionary link between bacterial and mammalian signaling systems. Both sAC isoforms differ biochemically from tmACs; they are insensitive to the known modulators of tmACs, e.g., forskolin and heterotrimeric G proteins (Buck et al., 1999). Rather, sAC proteins are directly stimulated by bicarbonate (Chen et al., 2000; Garbers et al., 1982; Garty and Salomon, 1987; Okamura et al., 1985; Visconti et al., 1990) and calcium (Hyne and Garbers, 1979; Jaiswal and Conti, 2003; Litvin et al., 2003), both of which are required for sperm capacitation in vitro (Tardif et al., 2003; Visconti et al., 1999). Furthermore, sAC activity (Neer and Murad, 1979), mRNA (Sinclair et al., 2000; Xie and Conti, 2004), and protein (Chen et al., 2000; Xie and Conti, 2004) are most prevalent in the mammalian testis relative to somatic tissues, suggesting that sAC has a critical role in spermatogenesis.
Involvement of sAC in one particular aspect of sperm function, motility, was confirmed by the recent genetic ablation of the sAC gene: sAC null sperm are morphologically normal but immotile (Esposito et al., 2004). We now demonstrate that although the sperm motility defect in sAC null sperm can be rescued with a membrane-permeable cAMP analog, these sperm remain infertile and do not display other hallmarks of capacitation. Thus, it appears that sAC null mice harbor additional defects, presumably due to the absence of sAC during spermatogenesis and/or epididymal maturation. To examine the specific functions of sAC in mature sperm, we developed a small molecule inhibitor specific for sAC. This inhibitor provides temporal control for inhibiting sAC activity, revealing that capacitation is actually comprised of separable processes, only some of which are dependent upon sAC function.
Results
Soluble AC Represents the Predominant Adenylyl Cyclase Activity in Male Germ Cells and Sperm
Cyclic AMP-dependent events are critical for spermatogenesis (Sassone-Corsi, 1998). To determine whether sAC is a source of cAMP during spermatogenesis, we used the distinct biochemical profiles of sAC and tmACs, i.e., stimulation by bicarbonate and forskolin, respectively, to quantitate their relative contributions to total cyclase activity in developing germ cells and in epididymal sperm. Cyclic AMP production in isolated germ cells and in sperm was stimulated 3- to 4-fold above baseline by bicarbonate (Figure 1A), revealing the presence of sAC. This bicarbonate-stimulated sAC activity was maximal in postmeiotic round and condensing spermatids, consistent with the essential role during spermiogenesis for CREMτ, a germ cell-specific member of the cAMP-responsive element modulator (CREM) family (Blendy et al., 1996; Nantel et al., 1996). In contrast, forskolin had little to no effect on cAMP levels. These results indicated that the vast majority of cAMP in developing germ cells and mature sperm is generated by sAC. Contrary to its name, soluble adenylyl cyclase, sAC activity was found in both supernatant and particulate fractions of isolated germ cell preparations and mature sperm, revealing that sAC is also responsible for the particulate AC activity initially reported in sperm (Braun and Dods, 1975).
Figure 1. Soluble AC Is in Male Germ Cells and Sperm.
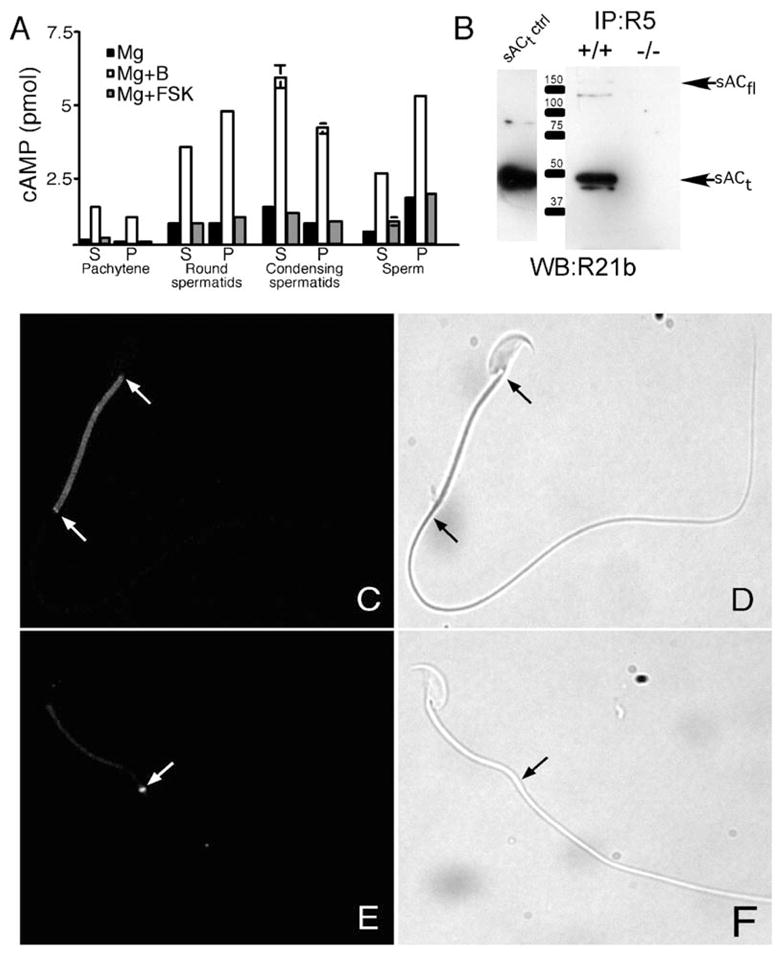
(A) AC assays of germ cells and sperm. The amount of cAMP produced in the presence of magnesium (Mg), magnesium + bicarbonate (Mg+B), and magnesium + forskolin (Mg+FSK) was assayed using soluble (S) and particulate (P) proteins prepared from pachytene spermatocytes, round spermatids, condensing spermatids, and sperm.
(B) Immunoblot (using biotinylated sAC monoclonal antibody R21) of immunoprecipitates (using sAC monoclonal antibody R5 recognizing a nonoverlapping epitope) from wild-type (+/+) and sAC null (−/−) testis cytosol. The first lane is a protein extract from sACt-overexpressing cells (sACt is the Mr ~50,000 isoform generated by alternative splicing).
(C) Immunofluorescent analysis of sAC in noncapacitated wild-type sperm using monoclonal antibody R21 (Zippin et al., 2003). Arrows refer to midplece region.
(D) Corresponding phase image of (C).
(E) Immunofluorescent analysis of sAC in capacitated wild-type sperm using the R21 antibody. Arrow refers to annular region.
(F) Corresponding phase image of (E).
If sAC regulates specific capacitation-associated events, we would expect it to be located in the subcellular regions of the polarized sperm where those processes occur. We used immunoblotting to confirm that our sAC monoclonal antibodies recognize sACfl and sACt, the two reported splice variants of sAC expressed in testis (Buck et al., 1999; Jaiswal and Conti, 2001). Detection required enrichment by immunoprecipitation prior to immunoblotting (Figure 1B), which is consistent with our need for large amounts of testicular protein to originally purify sACt from the supernatant of rat testes (Buck et al., 1999). Interestingly, immunoprecipitation with one sAC monoclonal antibody followed by immunoblotting with a second, nonoverlapping monoclonal antibody revealed the presence of additional sAC isoforms (Figure 1B).
