Summary
A Xenopus gene whose expression can be activated by the organizer-specific homeobox genes goosecoid and Xnot2 was isolated by differential screening. The chordin gene encodes a novel protein of 941 amino acids that has a signal sequence and four Cys-rich domains. The expression of chordin starts in Spemann’s organizer subsequent to that of goosecoid, and its induction by activin requires de novo protein synthesis. Microinjection of chordin mRNA induces twinned axes and can completely rescue axial development in ventralized embryos. This molecule is a potent dorsalizing factor that is expressed at the right time and in the right place to regulate cell-cell interactions in the organizing centers of head, trunk, and tail development.
Introduction
When the dorsal blastopore lip of an amphibian gastrula is transplanted to the opposite (ventral) side of a host embryo, a secondary body axis results. The transplanted tissue acts as an organizer center, recruiting neighboring host cells into various tissues of the secondary axis (Spemann and Mangold, 1924). The organizer provides patterning information, as revealed by observations that early dorsal lips induce head structures (Spemann, 1931), advanced lips induce trunk–tail structures (Spemann and Mangold, 1924; Hamburger, 1988), and cells of the tailbud hinge (which derive from the organizer) recruit host cells into tail structures (Gont et al., 1993). In addition to recruiting neighboring cells, the organizer cells themselves give rise mainly to the prechordal plate (head mesoderm), notochord, and tailbud hinge (Hamburger, 1988; Gont et al., 1993), tissues that correlate with the head, trunk, and tail organizer activities. Seventy years after the organizer experiment (Spemann and Mangold, 1924), the quest to isolate the factors released by the organizing centers to recruit neighboring cell continues. Understanding these signals is of great interest, for the secondary embryos can have all the anteroposterior and dorsoventral features of the primary axis. Thus, the organizer is the source of the signals that pattern the vertebrate body plan.
Recent evidence suggests that multiple inducing factors are active in the embryo. The Nieuwkoop center is a group of dorsovegetal cells of the Xenopus embryo that are responsible for inducing Spemann’s organizer in the overlying cells of the dorsal marginal zone at the early blastula stage, while they themselves do not give rise to axial mesoderm (Gerhart et al., 1991; Gilbert, 1994). Several secreted factors are candidate Nieuwkoop center signals, including activin-like molecules (Smith, 1993), Vg-1 (Thomsen and Melton, 1993), members of the Wnt family (McMahon and Moon, 1989; Sokol et al., 1991; Smith and Harland, 1991), and noggin (Smith and Harland, 1992).
A number of genes expressed specifically in Spemann’s organizer have been isolated recently in Xenopus. Several encode DNA-binding proteins; these are goosecoid (gsc; a divergent homeobox-containing gene with some similarities to Drosophila gooseberry and bicoid; Cho et al., 1991), Xlim-1 (encoding a homeobox protein containing two LIM domains; Taira et al., 1992), XFKH1 (encoding a transcription factor related to mammalian HNF-3β; Dirksen and Jamrich, 1992; Ruiz i Altaba and Jessel, 1992), and Xnot and Xnot2 (two related cDNAs that contain homeoboxes distantly related to Drosophila empty spiracles; von Dassow et al., 1993; Gont et al., 1993). In addition, a secreted protein, noggin, is specifically expressed in the dorsal lip and has potent dorsalizing activity (Smith and Harland, 1992).
Interestingly, gsc, Xlim-1, XFKH1, and Xnot expression can be induced by activin in the presence of protein synthesis inhibitors (Cho et al., 1991; Taira et al., 1992; Dirksen and Jamrich, 1992; von Dassow et al., 1993). Similar results are reported below for noggin. This suggests that these organizer-specific genes are primary response genes to the early Nieuwkoop center signal, which can be mimicked experimentally by activin. However, studies with the gsc homeobox gene indicate that secondary response genes (requiring synthesis of primary response proteins for expression) should also be involved in the organizer phenomenon.
gsc can induce secondary axes when its mRNA is microinjected in the ventral side of the embryo (Cho et al., 1991; Steinbeisser et al., 1993). Although all the organizer-specific transcription factors mentioned above are believed to play important roles in the organizer, so far axis-forming activity has only been reported for gsc. Cells injected with gsc can recruit neighboring uninjected cells to form tissues of the secondary axis, as in Spemann’s experiment (Niehrs et al., 1993). As gsc encodes a DNA-binding protein, these noncell-autonomous recruitment properties should be mediated by secreted or cell surface molecules encoded by downstream target genes regulated by gsc. Such molecules would be expected to be encoded by secondary response genes to activin, since protein synthesis inhibitors that block gsc translation should also block the expression of its downstream target genes.
In an attempt to isolate genes downstream of gsc that might participate in the organizer phenomenon, we carried out a differential screen for zygotic dorsal-specific cDNAs. We present here a gene that can be activated both by gsc and Xnot2 mRNA injections and is a secondary response gene to activin treatment. In situ hybridization analyses indicate that its expression closely follows the areas of the embryo that express gsc and Xnot2, namely the prechordal plate, the notochord, and the chordoneural hinge. Consequently, we named this gene chordin. It encodes a novel putative extracellular protein. Microinjected chordin mRNA has potent axis-forming activities, including the ability to recruit neighboring cells into secondary axes. chordin is expressed at the right time and in the right place to mediate inductive cell interactions in the head, trunk, and tail organizers. We propose that chordin may be a signaling factor that executes some of the functions of dorsal-specific homeobox genes in Spemann’s organizer phenomenon.
Results
Differential Screening for Dorsal-Specific Zygotic cDNAs
In Xenopus embryos, it is possible to increase or decrease the amount of organizer tissue experimentally. LiCl treatment of 32-cell embryos results in an expanded Spemann organizer comprising a ring spanning the entire marginal zone at the gastrula stage (Kao and Elinson, 1988) that expresses high levels of gsc transcripts (Cho et al., 1991). At later stages, these “dorsalized” embryos consist mainly of head structures, including radial eyes and cement glands. Irradiation of the vegetal pole of fertilized eggs with ultraviolet light (UV) results in “ventralized” embryos that lack an organizer (Stewart and Gerhart, 1990) and gsc expression (Cho et al., 1991).
To isolate dorsal-specific genes, duplicate filters of an unamplified dorsal lip cDNA library (Blumberg et al., 1991) were hybridized with probes synthesized either from dorsalized or ventralized gastrula mRNAs. To enrich for zygotically expressed genes, the probes were subtracted with maternal (8-cell embryo) mRNA. Screening of 25,000 plaques (see Experimental Procedures) yielded six independent groups of cDNAs enriched in LiCl-treated embryos.