In noncapacitated sperm, sAC was localized in a punctate pattern throughout the midpiece (Figure 1C). The midpiece is a region characterized by the presence of the axoneme, outer dense fibers, and the mitochondrial sheath. Because the axoneme and outer dense fibers are present in both the mid and principal pieces of the flagellum, the localization of sAC to the midpiece suggests that it is associated with mitochondria, a site consistent with one of its locations in somatic cells (Zippin et al., 2003). In addition, strong immunoreactivity was observed in the annulus, a region that demarcates the midpiece from the principal piece (Figure 2C). After sperm capacitation, the majority of sAC immuno-reactivity was in the annular region (Figure 1E). Interestingly, we did not detect sAC immunoreactivity in other regions of the sperm thought to undergo cAMP-dependent events during capacitation, e.g., the acrosome.
Figure 2. Sperm from sAC Null Animals Have Defects in Motility.
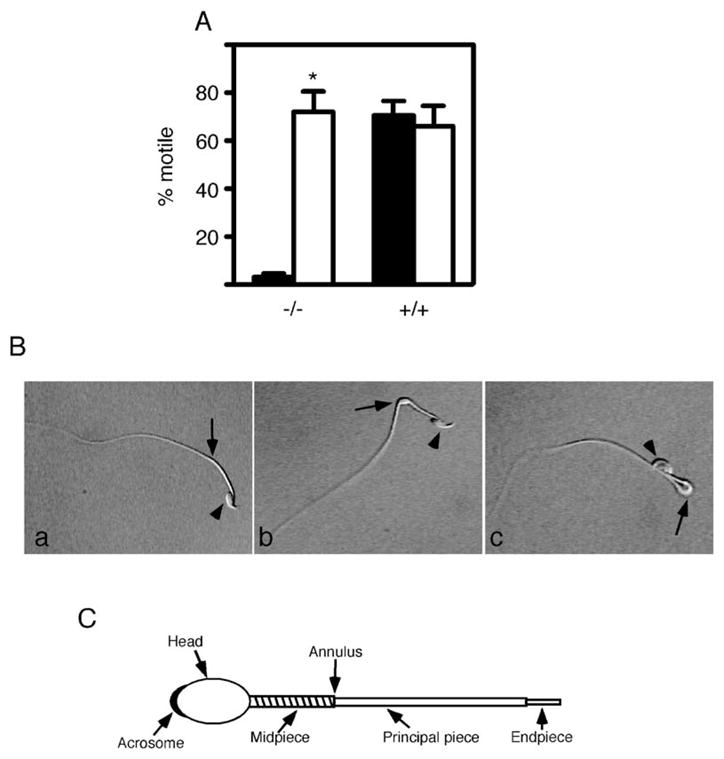
(A) Motility analysis of sAC null sperm. Sperm from +/+ and −/− animals were collected and capacitated in the absence (solid bars) and presence (open bars) of 1 mM dbcAMP. Progressive motility was determined as described at 1.5 hr. n = 4 for both +/+ and −/− animals. *p < 0.05.
(B) Flagellar angulation of sAC null sperm. (a) A wild-type sperm. (b) A sAC null sperm in the process of forming a hairpin at the annulus. (c) A sAC null sperm in which the head has bent back 180° onto the tail. In all three photomicrographs, the head (arrowhead) and annulus (arrow) are denoted.
(C) Schematic diagram of a sperm showing the annulus, a region that demarcates the midpiece from the principal piece and where the flagellar angulation occurs. Diagonal lines in the midpiece represent the mitochondrial sheath.
Sperm from sAC Null Mice Show Flagellar Angularity and Are Incapable of Fertilizing Eggs In Vitro
All detected sAC isoforms, including sACfl and sACt proteins, were absent in the testis from mice with exons 2–4 of the sAC gene deleted (Figure 1B), and consistent with a previous report (Esposito et al., 2004), these males were infertile (data not shown). Immediately after isolation and collection into capacitation medium, cauda epididymal sAC null sperm appeared morphologically normal; however, they were immotile, showing only a small vibratory movement compared to wild-type sperm (Figure 2A; see Movies S1 and S2 available in the Supplemental Data online). Furthermore, these sperm were unable to fertilize eggs in an in vitro fertilization (IVF) assay as indicated by pronuclear formation and cleavage to the 2-cell stage (Table 1; Esposito et al., 2004). When these sAC null sperm were collected in capacitation medium containing the membrane-permeable cAMP analog, dbcAMP, between 50% and 90% developed vigorous progressive motility (Figure 2A; Movie S3); these movements were similar to those described by Esposito et al. (2004).
Table 1.
In Vitro Fertilization with sAC +/+, +/−, and −/− Sperm
| +/+ | +/− | −/− | −/− +dbcAMP | |
|---|---|---|---|---|
| Total # of eggs | 123 | 116 | 96 | 114 |
| Unfertilized eggs | 45 | 85 | 91 | 113 |
| Fertilized eggs | 78 (63.4%) | 31 (26.7%) | 5 (5.2%) | 1 (0.9%) |
We extended these experiments by examining whether progressively motile sAC null sperm became hyperactive and could fertilize eggs in vitro. Sperm from sAC null males were capacitated in the presence of dbcAMP and then added to cumulus-enclosed eggs in medium not containing dbcAMP. Null sperm were motile under these conditions but fertilized only ~5% of the eggs (Table 1). In contrast, sperm from wild-type littermates fertilized >60% of the eggs. Therefore, simply bypassing the need for sAC activity by providing its end product, cAMP, did not restore fertilization competence to seemingly mature and motile sAC null sperm.
In examining the sAC null motile sperm in more detail, they did not become hyperactive. Furthermore, we observed that almost 100% of these sperm showed flagellar angularity within 10 min of isolation; nonmotile sperm showed a similar phenotype. The flagellum began to bend at the annular region (the midpiece-principal piece junction) (Figures 2B and 2C), which coincides with sAC localization in capacitated wild-type sperm (Figure 1E). Over time, the sperm folded back 180° until the head and midpiece formed a hairpin configuration with the rest of the sperm tail (Figure 2B; Movies S1 and S3). As expected, the majority of wild-type sperm displayed normal motility both in the presence and absence of dbcAMP and only a small percentage (<10%) folded back. Therefore, while progressive motility is perturbed in sAC null sperm, it is not their only defect; both the lack of hyperactive motility and the severe flagellar angularity would preclude motile sAC null sperm from fertilizing eggs. The existence of defects in sAC null sperm incubated in the presence of a membrane-permeable cAMP analog suggest that sAC is required during spermatogenesis and/or for sperm maturational events prior to capacitation that are essential for fertilization competence.
Protein Tyrosine Phosphorylation Is Altered in sAC Null Sperm
To determine if other capacitation-related events are altered in sAC null sperm, we examined protein tyrosine phosphorylation patterns. Sperm from wild-type littermates showed only the constitutively tyrosine-phosphorylated hexokinase band when incubated under noncapacitating conditions (Kalab et al., 1994), and when capacitated, they displayed the characteristic pattern of protein tyrosine phosphorylation (Figure 3A). In contrast, noncapacitated sAC null sperm showed a different qualitative and quantitative pattern of protein tyrosine phosphorylation, revealing that even prior to capacitation, morphologically normal looking sAC null sperm are different from wild-type. Incubating sAC null sperm in capacitating medium did not alter the protein tyrosine phosphorylation pattern nor did the inclusion of dbcAMP (Figure 3B); furthermore, the addition of IBMX, a PDE inhibitor, did not change the pattern (data not shown). The aberrant basal tyrosine phosphorylation pattern and the fact that sAC null sperm were recalcitrant to cAMP-induced phosphorylation events provide further evidence that sAC null sperm harbor a spermatogenic and/or maturational defect.