To identify cDNAs of interest, further screening was performed in three ways with the longest clone of each group. First, the ability of its sense RNA to induce secondary axes was explored by microinjection into ventral blastomeres. Second, whole-mount in situ hybridization (Harland, 1991) using a mixture of embryos of different stages was used to identify clones expressed in areas of known organizing activity. Third, the activation of candidate genes by organizer-specific homeobox products was tested by whole-mount in situ hybridization of embryos microinjected with gsc mRNA at concentrations known to induce formation of secondary axes. In initial microinjection studies (data not shown), only one clone showed weak axis-forming activity, and while it was first expressed in the dorsal lip, at later stages it was expressed in the endoderm. More importantly for this study on organizer target genes, this clone (designated endodermin) was not activated by microinjected gsc mRNA; the properties of endodermin will be presented elsewhere (Y. S., H. S., B. L., and E. M. D. R., unpublished data). In situ hybridization identified only one group, consisting of three clones (which eventually proved not to be full length), that was exclusively expressed in cells with Spemann’s organizer activity. The gene encoding these cDNAs, chordin, was found to be activated by gsc and was chosen for further study.
chordin Is Expressed in Head, Trunk, and Tail Organizer Regions
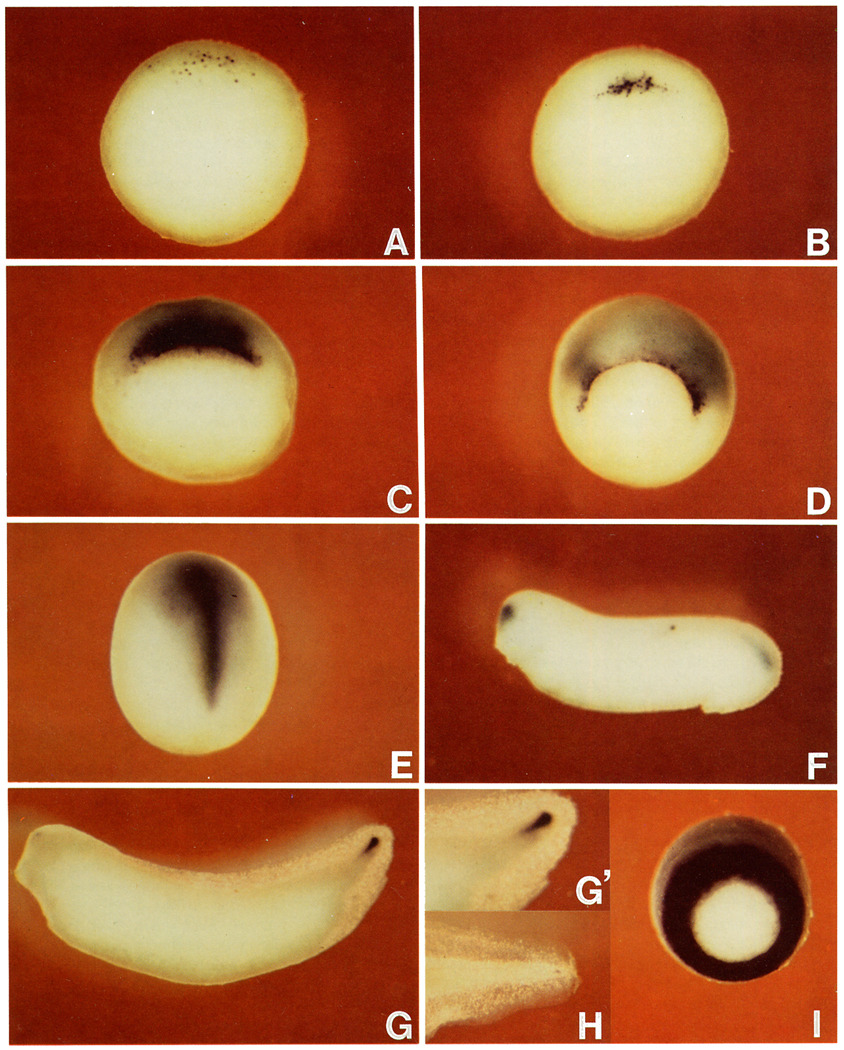
Figure 1 shows the spatial and temporal pattern of expression of Xenopus chordin. By in situ hybridization, chordin transcripts are first detected 1 hr before gastrulation (stage 9.5; Nieuwkoop and Faber, 1967) in nuclei scattered in the dorsal marginal zone (Figure 1A). When the dorsal lip is first detected at stage 10.25, the chordin transcript is found exclusively on the forming lip (Figure 1B), and cytoplasmic staining can also be observed. Once the dorsal lip is fully formed (stage 10.75), chordin expression is intense both in superficial and involuted cells (Figure 1C). By the midgastrula stage, when a circular blastopore is formed (stage 11), most cells expressing chordin have involuted (explaining the more diffuse appearance of the chordin signal), except for cells in the dorsal half of the edge of the lip itself (Figure 1D).
Figure 1. chordin Is Expressed in Regions with Head, Trunk, and Tail Organizer Activity.
Digoxygenin-labeled antisense chordin RNA was hybridized to embryos at stage 9.5 (A); stage 10.25 (B); stage 10.75 (C); stage 11.5 (D); stage 13, note expression in the prechordal plate and notochord (E); stage 26 (F); stage 33, note expression in the chordoneural hinge of the tailbud (G); stage 33, enlarged view of the tailbud region (G’); stage 42, enlarged view of the tail region (H); LiCl-treated embryo at stage 11.5 (I), compare with (D). (A)–(D) and (I) are vegetal views, dorsal side is at the top. (E) is viewed from the dorsal side with anterior at top. (F)–(H) are lateral views.
At early neurula (stage 13), strong chordin expression is detected in the prechordal plate (head mesoderm) and the notochord (Figure 1E). At the early tailbud stage (stage 26), chordin is transiently expressed in the forebrain, fading from the prechordal plate and anterior notochord but remaining in the posterior notochord and tailbud hinge (Figure 1F). Later on (stage 33, corresponding to 42 hr of development), the chordin signal is detected exclusively in the tailbud (Figure 1G). Closer examination reveals that the expression is localized in a specific region in the tailbud, the chordoneural hinge(Figure 1G’). This is of interest because transplantation experiments have shown that the chordoneural hinge retains organizer activity at this stage (Gont et al., 1993). Expression continues in the tip of the tail in swimming tadpoles (stage 42, 72 hr after fertilization; Figure 1H). In embryos dorsalized by LiCl treatment, chordin expression is enhanced, forming a ring that spans the entire marginal zone (compare Figures 1I and 1C); this explains why the gene was isolated in the differential screen. Although Figure 1 shows only external views of embryos, all observations mentioned above were confirmed in embryos rendered transparent by clearing solution and in histological sections (data not shown).