Figure 3. Sperm from sAC Null Animals Show an Aberrant Protein Tyrosine Phosphorylation Pattern.

Sperm from +/+ and −/− animals were collected. Both noncapacitated (NC) and capacitated (CAP) sperm were prepared in the presence and absence of 1 mM dbcAMP. Proteins were solubilized, separated by gel electrophoresis, and assayed for the presence of protein tyrosine phosphorylation by immunoblotting with anti-phosphotyrosine antibody.
(A) Protein from +/+ sperm. The band at Mr ~116,000 (arrow) is hexokinase that is constitutively tyrosine-phosphorylated (Kalab et al., 1994).
(B) Protein from −/− sperm. Both noncapacitated and capacitated sperm from −/− animals had an aberrant pattern of protein tyrosine phosphorylation pattern that did not change with the addition of dbcAMP.
Acrosomal Exocytosis Occurs in sAC Null Sperm
Normally, sperm that are fully capacitated have acquired the ability to undergo acrosomal exocytosis. AE has been shown to be a cAMP-dependent process postulated to be regulated by tmACs (Leclerc et al., 1996). To determine if sAC is required for AE, sperm from sAC null males and wild-type littermates were incubated under capacitating conditions and then either left untreated or treated with the calcium ionophore, A23187, to induce AE. In uninduced sperm, AE was observed in 39% (43/109) of wild-type and 38% (43/113) of null sperm. After induction with A23187, AE was observed in 70% (70/100) of wild-type and 70% (74/105) of null sperm. In separate experiments, the percentage of sAC null sperm undergoing AE was the same whether treated with solubilized zona pellucida, 54% (55/102), or with A23187, 51% (40/79). Therefore, sAC null sperm, which were not capacitated as assessed by their pattern of protein tyrosine phosphorylation (Figure 3B), are able to physiologically undergo AE. These data reveal that capacitation consists of separable signaling events, not all of which require sAC activity.
Treatment of Wild-Type Sperm with a sAC-Specific Inhibitor Affects cAMP Production
Because sAC seems to have a role during spermatogenesis or epididymal maturation, the study of sAC null sperm is uninformative for elucidating its functions in mature spermatozoa. To overcome this limitation, we screened a combinatorial chemical compound library of 15,312 small lipophilic molecules to identify a sAC-selective inhibitor that would provide temporal control of sAC activity in wild-type sperm. A number of structurally and functionally distinct molecules capable of inhibiting the activity of purified sACt protein were identified, a subset of which were highly selective for sAC relative to tmACs. One of these, KH7 (Figure S1A), displayed an IC50 between 3 and 10 μM toward both recombinant purified human sACt protein (SOM, Figure 1B) and heterologously expressed sACt in cellular assays (SOM, Figure 1C). KH7 (50 μM) also completely inhibited the cyclase activity present in sAC immunoprecipitates from testis cytosol (data not shown). KH7 was noncompetitive with either the substrate (Mg2+-ATP) or the activators calcium or bicarbonate (data not shown). KH7 is inert toward tmAC activities in vitro (data not shown) and in vivo, in a whole-cell context (SOM, Figure 1D), and it did not inhibit soluble guanylyl cyclase in vitro (A. Beuve, UMDNJ, New Jersey Medical School, personal communication) at concentrations up to at least 100 μM.
To determine the efficacy of KH7 in sperm, cAMP production was measured in wild-type sperm incubated in noncapacitating or capacitating medium ± KH7. Consistent with previous reports (Visconti et al., 1999; White and Aitken, 1989), capacitation increased cAMP levels in wild-type sperm (Figure 4). Concentrations of KH7 near its in vivo IC50 (10 μM) blocked this capacitation-induced cAMP increase. At higher concentrations (50 μM), 5- to 10-fold above its IC50 but still selective for sAC relative to tmACs, KH7 resulted in a significant decrease in the basal cAMP accumulation in sperm regardless of the incubation medium. These data are consistent with our finding (Figure 1) that sAC represents the predominant source of cAMP in sperm, and they demonstrate that sAC is the source of cAMP generated during capacitation.
Figure 4. KH7-Treated Sperm Show a Reduction in cAMP Levels.
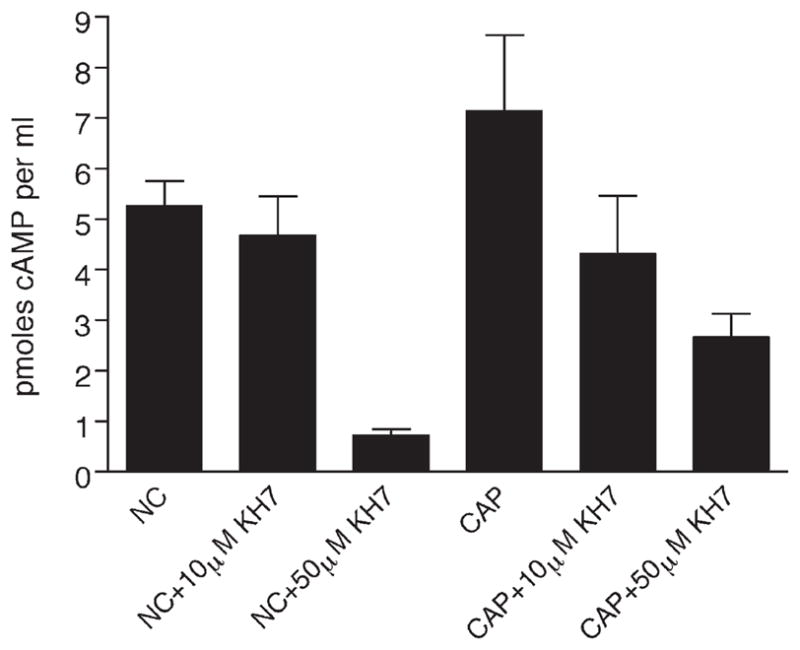
Noncapacitated (NC) and capacitated (CAP) sperm were prepared and either left untreated or treated with 10 μM or 50 μM KH7. The amount of cAMP produced was determined, and data points correspond to averages of duplicate determinations with standard deviations indicated. This experiment was repeated two times with similar results.
Capacitation Events Are Inhibited by KH7
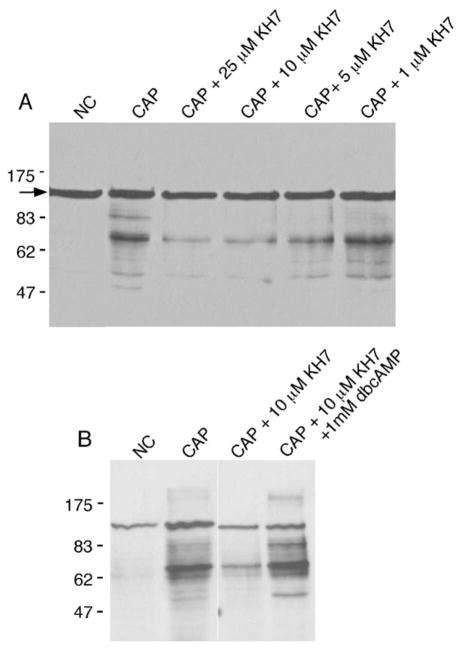
We next used KH7 to determine whether specific events of capacitation are sAC dependent. KH7 prevented capacitation-induced protein tyrosine phosphorylation in wild-type sperm (Figure 5A). Interestingly, 10 μM KH7, which completely blocked the capacitation-induced rise in cAMP (Figure 4), maximally inhibited the capacitation-induced pattern of protein tyrosine phosphorylation (Figure 5A). Thus, pharmacological inhibition of sAC activity matches the phenotype of sAC null sperm; both experiments demonstrate that sAC is required for capacitation-induced changes in protein tyrosine phosphorylation (Figures 3 and 5A). However, there is a revealing difference between pharmacologic and genetic experiments. In contrast to our findings with sAC null sperm (Figure 3), dbcAMP restored the normal pattern of tyrosine-phosphorylated proteins in KH7-treated wild-type sperm (Figure 5B). Rescue by dbcAMP confirmed that KH7 was not simply toxic to sperm, and the difference between knockout and KH7-inhibited sperm once again revealed that there must be a spermatogenic/epididymal maturation defect in sAC null sperm.
Figure 5. Effect of KH7 on Protein Tyrosine Phosphorylation in Sperm.
Epididymal sperm were collected and incubated under noncapacitating (NC) and capacitation (CAP) conditions in the presence and absence of KH7. Proteins were solubilized, separated by gel electrophoresis, and assayed for the presence of protein tyrosine phosphorylation by immunoblotting with the anti-phosphotyrosine antibody.
(A) Sperm coincubated with various concentrations of KH7. The band at Mr ~116,000 (arrow) is hexokinase, which is constitutively tyrosine phosphorylated.
(B) In the presence of 10 μM KH7 and 1 mM dbcAMP, the prototypical protein tyrosine phosphorylation pattern was observed.
We next wanted to examine whether wild-type sperm treated with KH7 showed motility defects, similar to those seen in sAC null sperm (Figure 2; Esposito et al., 2004). Noncapacitated sperm incubated in 10 μM KH7 displayed a normal motility pattern (data not shown), which is consistent with our observation that 10 μM KH7 did not affect resting, unstimulated cAMP levels (Figure 4). In contrast, when we incubated sperm in high concentrations of KH7, which were sufficient to lower resting cAMP levels (Figure 4), motility was significantly altered. In the presence of 50 μM KH7, noncapacitated sperm displayed either a sine wave pattern typical of normal motility (Yanagimachi, 1994), but at a much lower frequency, or were simply “twitching” with no forward progression (data not shown). In contrast to the sAC null sperm, KH7-treated sperm did not display any flagellar angularity.
Incubation in capacitating medium induced an alteration in the motility pattern of wild-type sperm—they display a physiological “hyperactivated” pattern of motility, i.e., a high amplitude, asymmetric waveform (Ho et al., 2002). At high concentrations of inhibitor (50 μM), capacitating conditions did not support either forward progressive motility or hyperactive motility. In contrast, low concentrations (10 μM) of KH7, which blocked capacitation-induced elevation of cAMP (Figure 4) and the protein tyrosine phosphorylation pattern (Figure 5), did not affect induction of hyperactivated motility. Thus, we have distinguished hyperactivated motility from these other hallmarks of sperm capacitation by showing that the elevation of cAMP and the prototypical pattern of protein tyrosine phosphorylation induced by capacitating medium are not causally linked to the conversion to a hyperactivated motility pattern.
In Vitro Fertilization Is Inhibited by KH7
The spermatogenic defect in sAC null sperm discredited their use for determining whether sAC is required in mature sperm for successful fertilization. To circumvent this limitation, we assayed the in vitro fertilization ability of wild-type sperm capacitated in the presence of KH7 (50 μM) ± dbcAMP. Wild-type sperm in the presence of DMSO (vehicle control) fertilized ~50% of eggs (Table 2) while sperm capacitated in the presence of KH7 showed a greatly reduced incidence of fertilization (13%; p < 0.0001). Unlike with sAC null sperm (Table 1), fertilization by KH7-treated sperm was fully restored by coincubation with dbcAMP (Table 2); therefore, KH7 is not toxic, and its effects are specific to inhibiting sAC-mediated generation of cAMP. Finally, KH7 did not inhibit the ability of wild-type sperm to undergo AE in the presence of solubilized zona pellucida (data not shown), indicating that the KH7-mediated inhibition of fertilization was not due to effects on AE. These data are consistent with our previous results that sAC null sperm underwent normal AE. In summary, KH7 is a specific inhibitor of cAMP synthesis by sAC in sperm, and its use demonstrates that inhibition of sAC in mature sperm is an effective means to block fertilization by mature spermatozoa.
Table 2.
In Vitro Fertilization with KH7-Treated Wild-Type Sperm
| DMSO | KH7 | KH7+dbcAMP | |
|---|---|---|---|
| Total # of eggs | 115 | 136 | 93 |
| Unfertilized Eggs | 58 | 118 | 50 |
| Fertilized Eggs | 57 (49.6%) | 18 (13.2%) | 43 (46.2%) |
Discussion
Sperm capacitation is associated with changes in calcium and bicarbonate levels and with increases in cAMP. These characteristics suggest that bicarbonate- and calcium-regulated sAC (Chen et al., 2000; Jaiswal and Conti, 2003; Litvin et al., 2003) plays a critical role in this process essential for fertilization. This hypothesized role has now been supported genetically, by deletion of three exons encoding the amino-terminal catalytic domain of testis sAC (Esposito et al., 2004), and pharmacologically, by using a small molecule, sAC- selective inhibitor. The sterility in sAC null males had been ascribed to a motility defect in their sperm. However, we now show that although motility can be restored to sAC null sperm by dbcAMP, capacitation-associated hallmarks (e.g., the protein tyrosine phosphorylation pattern and fertility competence) are not. Therefore, we conclude that immotility is not the only defect in sAC null sperm.
The observation that sAC null sperm show flagellar angularity, an irreversibly altered protein tyrosine phosphorylation pattern, and an inability to fertilize eggs in vitro reveals a role for sAC during spermatogenesis and/or epididymal maturation. Gene expression during spermatogenesis is dependent upon CREMτ, and genetic ablation of this transcription factor results in spermatogenic arrest early during spermiogenesis (Blendy et al., 1996; Nantel et al., 1996). Flagellar angularity was reported in sperm from mice with the activator of CREM in testis (ACT) gene deleted (Kotaja et al., 2004). These results suggest that genes encoding flagellar proteins are under the control of CREM and its coactivator ACT during spermatogenesis and that cAMP-dependent transcriptional differences would be found between germ cells from sAC null and wild-type mice.
The inability of motile, sAC null sperm to fertilize eggs in vitro can be explained by both their lack of hyperactive motility and their flagellar angulation, i.e., they formed a hairpin structure in which the head and midpiece fold back 180°. This bending occurrs at the annular region (the annulus demarcates the midpiece—principal piece junction) of the tail where sAC appears to translocate during the capacitation of wild-type sperm (Figure 1C). Although few proteins have been localized to the annulus, the α1A calcium channel subunit appears abundant in this region (Westenbroek and Babcock, 1999). Localization of this protein, together with calcium-activated sAC, which is relocalized to this region following capacitation, could provide a mechanism for cAMP generation in a spatially restricted fashion.