Taken together, these descriptive studies show that chordin is expressed initially in the dorsal lip and then in tissues derived from the organizer. The expression in the dorsal lip and prechordal plate overlaps in part with that of the homeobox gene gsc. The later expression of chordin in the notochord and chordoneural hinge does not coincide with that of gsc but does overlap with the expression of another homeobox gene, Xnot2 (Gont et al., 1993). These data led to the hypothesis that the chordin gene might be positively regulated (directly or indirectly) by both homeodomain proteins.
chordin Is Activated by gsc and Xnot2
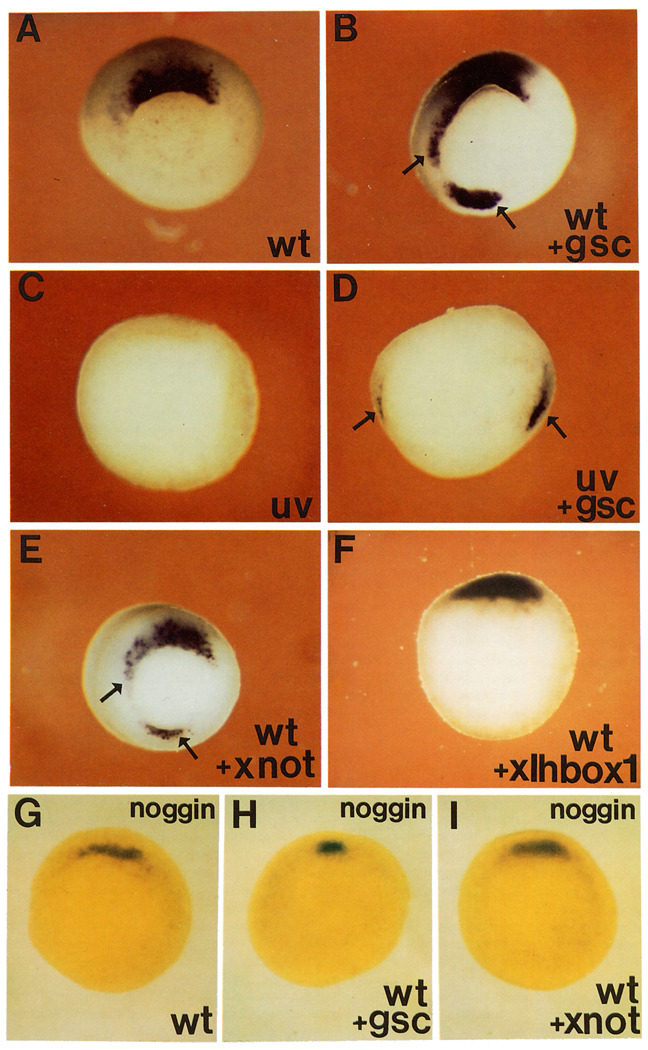
To test this hypothesis, synthetic gsc or Xnot2 mRNAs were microinjected radially into all blastomeres of 4-cell embryos and hybridized with a chordin probe at the gastrula stage. Both gsc mRNA (compare Figures 2A and 2B) and Xnot2 mRNA (Figure 2E) were able to induce ectopic patches of chordin mRNA in the ventral and lateral marginal zone. We also tested whether chordin could be activated in embryos lacking an organizer. In UV-treated embryos, chordin expression is eliminated (Figure 2C), in keeping with the dorsal character of this gene. When UV-treated embryos were injected diagonally into two blastomeres at the 4-cell stage, two patches of chordin expression could be induced both by gsc (Figure 2D) and Xnot2 (data not shown) mRNAs.
Figure 2. chordin, but Not noggin, Expression Is Activated by gsc and Xnot2 Homeobox-Containing mRNAs.
Embryos were injected in the equator with the indicated synthetic mRNA at the 8-cell stage, cultured until stage 11, and hybridized with a chordin (A–F) or noggin (G–I) antisense RNA probe. Four radial injections were given to wild-type embryos, and two diagonal ones to UV-treated embryos. (A) Wild-type (wt) uninjected embryo hybridized with the chordin probe. Staining is only seen in the organizer region. (B) Sibling embryo radially injected with gsc mRNA (80 pg per blastomere). Arrows indicate ectopic expression of chordin. (C) UV-treated embryo, chordin expression is abolished. (D) UV-treated embryo injected diagonally at two sites with gsc mRNA, arrows indicate two spots in which chordin expression is activated. (E) Embryo injected radially with Xnot2 mRNA (100 pg per blastomere). Arrows indicate regions of ectopic chordin expression. (F) Embryo injected radially with a biologically active homeobox mRNA of the Antennapedia type (XIHbox-1); no ectopic expression of chordin was detected even at 500 pg per blastomere. (G) Wild-type embryo hybridized with the noggin probe. Because the noggin signal is deep, embryos were cleared in Murray’s solution. (H) Embryo treated as in (G), but radially injected with gsc mRNA. Note that noggin is not activated by gsc. (I) Embryo treated as (G), but radially injected with Xnot2 mRNA, there is no ectopic expression of noggin. None of the RNAs injected in this figure cross-hybridized with the probes used, as determined in control experiments. Photos were taken from the vegetal side.
The activation by these homeobox genes appears to be specific for chordin. Radial injection of gsc or Xnot2 mRNA did not cause ectopic expression of the organizer-specific gene noggin (Figures 2G–2I). In addition to providing a convenient negative control, the inability of gsc to activate noggin in this assay suggests that noggin is unlikely to mediate the noncell-autonomous effects of gsc. As a further control, a biologically active homeobox mRNA of the Antennapedia type (XIHbox-1; Wright et al., 1989) was injected and found not to activate chordin expression (even at 5-fold higher concentrations than those used for gsc and Xnot2 mRNA; Figure 2F). An unrelated control synthetic mRNA (human prolactin; Amaya et al., 1991) also failed to activate chordin.
We conclude that expression of chordin, but not that of noggin, can be activated by the gsc and Xnot2 homeobox gene products. The expression patterns of these transcription factors partially overlap with that of chordin; the effects of Xlim-1 and XFKH1, which also overlap in expression with chordin, were not tested in this study. While the results do not address the issue of whether this activation is direct or requires additional intermediate steps, they suggest that chordin may function downstream of dorsal transcription factors in the organizer.
chordin Induction by Activin Requires De Novo Protein Synthesis
Activin is a potent inducer of dorsal mesoderm (Smith, 1993). Several genes can be activated by this growth factor even in the absence of de novo protein synthesis. Such Xenopus primary response genes include Mix-1 (Rosa, 1989), Brachyury (Smith et al., 1991), gsc (Cho et al.,1991; Tadano et al., 1993), Xlim-1 (Taira et al., 1992), XFKH1 (Dirksen and Jamrich, 1992), and Xnot (von Dassow et al., 1993). Since gsc and Xnot2 can induce ectopic expression of chordin mRNA, it was of interest to test whether chordin is also a primary response gene to mesoderm induction or whether it is activated subsequent to the expression of organizer-specific homeobox genes.
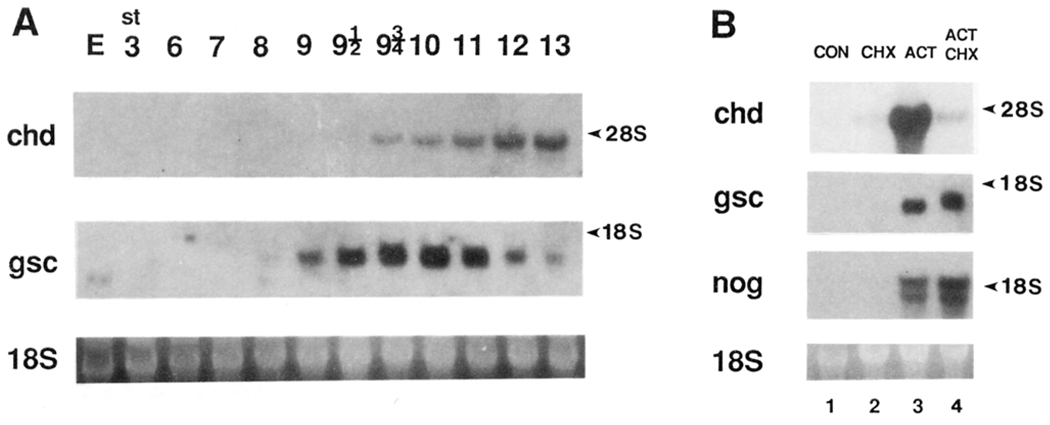
Figure 3A compares the temporal expression patterns of gsc and chordin by Northern blot analysis. gsc has a small amount of maternal transcripts, and zygotic ones become detectable at stage 9, 2 hr before gastrulation starts. In contrast, chordin expression is not detectable until stage 9.5, 1 hr before gastrulation starts. Thus, while gsc transcripts accumulate shortly after zygotic transcription starts at midblastula, those of chordin accumulate 1 hr later.
Figure 3. chordin Induction by Activin Requires De Novo Protein Synthesis.
(A) Temporal pattern of chordin expression. Northern blot analysis was performed with total RNA (7.5 µg) from various stages of early Xenopus embryos. Full-length chordin (chd) or gsc (gsc) cDNA were used as probes. 18S RNA stained with ethidium bromide is shown below as a loading control. The chordin transcript was first detected at stage 9.5, 1 hr before the onset of gastrulation. The accumulation of zygotic gsc RNA was detected earlier, at stage 9, 2 hr before gastrulation, as previously described (Cho et al., 1991). Maternal transcripts (E, egg) are present in the case of gsc but not in that of chordin, even after longer exposure.
(B) CHX inhibits activation of the chordin gene by activin. Animal caps (stage 8) were treated with 30 ng/ml activin for 2.5 hr (corresponding to stage 10) in the presence or absence of a protein synthesis inhibitor CHX (5 µg/ml) (Rosa, 1989; Cho et al., 1991). Total RNA (10 µg) was loaded in this Northern blot. Lane 1, untreated control; lane 2, CHX alone; lane 3, activin alone; lane 4, activin in the presence of CHX. Note that while chordin (chd) induction is inhibited by blocking protein synthesis, the induction of gsc and of noggin (nog) is somewhat increased. This indicates that while noggin and gsc are primary response genes to activin treatment, chordin is a secondary response gene.
To test whether chordin is a primary or a secondary response gene, animal cap explants were incubated with activin in the presence or absence of cycloheximide (CHX), which inhibited protein synthesis by 95% (see Experimental Procedures). chordin was induced by activin, but this induction was significantly decreased by CHX (Figure 3B). In contrast, gsc induction by activin was somewhat increased by CHX, in agreement with previous observations (Tadano et al., 1993). As noggin is an organizer-specific secreted factor, it was important, in the wider context of this study, to determine whether noggin is a primary response gene. As shown in Figure 3B, noggin transcripts were induced by activin in the presence of CHX (even to a higher level than in its absence), indicating that noggin, like gsc, is a primary response gene.
Together with the time course, the animal cap studies indicate that the induction of chordin by activin treatment involves intermediate steps requiring de novo protein synthesis. Thus, the induction mechanism of chordin differs from that of other organizer-specific genes described to date, including noggin.