Results using the sAC null sperm are in stark contrast to those using wild-type sperm treated with the sAC-specific inhibitor KH7. The defects seen with the chemical block include events associated with capacitation and fertilization competence and can be rescued by providing sperm with an alternative source of cAMP, i.e., addition of membrane-permeable dbcAMP. Furthermore, flagellar angularity was not seen in KH7-treated sperm. Therefore, the sAC-specific inhibitor does not appear to cause any non-sAC-related side effects, and in addition to its role in motility (Esposito et al., 2004; also this report), sAC must be able to generate cAMP in both germ cells and mature sperm for fertilization competence.
Our successful development of a small molecule inhibitor provided two advantages compared to genetic ablation for the study of sAC in capacitation. First, we were able to temporally control sAC activity, permitting the specific examination of capacitation-related processes. Second, using a small molecule inhibitor revealed dose-dependent effects not possible with genetic ablation. High doses of KH7 blocked most capacitation-related events. In contrast, low doses of the inhibitor blocked protein tyrosine phosphorylation but not hyperactivated motility, indicating that capacitation is defined by separable events. Of interest, small molecule inhibitors offer the advantage of reversibility when considering contraceptive possibilities.
The major disadvantage of small molecule inhibitors relative to gene targeting strategies is the possibility that the small molecule affects additional cellular targets that could lead to false interpretation of effects or nonspecific toxicity. This does not appear to be a concern because the inhibitory effects of KH7 were rescued with cAMP, the end product of sAC activity. Furthermore, there is considerable agreement between the effects of the sAC inhibitor and sAC gene deletion on a variety of sperm functions (Table 3).
Table 3.
Comparison of the Effect of Genetic and Chemical Ablation of sAC on Capacitation-Related Events in Sperm
| sAC −/− Sperm | KH7-Treated Wild-Type Sperm | |
|---|---|---|
| Bicarbonate-induced cAMP production | Absent | Blocked |
| Bicarbonate-induced phosphotyrosine pattern | Aberrant pattern; NOT affected by bicarbonate; NOT affected by dbcAMP | Blocked; rescued by dbcAMP |
| Motility | Absent; rescued by dbcAMP | Blocked (at high concentrations of inhibitor); rescued by dbcAMP |
| Capacitation-associated hyperactivated motility | Absent in the presence of dbcAMP | Normal (at low concentrations of inhibitor) |
| Structure of flagellum | Angulation in presence and absence of dbcAMP | Normal in presence of inhibitor |
| Fertilization | Blocked; NOT rescued by dbcAMP | Blocked; rescued by dbcAMP |
| Acrosomal exocytosis | Normal | Normal |
Using the sAC inhibitor, we showed that the poorly characterized group of processes, first referred to over 50 years ago as capacitation (Austin, 1952; Chang, 1951), is in fact multiple, separable events, only some of which require sAC activity. AE is unique in that it does not appear to require sAC activity; this finding was confirmed with sAC null sperm and is consistent with the lack of sAC immunoreactivity in the sperm head (Figure 1). In fact, several lines of evidence implicate tmACs as the source of cAMP important in AE. Forskolin, the specific activator of tmACs, was reported to stimulate AE (Leclerc et al., 1996), and there is evidence for tmAC activity in the head region of capacitated sperm (Liguori et al., 2004). Finally, the recent genetic ablation of the type 3 tmAC gene showed that null males are subfertile and their sperm show alterations in spontaneous AE (Livera et al., 2005). Furthermore, few embryos are produced by sperm from these mice by in vitro fertilization unless the zona pellucida is removed.
In contrast to AE, capacitation-induced protein tyrosine phosphorylation was dependent upon sAC activity. Because a number of constituents of the sperm flagellum are tyrosine phosphorylated during capacitation, it was thought that the phosphorylation of these proteins might be related to motility regulation (Carrera et al., 1996; Ficarro et al., 2003). However, motility does not appear to be causally linked to the tyrosine phosphorylation of proteins observed in our immunoblot assays; low concentrations of KH7 were sufficient to inhibit the capacitation-induced elevation of cAMP and induction of the prototypical pattern of protein tyrosine phosphorylations without affecting hyperactivation. Similarly, hyperactivated motility in bull sperm is mediated by a signaling pathway that is independent of acrosomal responsiveness and does not appear to involve protein tyrosine phosphorylation (Marquez and Suarez, 2004). Regardless, although we do not yet understand the role of these protein tyrosine phosphorylation events, the pattern was strongly correlated with fertilizing ability in vitro.
Thus, we have pharmacologically distinguished sAC-dependent from sAC-independent processes during sperm capacitation, demonstrating that it is comprised of molecularly separate events. Sperm require sAC for normal motility, and the process of capacitation induces sAC-dependent cAMP elevation that promotes the prototypical pattern of protein tyrosine phosphorylation. Capacitation also induces a change in motility to a hyperactivated pattern, but the sAC-dependent increases in cAMP and tyrosine-phosphorylated proteins are not required for this change. Finally, the cAMP required for acrosomal exocytosis seems to be provided by a tmAC and not by sAC.
Experimental Procedures
Animals
sAC null mice, in which coding sequence exons 2–4 were deleted, were generated at Lexicon Genetics (The Woodlands, TX). These mice were previously shown to exhibit male-specific sterility, to have lost detectable bicarbonate-stimulated adenylyl cyclase activity in their testes, and to have immotile sperm (Esposito et al., 2004).
Retired male mice breeders, CD-1 strain, were purchased from Charles River Breeding Laboratories, Inc. (Wilmington, MA). CF-1 females (6–8 weeks old) were purchased from Harlan (Indianopolis, IN). B6SJLF1/J males for IVF were purchased from Jackson Laboratories (Bar Harbor, ME).
Generation and Characterization of a Specific Inhibitor of sAC
A compound collection from Chemical Diversity (San Diego, CA) was screened at the High Throughput Screening Resource Center of Rockefeller University. All compounds (at ~30 μM) were tested for their ability to inhibit cAMP production of purified sAC protein. We tested four different compounds per well during the primary screen using the Correlate-EIA cAMP assay (Assay Design) in microtiter (96-well) plates. Using a cutoff of 50% inhibition, 135 positive wells were selected from among the 15,312 compounds tested. On secondary screen, the four compounds per well were deconvoluted and assayed in duplicate. During this secondary screen, 50 independent “hits” were confirmed. Of these, eight of the most structurally unique compounds (i.e., the ones that seemed to be most potent and most dissimilar from each other) were analyzed further. IC50 values for each compound were determined, and the compounds were counterscreened for their ability to inhibit solubilized versions of tmACs, consisting of only the catalytic domains (Chen et al., 2000; Tang et al., 1995). Some of the identified compounds also inhibited tmAC activity, and while they may prove useful as inhibitors of all types of mammalian AC, their analysis is not yet complete. One inhibitor, KH7, proved equipotent against sAC activity in vitro and in a cellular context while displaying little to no effect toward tmACs and was used in this study.
Preparation of Protein from Germ Cells and Sperm
Pachytene spermatocytes, round spermatids, and condensing spermatids were separated into discrete populations from decapsulated testes of adult mice by sedimentation velocity at unit gravity as described previously (Bellve et al., 1977a, 1977b). The pachytene spermatocyte and round spermatid populations were each at least 85%–90% pure, and the condensing spermatid population was ~40%–50% pure (contaminated primarily with anucleated residual bodies and some round spermatids).