chordin Encodes a Novel Putative Secreted Protein
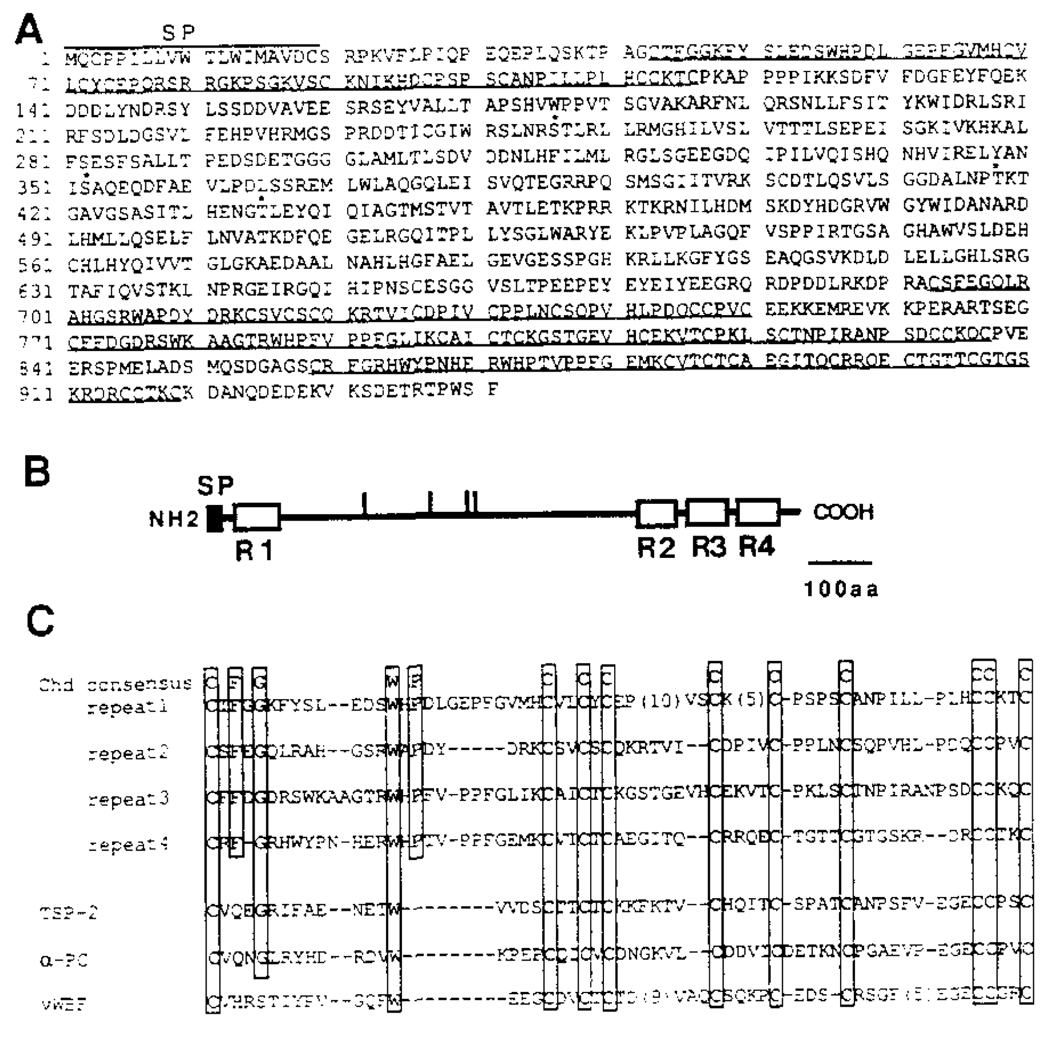
The three chordin cDNAs isolated in the initial screen were partial clones resulting from reverse transcriptase priming within an A-rich stretch within the coding sequence. These clones failed to reveal any biological activity in extensive microinjection experiments performed before sequence information was available. We rescreened the cDNA library, and a full-length 3.8 kb chordin cDNA was isolated and sequenced. The deduced protein sequence of the longest open reading frame is shown in Figure 4A. chordin encodes a large protein (predicted molecular mass of 105 kDa) of 941 amino acids. Hydropathy analysis showed a single hydrophobic segment comprising the 19 amino-terminal amino acids, followed by a putative signal sequence cleavage site. The presence of a signal peptide and the lack of possible transmembrane segments suggest that chordin is a secreted protein. There are four possible N-glycosylation (NXS/T) sites. When compared with its own sequence by dot matrix alignment, the chordin protein was found to contain four internal repeats of 58–74 residues (Figures 4B and 4C). Each repeat contains ten Cys residues at conserved positions as well as four other conserved amino acids, which are boxed in Figure 4C.
Figure 4. Xenopus chordin Encodes a Putative Secreted Protein.
(A) The amino acid sequence deduced from the nucleotide sequence of a full-length Xenopus chordin cDNA clone is shown. The initiator Met was assigned to the first ATG in the longest reading frame, which has an in-frame stop codon 130 bp upstream of this ATG. Four potential N-glycosylation consensus sequences are indicated by asterisks. Four internal repeats are underlined. A hydrophobic signal peptide segment is found at the amino terminus (residues 1–19).
(B) Schematic structure of chordin protein. The potential signal peptide and four internal Cys-rich repeats are shown by closed and open boxes, respectively. Vertical bars indicate potential N-glycosylation sites. SP, signal peptide.
(C) Comparison of Cys-rich repeats in chordin and those in some secreted proteins. The residues conserved among four repeats are boxed. chd, Xenopus chordin; TSP-2, mouse thombospondin-2; α-PC, human α1 procollagen type I; vWBF, human von Willebrand factor C1 domain.
When the Cys-rich repeats were used to search the BLAST network data bases, it was found that similar Cys-rich repeats are present in several extracellular proteins. The conservation is restricted primarily to the spacing of Cys residues (Figure 4C). Thrombospondin 1 and 2 and α1 procollagen types I and III are extracellular matrix proteins that contain a single Cys-rich domain near the amino terminus (Bornstein, 1992). Interestingly, these proteins are trimeric, and the Cys-rich domains may be involved in their multimerization (Bornstein, 1992). von Willebrand factor, a protein that facilitates adhesion of platelets during blood clotting, contains two Cys-rich domains at its carboxyl terminus (Hunt and Barker, 1987). Since the Cys-rich repeats are found in extracellular proteins and chordin contains such repeats at the both termini, the presence of these structural motifs supports the view that chordin may be a secreted protein.
Outside of the Cys-rich repeats, the rest (681 amino acids) of the chordin protein does not have significant homology to any sequences in the data bases. We conclude from these sequence comparisons that chordin mRNA encodes a novel putative secreted protein. Because chordin is specifically expressed in regions of the embryo that have organizer activity, we next tested whether this molecule is active in inductive signaling.
chordin mRNA induces Secondary Axes
For phenotypic analysis, the chordin cDNA was subcloned into an expression vector (Amaya et al., 1991) and RNA synthesized with SP6 polymerase was injected into a single blastomere of Xenopus embryos. chordin mRNA induced secondary axes at substantial frequencies when injected into ventrovegetal blastomeres (59% at the 8-cell stage, n = 46; 37% at the 32-cell stage, n = 27). When injected into dorsal or animal (top) blastomeres, a high proportion of dorsalized embryos resulted. Embryos injected with a control mRNA encoding an unrelated secreted protein (human prolactin in the same vector; Amaya et al., 1991) were unaffected.
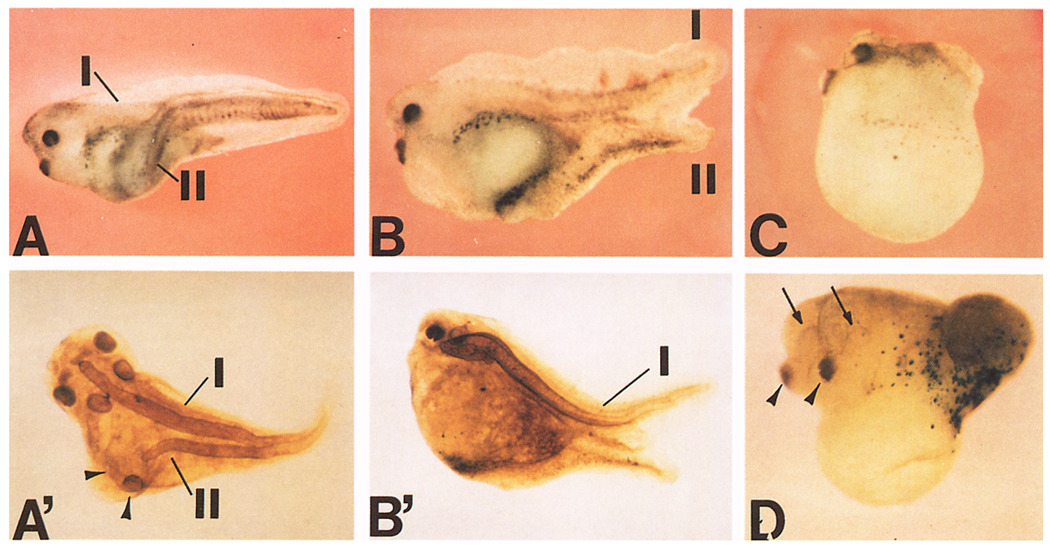
Figure 5A shows a typical secondary axis induced by a single ventral injection of chordin mRNA at the 8-cell stage. Immunostaining with a notochord marker showed that this secondary axis (Figure 5A’) contained a notochord and lacked anterior structures such as eyes and cement glands, but had auditory vesicles, implying that the axis extended anteriorly at least as far as the hindbrain. Of 37 twinned embryos (injected at the 8-cell stage) stained with this antibody, 64% had a notochord in the secondary axis and 54% had secondary auditory vesicles. In Xenopus, the absence of a differentiated notochord in experimentally manipulated embryos is not uncommon (Steinbeisser et al., 1993). Many axes were similar to those induced by ectopic expression of gsc or activin (Steinbeisser et al., 1993), but differences were also noted. In particular, embryos with double tails were found in 11% of the twinned embryos (n = 223), suggesting that chordin can induce tail-organizing activity in some cases; this phenotype has not been observed in secondary axes induced by gsc (e.g., Cho et al., 1991; Steinbeisser et al., 1993). Figures 5B and 5B’ show one such embryo with double tails in which the secondary tail does not express the notochord marker. Embryos were scored as having double tails only when the primary and secondary axes were separate throughout their entire length; in general, the secondary tailbud formed in the ventral side, directly opposite to the primary axis (Figure 5B). Twinned embryos with duplicated anterior structures were observed at a low frequency (15% with double cement glands and 4.5% with extra eyes; Figure 5G). On the other hand, enlarged head structures, such as cement glands, were commonly observed in dorsalized embryos caused by chordin mRNA injections into dorsal or animal blastomeres (Figure 5C).
Figure 5. chordin mRNA Induces Secondary Axes.
(A) Secondary axis formed after microinjection of chordin RNA (200 pg) into a ventrovegetal blastomere of an 8-cell embryo. (A’) Immunostaining with a notochord marker (MZ-15) of the same embryo shown in (A). A second notochord (II) and extra auditory vesicles (arrowheads) are present.
(B) Secondary tail formed after microinjection of chordin RNA into the animal pole. (B’) Immunostaining with MZ-15 notochord antibody. No mature second notochord was found in this secondary tail.
C) A dorsalized embryo resulting from the injection of chordin RNA (200 pg) into a dorsovegetal blastomere of an 8-cell embryo. Notochord staining showed a short and thick double-barreled notochord (data not shown).
D) A double-headed embryo induced by chordin. chordin RNA (150 pg) was coinjected with β-galactosidase RNA into a A4 blastomere of a 32-cell embryo. Cement glands (arrowheads) and eyes (arrows) are duplicated. Strong β-galactosidase activity was detected in the posterior epidermis but not in the secondary axis (confirmed by histological section; data not shown), indicating that chordin has noncell-autonomous effects on uninjected cells. In all cases, injection of β-galactosidase or prolactin control RNAs showed no particular phenotypes.
To examine the fate adopted by injected and uninjected cells, single blastomeres were injected at the 32-cell stage with a mixture of chordin and β-galactosidase (lineage tracer) mRNAs. It appears that chordin mRNA has both long- and short-range effects. Figure 5D shows an embryo injected into the A4 blastomere, in which the injected cells remained in the ectoderm and did not contribute to the secondary axis. When a ventromarginal blastomere, C4, was injected, almost all embryos with secondary axes displayed labeling in part of the secondary axis itself (data not shown, but see Figures 6E–6G below), suggesting that the chordin-injected cells had their fate changed into that of Spemann’s organizer.
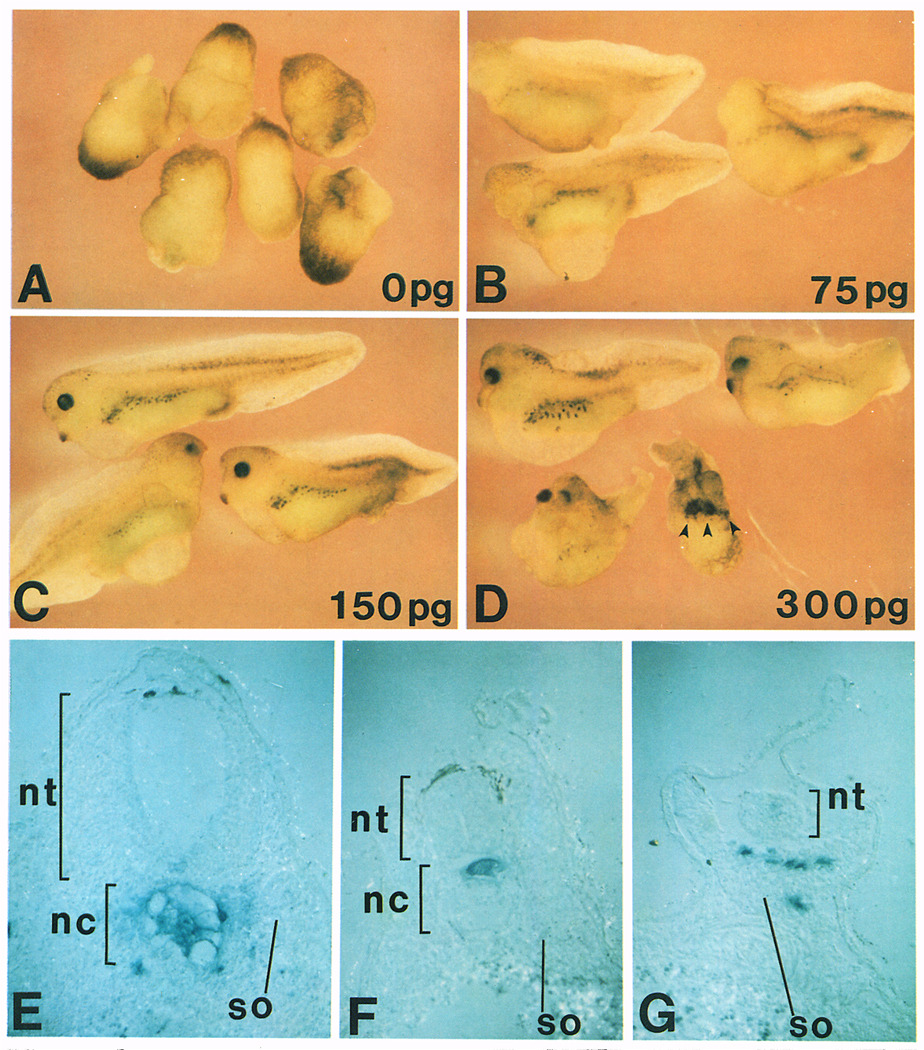
Figure 6. Dose-Dependent Axial Rescue by chordin mRNA.