Sperm were obtained from retired male breeders by incising the cauda epididymides in PBS (pH 7.4) at 37°C. After a 10 min swim out, sperm were washed in PBS. Cauda sperm were pelleted at 100 × g for 8 min, resuspended in PBS with 1× Complete protease inhibitor Cocktail (Roche, Nutley, NJ), and homogenized 10 strokes on ice with a Dounce homogenizer. Protein was prepared as described above.
Germ cells and sperm were fractionated into soluble and particulate fractions by centrifugation at 100,000 × g for 1 hr.
Adenylyl Cyclase Assay with Germ Cells and Sperm
Purified populations of germ cells and noncapacitated and capacitated sperm were prepared and then sonicated in 20 mM Tris/HCl (pH 7.4) in the presence of proteinase inhibitors. 50 μg of protein was incubated for 20 min at 30°C with 10 mM Mg2+-ATP in the presence or absence of 40 mM sodium bicarbonate or 100 μM forskolin. cAMP was determined using the Cyclic AMP [3H] Biotrak Assay System (Amersham Biosciences, Piscataway, NJ).
Sperm Capacitation
Sperm were capacitated as described previously (Travis et al., 2001). Briefly, cauda epididymal sperm were collected by a swim-out procedure on a 37°C slide warmer containing Modified Whitten’s (MW) medium (Whitten, 1971) (MW = 15 mM HEPES disodium salt, 1.2 mM MgCl2, 100 mM NaCl, 4.7 mM KCl, 1 mM pyruvate, 4.8 mM lactic acid hemicalcium salt [pH 7.35]) without glucose and bicarbonate for 10 min. After removing tissue debris, the sperm was pelleted and resuspended to a concentration of 2 × 106 sperm in 30 μl in MW medium without glucose and bicarbonate and then diluted to a final volume of 300 μl of MW medium containing 5.5 mM glucose and with or without capacitating agents. The capacitating medium contained 9 mM NaHCO3 and 3 mM 2-hydroxypropyl-β-cyclodextrin. When appropriate, KH7 or DMSO was added to the appropriate tubes so that a final concentration of 1% DMSO was maintained. The sperm were incubated for 1 hr in a 37°C humidified incubator with 5% CO2.
Sperm from sAC null animals were isolated and capacitated by the same protocol. Because these sperm are immotile, they did not disperse in medium; rather, a clump of sperm was collected and used for further experiments.
In Vitro Fertilization Assays
Sperm from sAC null animals were collected directly into capacitation medium ± 1 mM dbcAMP. CF-1 females were superovulated as described previously (Manejwala et al., 1986), and metaphase II-arrested eggs were collected 13–14 hr after administration of human chorionic gonadotropin. For IVF assays with sperm from sAC null males or their littermates, cumulus-enclosed eggs were placed into 50 μl drops of Whitten’s medium supplemented with 15 mg/ml BSA. The sperm were collected and capacitated as described above. Sperm (5 × 105) were added to the 50 μl fertilization drop and fertilization was allowed to proceed for 3 hr; afterward, the eggs were removed, washed free of unbound sperm, and incubated at 37°C in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2. The inseminated eggs were examined morphologically for pronuclear formation and cleavage to the 2-cell stage. The experiment was performed three times.
For IVF assays using KH7, the sperm were from B6SJLF1/J males (IVF males), and the eggs underwent cumulus cell removal by brief incubation in Whitten’s medium containing 0.1% hyaluronidase. Sperm from IVF males were allowed to swim out into 1 ml of MW medium without glucose and bicarbonate for 10 min on a 37°C slide warmer. After removing the epididymis, sperm were dispersed, and 7 × 106 sperm were removed and capacitated in 250 μl MW medium containing 9 mM NaHCO3, 3 mM 2-hydroxypropyl-β-cyclodextrin, and 5.5 mM glucose in the presence of either 0.5% DMSO (vehicle for KH7), 50 μM KH7, or 50 μM KH7 + 1 mM dbcAMP. In some experiments, the KH7:sperm ratio was increased by capacitating 2 × 106 sperm in 500 μl as above and then concentrating the sperm by centrifugation at 300 × g for 2 min. In both procedures, after incubating for 1 hr, 1 × 106 sperm were added to 15–50 eggs in a 500 μl drop of Whitten’s medium containing 15 mg/ml BSA (because sperm were added to drug-free IVF media, we estimate that eggs were inseminated with aliquots of treated sperm in the presence of <5 μM KH7 in the fertilization drop). Sperm were incubated with the eggs for 1.5–2.0 hr, and then the eggs were washed and assayed for fertilization as described above. The experiment was performed five times.
Sperm Motility Assays
Sperm were prepared for IVF as described above (1 hr capacitation treatment followed by dilution in Whitten’s IVF medium) ± 1 mM dbcAMP. After 1.5 hr in IVF medium, 20 μl samples were placed in slide chambers made by sticking two strips of Parafilm on a slide to support a coverslip at a chamber depth of approximately 50 μm. Both slides and coverslips had been coated with agarose to prevent sticking of sperm. The samples were transferred to a 37°C stage on a Zeiss Axiovert microscope (Carl Zeiss, Thornbrook, NY). Sperm were videotaped using differential interference contrast optics with a 40× objective, combined with stroboscopic illumination at 60 Hz (Chadwick-Helmuth, El Monte, CA). Superimposed on the recorded image was time-date information provided by a videotimer (Model VTG33; For-A Co., Ltd., Newton, MA). A black-and-white video camera (model CCD72, Dage-MTI, Inc., Michigan City, IN) was used with a Panasonic AG-7300 Super VHS videocassette recorder (Panasonic Industrial Co., Secaucus, NJ).
Gel Electrophoresis, Immunoblotting, and Immunoprecipitation
Testes from both wild-type and sAC null mice were homogenized in detergent-containing lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP40, 1 mM PMSF, 10 μg/ml aprotinin and leupeptin) by tissue homogenizer and spun 1 hr at 100,000 × g. Supernatant proteins (3 mg) were incubated with 50 μg mouse IgG (Pierce) plus 50 μl Protein G Sepharose 4B (70% slurry, Amersham Biosciences) for 2 hr and spun at 14,000 × g for 15 min. Precleared supernatant was incubated overnight with 50 μg mouse monoclonal antibody (R5 raised against human sACt and recognition sequence mapped to amino acids 435–449) or nonspecific IgG. Immunoprecipitate was recleared by spinning at 14,000 × g for 15 min, and immune complexes were captured by incubation with 50 μl Protein G Sepharose. Beads were washed twice with detergent-containing lysis buffer and twice with detergent-containing lysis buffer plus 150 mM NaCl. Immune precipitates were separated by SDS-PAGE, analyzed by immunoblotting using biotinylated mouse monoclonal antibody R21 (raised against human sACt and recognition sequence mapped to amino acids 203–217), and detected by ECL (Bio-Rad Laboratories, Hercules, CA) using Steptavidin-HRP (Pierce). Protein tyrosine phosphorylation was assayed as described previously (Travis et al., 2001).
Immunofluorescence of Sperm
Noncapacitated and capacitated sperm (20 × 106/ml) were prepared and allowed to settle on coverslips in a humidity chamber for 30 min at room temperature and fixed in 4% paraformaldehyde in 1× PBS for 15 min at room temperature. After washing 4 × 2 min in PBS, the sperm were permeablized in −20°C methanol for 2 min and washed 4 × 2 min in PBS. The sperm were blocked in 10% normal goat serum in PBS for 1 hr at 37°C in a humidity chamber, washed 4 × 2 min in PBS, and incubated with a monoclonal antibody to sAC (Zippin et al., 2003) (R21; 1:50 dilution in blocking solution) for 1 hr at 37°C in a humidity chamber. After washing 4 × 2 min in PBS, the sperm then were incubated with streptavidin-FITC (Roche) (1:500 in blocking solution) for 1 hr at 37°C in a humidity chamber. Sperm were washed 4 × 2 min in PBS, mounted with Fluoromount-G (Southern Biotechnology Assoc., Birmingham, AL), and imaged with Metamorph software on a Nikon Inverted Microscope.