Increasing doses of chordin mRNA were injected into one vegetal blastomere of an 8-cell embryo after UV irradiation at the 1-cell stage. (A) Uninjected embryos that have been completely lost axial structures after UV treatment (DAI = 0.1, n = 10); (B) 75 pg of chordin RNA (DAI = 2.1, n = 17); (C) 150 pg of chordin RNA (DAI = 4.2, n = 10); (D) 300 pg of chordin RNA (DAI = 6.2, n = 8). Multiple cement glands are indicated by arrowheads. Injection of control prolactin RNA did not rescue the UV-ventralized phenotype. (E–G) transverse histological sections of embryos injected with β-galactosidase and chordin mRNA into the C-tier region and stained with X-Gal. Note that the injected cells populate in the dorsal axis and recruit uninjected cells into the axis. nt, neural tube; nc, notochord; so, somite.
chordin Rescues Complete Axes in Ventralized Embryos
To test further the axis-forming activity of chordin, a UV-rescue assay was used. Due to its high level of sensitivity, this method is frequently favored in Xenopus embryology (Smith and Harland, 1991, 1992; Steinbeisser et al., 1993; Thomsen and Melton, 1993). Figure 6A shows ventralized embryos resulting from UV treatment. They lack axial structures as indicated by a dorsoanterior index (DAI; Kao and Elinson, 1988) of 0.1. (In this scale, a DAI of 0 corresponds to embryos with no axis, and a value of 5 to a normal embryo.) When 75 pg of synthetic chordin mRNA was injected into a single vegetal blastomere of UV-treated embryos at the 8-cell stage, substantial rescue of trunk and tail structures occurred (Figure 6B), although the embryos still lacked the most anterior head structures (DAI = 2.1). These phenotypes were similar to those observed in rescue experiments with gsc or activin mRNA (Steinbeisser et al., 1993). When 150 pg of chordin mRNA was injected (Figure 6C), the entire axis was rescued (DAI = 4.2), including eyes and cement glands. When the amount of chordin RNA was doubled (Figure 6D), embryos with exaggerated dorsoanterior structures, such as multiple cement glands (indicated by arrowheads), resulted (DAI = 6.2).
When UV-treated embryos were microinjected into a blastomere of the C-tier region with a mixture of chordin and β-galactosidase mRNAs as a lineage tracer, the labeled chordin-injected cells were located in the dorsal axis (n = 19), usually in anterior regions, or in endodermal cells (n = 5) (data not shown). Histological analysis showed that the injected cells contribute preferentially to dorsal tissues, i.e., notochord and somites (Figure 6E). This implies that expression of chordin changes the fate of the injected cells into organizer-like tissue. Most of the dorsal axis, however, was recruited from uninjected cells, including most of the somite and all of the neural tissue shown in Figure 6E. The noncell-autonomous effects of chordin on neighboring cells are best illustrated by the embryo shown in Figure 6F, in which only a small sector of notochord was derived from injected cells, while most of the notochord (as well as the rest of the dorsal axis) was recruited from uninjected cells. Some rescued axes, particularly at low chordin mRNA concentrations, lacked a notochord and the somites were fused. In such cases (Figure 6G), the injected cells were found in the somite, principally in the midline underlying the neural tube, indicating that chordin can rescue axial structures even in the absence of notochord tissue.
We conclude that chordin mRNA can completely rescue axis formation in ventralized embryos. The injected cells preferentially give rise to organizer derivatives and are able to recruit neighboring cells to form multiple dorsal tissues, including notochord.
chordin Modifies Mesoderm Induction
To test whether chordin mRNA has mesoderm-inducing activity, embryos were injected in the animal pole and animal cap explants were prepared at midblastula. Animal caps injected with control prolactin or with chordin mRNA failed to elongate and (as determined by histological analysis) consisted of atypical epidermis and lacked mesodermal tissues (Table 1). Thus, chordin lacks mesoderm induction activity per se. However, microinjection of chordin mRNA can dorsalize ventral marginal zone (VMZ) explants (Table 1), which have received the inductive signals that lead to formation of ventral mesoderm. A class of molecules, called competence modifiers, which cannot induce mesoderm on their own but can regulate the response of embryonic cells to induction, has been described (Moon and Christian, 1992).
Table 1.
chordin RNA Dorsalizes Ventral Mesoderm
| Explant | RNA | bFGF | AtEP | Bl | Mst | Mus | Noto | (n) |
|---|---|---|---|---|---|---|---|---|
| AC | prolactin | − | 100% | 0% | 0% | 0% | 0% | (18) |
| AC | chordin | − | 100% | 0% | 0% | 0% | 0% | (20) |
| AC | prolactin | + | 16% | 52% | 72% | 4% | 0% | (25) |
| AC | chordin | + | 2% | 2% | 4% | 31% | 40% | (48) |
| VMZ | prolactin | − | 0% | 85% | 95% | 15% | 0% | (20) |
| VMZ | chordin | − | 0% | 5% | 19% | 57% | 52% | (21) |
Animal caps were explanted at stage 8, incubated with or without 50 ng/ml of bFGF in 0.3× MBS solution containing 0.1 mg/ml BSA for 2 hr, and cultured in 0.3× MBS for 2 days. Microinjection of chordin mRNA did not produce elongation of animal cap explants by itself, but did cause elongation of most of the explants in the presence of bFGF. VMZs excised at stage 10.5, and the explants were cultured for 2 days. Microinjection of chordin mRNA caused extensive elongation of VMZs. In the animal cap experiments, 300 pg of synthetic chordin mRNA was injected radially into each of the four animal pole blastomeres at the 8-cell stage. In the VMZ experiments, 200 pg of chordin mRNA was injected radially into each of the four vegetal blastomeres of 8-cell embryos. In both experiments, explants were fixed when sibling embryos reached stage 42. All explants were stained in whole-mount with the MZ-15 antibody marker, scored for notochord, and then embedded in paraffin, sectioned and scored by histology. The frequency of each type of tissue in the explants is shown. The data indicate that chordin per se is not a mesoderm inducer but acts as a modifier (dorsalizer) of mesoderm differentiation. AtEp, atypical epidermis; Bl, blood; Mst, mesothelium; Mus, skeletal muscle; Noto, notochod; Neur, neural tissue.