Purification of Zona Pellucidae Proteins
To purify solubilized zona pellucidae proteins, the procedure of Leyton et al. (1989) was used. Briefly, ovaries from 30 3-week-old CD-1 females were collected and homogenized in 2 ml of homogenization buffer (HB) (25 mM Triethanolamine [pH 8.5], 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 300 ng DNase, 1× Complete Protease Inhibitor Cocktail [Roche]). After adding 200 μl 10% NP-40 and 200 μl 10% deoxycholate, the sample was homogenized again and layered onto a 3-step Percoll (Sigma) gradient consisting of 2 ml of 2% Percoll, 2 ml of 10% Percoll, and 3 ml of 22% Percoll. After centrifugation for 2 hr at 200 × g at 4°C in a swinging bucket rotor, the 10% fraction was collected and the intact zona pellucidae counted (to determine zonae equivalents). The sample was diluted with 25 ml HB, collected by centrifugation at 16,000 × g for 10 min at 4°C, and washed in HB and then in modified Whitten’s medium. The sample was acidified by adding 1 N HCl (2 μl/1.5 ml sample) to reduce the pH to ~2.5 and incubated at 37°C for 15 min. After centrifugation at 16,000 × g for 10 min at 4°C, the supernatant was collected and neutralized with 2 μl 1 N NaOH and frozen.
Assay for Acrosomal Exocytosis
Sperm from sAC null animals were capacitated for 1.5 hr as described above. Sperm (~2–5 × 106) either were untreated or treated with 20 μM A23187 (in DMSO) (Calbiochem, San Diego, CA) for 30 min at room temperature to induce AE. Both groups of sperm were allowed to settle for 30 min onto coverslips. After washing cells with PBS, they were fixed for 15 min in 4% paraformaldehyde and permeabilized with 100% MeOH at −20°C for 2 min. The sperm were washed with PBS, blocked with 10% goat serum in PBS for 1 hr at room temperature, and incubated with anti-sp56 (QED Bioscience, San Diego, CA) (1:50 in 10% goat serum in PBS) for 1 hr at 37°C in a humidified chamber. Anti-sp56 is an antiserum that recognizes the acrosomal matrix protein sp56 (Kim et al., 2001). After washing with PBS, sperm then were incubated with anti-mouse Alexa-Fluor 488 (1:100 in 10% goat serum in PBS) for 1 hr at 37°C in a humidified chamber. The sperm were washed, mounted with Vectashield (Vector Laboratories, Burlingame, CA) containing 1.5 μg/ml propidium iodide to visualize DNA, and viewed using an epifluorescence-equipped Nikon Eclipse inverted microscope with a 100X PlanFluor oil objective. The number of sperm in a field was determined by propidium iodide staining, whereas the number of sperm that had not undergone AE was determined by the presence of positive sp56 staining in the acrosomal region of the sperm head.
To assess acrosomal status in wild-type KH7-treated sperm, epididymal sperm from a transgenic mouse line that accumulates enhanced green fluorescent protein (EGFP) in the sperm acrosome were used (Nakanishi et al., 1999) (a generous gift of Dr. George Gerton). The visualization of EGFP in a sperm is an indication of an intact acrosome, while the loss of EGFP represents a sperm that has undergone AE. Sperm were capacitated as described above in the presence or absence of 10 μM and 50 μM KH7. After incubating for 1 hr, sperm were removed, fixed at a final concentration of 1% paraformaldehyde, and mounted on coverslips with Vectashield. AE was determined by the presence or absence of EGFP fluorescence in the acrosomal region of the sperm.
To assess acrosomal exocytosis with solubilized zona pellucidae proteins, both wild-type (containing the EGFP transgene) and sAC null epididymal sperm were capacitated and incubated with 5 zona pellucidae equivalents for 30 min at 37°C. Each population of sperm were then processed as described above.
Supplementary Material
Acknowledgments
We are grateful to Dr. Edward Hyde for assistance screening the Tri-institutional Chemical Library, ChemDiv Inc., for original synthesis of the compound library, and Peter Meltzer and Organix, Inc., for independent synthesis and verification of the structure of KH7. We also thank Dr. Annie Beuve and Fu-Jung Chang for testing KH7 against soluble guanylyl cyclase. This work was supported by NIH HD38722 and the Hirschl Weil-Caulier Trust (L.R.L.), NIH HD42060, GM62328, and the Ellison Medical Foundation (J.B.), NSF MCB-0421855 (S.S.S.), NIH HD044740 (C.J.W.), and NIH HD06427 (S.B.M.). B.M. was supported by NIH 5 F31 HD43693. The authors declare that they have no competing financial interests.
Footnotes
Supplemental Data include one figure, one table, and three movies and can be found with this article online at http://www.developmentalcell.com/cgi/content/full/9/2/249/DC1/.
References
- Austin CR. The “capacitation” of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Baxendale RW, Fraser LR. Evidence for multiple distinctly localized adenylyl cyclase isoforms in mammalian spermatozoa. Mol Reprod Dev. 2003;66:181–189. doi: 10.1002/mrd.10344. [DOI] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977a;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Millette CF, Bhatnagar YM, O’Brien DA. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977b;25:480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schutz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- Braun T, Dods RF. Development of a Mn-2+-sensitive, “soluble” adenylate cyclase in rat testis. Proc Natl Acad Sci USA. 1975;72:1097–1101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera A, Moos J, Ning XP, Gerton GL, Tesarik J, Kopf GS, Moss SB. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin-dependent mechanism: identification of A kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol. 1996;180:284–296. doi: 10.1006/dbio.1996.0301. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Defer N, Marinx O, Poyard M, Lienard MO, Jegou B, Hanoune J. The olfactory adenylyl cyclase type 3 is expressed in male germ cells. FEBS Lett. 1998;424:216–220. doi: 10.1016/s0014-5793(98)00178-1. [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, Van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, Kalab P, Marto JA, Shabanowitz J, Herr JC, Hunt DF, Visconti PE. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–11589. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- Fisch JD, Behr B, Conti M. Enhancement of motility and acrosome reaction in human spermatozoa: differential activation by type-specific phosphodiesterase inhibitors. Hum Reprod. 1998;13:1248–1254. doi: 10.1093/humrep/13.5.1248. [DOI] [PubMed] [Google Scholar]
- Fraser LR. Dibutyryl cyclic AMP decreases capacitation time in vitro in mouse spermatozoa. J Reprod Fertil. 1981;62:63–72. doi: 10.1530/jrf.0.0620063. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS. Bovine sperm capacitation: assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol Reprod Dev. 2004;67:487–500. doi: 10.1002/mrd.20034. [DOI] [PubMed] [Google Scholar]
- Garbers DL, Tubb DJ, Hyne RV. A requirement of bicarbonate for Ca2+-induced elevations of cyclic AMP in guinea pig spermatozoa. J Biol Chem. 1982;257:8980–8984. [PubMed] [Google Scholar]
- Garty NB, Salomon Y. Stimulation of partially purified adenylate cyclase from bull sperm by bicarbonate. FEBS Lett. 1987;218:148–152. doi: 10.1016/0014-5793(87)81036-0. [DOI] [PubMed] [Google Scholar]
- Gautier-Courteille C, Salanova M, Conti M. The olfactory adenylyl cyclase III is expressed in rat germ cells during spermiogenesis. Endocrinology. 1998;139:2588–2599. doi: 10.1210/endo.139.5.5967. [DOI] [PubMed] [Google Scholar]
- Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol. 2002;250:208–217. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- Hyne RV, Garbers DL. Regulation of guinea pig sperm adenylate cyclase by calcium. Biol Reprod. 1979;21:1135–1142. doi: 10.1095/biolreprod21.5.1135. [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, Conti M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J Biol Chem. 2001;276:31698–31708. doi: 10.1074/jbc.M011698200. [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LR. Essential Medical Physiology. 2. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- Kalab P, Visconti P, Leclerc P, Kopf GS. p95, the major phosphotyrosine-containing protein in mouse spermatozoa, is a hexokinase with unique properties. J Biol Chem. 1994;269:3810–3817. [PubMed] [Google Scholar]
- Kim KS, Cha MC, Gerton GL. Mouse sperm protein sp56 is a component of the acrosomal matrix. Biol Reprod. 2001;64:36–43. doi: 10.1095/biolreprod64.1.36. [DOI] [PubMed] [Google Scholar]
- Kotaja N, De Cesare D, Macho B, Monaco L, Brancorsini S, Goossens E, Tournaye H, Gansmuller A, Sassone-Corsi P. Abnormal sperm in mice with targeted deletion of the act (activator of cAMP-responsive element modulator in testis) gene. Proc Natl Acad Sci USA. 2004;101:10620–10625. doi: 10.1073/pnas.0401947101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3#,5#monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- Lefievre L, de Lamirande E, Gagnon C. Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol Reprod. 2002a;67:423–430. doi: 10.1095/biolreprod67.2.423. [DOI] [PubMed] [Google Scholar]
- Lefievre L, Jha KN, de Lamirande E, Visconti PE, Gagnon C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J Androl. 2002b;23:709–716. [PubMed] [Google Scholar]
- Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971;213:557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton L, Robinson A, Saling P. Relationship between the M42 antigen of mouse sperm and the acrosome reaction induced by ZP3. Dev Biol. 1989;132:174–178. doi: 10.1016/0012-1606(89)90215-7. [DOI] [PubMed] [Google Scholar]
- Liguori L, Rambotti MG, Bellezza I, Minelli A. Electron microscopic cytochemistry of adenylyl cyclase activity in mouse spermatozoa. J Histochem Cytochem. 2004;52:833–836. doi: 10.1369/jhc.3B6141.2004. [DOI] [PubMed] [Google Scholar]
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- Livera G, Xie F, Garcia MA, Jaiswal B, Chen J, Law E, Storm DR, Conti M. Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol Endocrinol. 2005;19:1277–1290. doi: 10.1210/me.2004-0318. [DOI] [PubMed] [Google Scholar]
- Manejwala F, Kaji E, Schultz RM. Development of activatable adenylate cyclase in the preimplantation mouse embryo and a role for cyclic AMP in blastocoel formation. Cell. 1986;46:95–103. doi: 10.1016/0092-8674(86)90863-9. [DOI] [PubMed] [Google Scholar]
- Marquez B, Suarez SS. Different signaling pathways in bovine sperm regulate capacitationand hyperactivation. Biol Reprod. 2004;70:1626–1633. doi: 10.1095/biolreprod.103.026476. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Ikawa M, Yamada S, Parvinen M, Baba T, Nishimune Y, Okabe M. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999;449:277–283. doi: 10.1016/s0014-5793(99)00433-0. [DOI] [PubMed] [Google Scholar]
- Nantel F, Monaco L, Foulkes NS, Masquilier D, LeMeur M, Henriksen K, Dierich A, Parvinen M, Sassone-Corsi P. Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature. 1996;380:159–162. doi: 10.1038/380159a0. [DOI] [PubMed] [Google Scholar]
- Neer EJ, Murad F. Separation of soluble adenylate and guanylate cyclases from the mature rat testis. Biochim Biophys Acta. 1979;583:531–534. doi: 10.1016/0304-4165(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 1985;260:9699–9705. [PubMed] [Google Scholar]
- Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, Kopf GS. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A- mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod. 1999;5:1017–1026. doi: 10.1093/molehr/5.11.1017. [DOI] [PubMed] [Google Scholar]
- Pitts RF. Physiology of the Kidney and Body Fluids. 3. Chicago: Year Book Medical Publishers; 1974. [Google Scholar]
- Sassone-Corsi P. Coupling gene expression to cAMP signalling: role of CREB and CREM. Int J Biochem Cell Biol. 1998;30:27–38. doi: 10.1016/s1357-2725(97)00093-9. [DOI] [PubMed] [Google Scholar]
- Sinclair ML, Wang XY, Mattia M, Conti M, Buck J, Wolgemuth DJ, Levin LR. Specific expression of soluble adenylyl cyclase in male germ cells. Mol Reprod Dev. 2000;56:6–11. doi: 10.1002/(SICI)1098-2795(200005)56:1<6::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Stanzel M, Gilman AG. Truncation and alanine-scanning mutants of type I adenylyl cyclase. Biochemistry. 1995;34:14563–14572. doi: 10.1021/bi00044a035. [DOI] [PubMed] [Google Scholar]
- Tardif S, Dube C, Bailey JL. Porcine sperm capacitation and tyrosine kinase activity are dependent on bicarbonate and calcium but protein tyrosine phosphorylation is only associated with calcium. Biol Reprod. 2003;68:207–213. doi: 10.1095/biolreprod.102.005082. [DOI] [PubMed] [Google Scholar]
- Tash JS, Means AR. cAMP-dependent regulatory processes in the acquisition and control of sperm flagellar movement. Prog Clin Biol Res. 1988;267:335–355. [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001;276:7630–7636. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Muschietti JP, Flawia MM, Tezon JG. Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim Biophys Acta. 1990;1054:231–236. doi: 10.1016/0167-4889(90)90246-a. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995a;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995b;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Stewart-Savage J, Blasco A, Battaglia L, Miranda P, Kopf GS, Tezon JG. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol Reprod. 1999;61:76–84. doi: 10.1095/biolreprod61.1.76. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Babcock DF. Discrete regional distributions suggest diverse functional roles of calcium channel alpha1 subunits in sperm. Dev Biol. 1999;207:457–469. doi: 10.1006/dbio.1998.9172. [DOI] [PubMed] [Google Scholar]
- White DR, Aitken RJ. Relationship between calcium, cyclic AMP, ATP, and intracellular pH and the capacity of hamster spermatozoa to express hyperactivated motility. Gamete Res. 1989;22:163–177. doi: 10.1002/mrd.1120220205. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Nutrient requirements for the culture of preim-plantation embryos in vitro. Adv Biosci. 1971;6:129–139. [Google Scholar]
- Xie F, Conti M. Expression of the soluble adenylyl cyclase during rat spermatogenesis: evidence for cytoplasmic sites of cAMP production in germ cells. Dev Biol. 2004;265:196–206. doi: 10.1016/j.ydbio.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press Ltd; 1994. pp. 189–317. [Google Scholar]
- Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.