We next tested whether chordin can dorsalize mesoderm induced by basic fibroblast growth factor (bFGF; Slack, 1991). When animal caps were injected with either control prolactin or chordin mRNA and treated with bFGF (Table 1), the control caps formed ventral mesoderm (blood and mesothelium), while chordin-injected caps formed dorsal mesoderm (notochord and muscle) as well as dorsoanterior ectodermal inductions such as cement glands and blocks of neural tissue.
We conclude that while chordin mRNA alone is unable to induce mesodermal tissues in animal caps, it is able to synergize with bFGF, leading to the dorsalization of ventral mesoderm.
Discussion
In an attempt to identify genes that might mediate the noncell-autonomous functions of gsc (Niehrs et al., 1993), we searched for cDNA clones enriched in dorsalized (LiCl-treated) and not in ventralized (UV-treated) embryos. By subtracting with maternal mRNA, we selected for zygotically expressed genes. The chordin cDNA was chosen for further study because it is expressed specifically in the regions of the embryo that have organizer activity and is activated by organizer-specific homeobox genes.
chordin Encodes a Novel Putative Extracellular Protein
chordin encodes a novel putative secreted protein of 941 amino acids. It has four Cys-rich domains similar to those found in extracellular proteins such as thrombospondin, the propeptide of α procollagen, and von Willebrand factor. The conservation is limited mostly to the spacing of the Cys residues; it appears that the Cys-rich domains define a superfamily of extracellular matrix or cell surface proteins. A role for extracellular matrix proteins in patterning signals would not be unprecedented; recently, one of the signals involved in axon guidance by the floor plate has been identified as netrin, a protein with homology to laminin B2 (Serafini et al., 1994).
chordin as a Target for Organizer-Specific Homeobox Genes
A number of organizer-specific transcription factors have been studied in Xenopus embryos (Cho et al., 1991; Taira et al., 1992; Dirksen and Jamrich, 1992; von Dassow et al., 1993). All can be induced by activin even in the absence of protein synthesis, suggesting that they are primary response genes to the signals released by the Nieuwkoop center. In contrast, expression of chordin mRNA starts subsequently to that of gsc and requires de novo protein synthesis. gsc and Xnot2 can activate ectopic expression of chordin (Figure 2). The experiment does not distinguish whether this activation is direct or involves intermediate steps; in the future, it will be of interest to determine whether gsc or Xnot2 proteins can bind to the chordin promoter. Because both gsc and chordin mRNA are able to induce secondary axes when injected in the ventral side of the embryo, it is conceivable that gsc might cause, in part, its effects on neighboring cells via activation of chordin. noggin is unlikely to mediate the recruiting function of gsc because its expression is not activated by gsc mRNA (Figure 2).
chordin Has Potent Axis-Forming Activity
Since chordin is expressed in the Spemann organizer, a number of embryological assays were performed to test whether it had biological activities congruent with its distribution of the embryo. Injection of chordin mRNA results in the formation of secondary axes that contain dorsal structures such as muscle and notochord. Typical secondary axes end anteriorly at the level of hindbrain (marked by auditory vesicles), as is the case for secondary axes induced by gsc and activin mRNAs (Steinbeisser et al., 1993), although at lower frequencies head or tail structures can be found (Figure 5).
In UV-treated embryos, a single injection of synthetic chordin mRNA can completely rescue axial development (Figure 6). This rescue is dose dependent and sensitive to 2-fold differences in the amount of injected mRNA. At low doses, the embryos lack head structures and notochord, and at high doses, the embryos have exaggerated dorsoanterior structures. In this UV rescue assay, the effect of chordin is similar to that of Xwnt-8 mRNA (Sokol et al., 1991; Smith and Harland, 1991), noggin (Smith and Harland, 1992), and the processed Vg-1 product (Thomsen and Melton, 1993).
Although the phenotypes are similar, there are significant differences in the temporal and spatial expression of these genes. Xwnt-8 is expressed in the ventral and lateral marginal zone but not in the organizer region and, therefore, is unlikely to act as a dorsalizing signal in vivo (Christian and Moon, 1993). Vg-1 is exclusively maternal and is expressed in the vegetal-most cells (Rebagliati et al., 1985) and, therefore, is likely to function in the Nieuwkoop signaling center rather than in the organizer proper. noggin expression overlaps in part with that of chordin; the similarities and differences between these two genes are discussed in the next section.
Comparison of chordin and noggin
Since noggin and chordin are dorsalizing factors with similar phenotypic effects, it may be useful to examine whether some differences are also found. noggin is expressed maternally and zygotically, the latter expression being localized in the organizer (Smith and Harland, 1992). Therefore, noggin could function in the Nieuwkoop center, the Spemann organizer, or both. On the other hand, chordin is expressed only zygotically and exclusively in the organizer region, so that it could not function in vivo in the Nieuwkoop center. There are no sequence similarities between the two proteins; noggin encodes a secreted polypeptide of 222 amino acids, while chordin is four times as large.
chordin and noggin display clear-cut differences in their modes of activation. noggin is a primary response gene to activin induction, while chordin is a secondary response gene requiring de novo protein synthesis (Figure 3B). chordin is activated by the organizer-specific homeobox genes gsc and Xnot2, while noggin is not (Figure 2). Thus, despite some phenotypic similarities in embryological assays, chordin and noggin appear to represent two parallel signaling pathways in Spemann’s organizer.
chordin Dorsalizes Mesoderm Differentiation
To investigate the mechanism underlying the biological activity of chordin, we studied mesoderm induction in animal cap explants. Injection of chordin mRNA did not induce mesoderm by itself, but promoted induction of notochord and neural tissues in animal caps treated with bFGF. Similar observations, in which dorsalizing agents cannot induce mesoderm in animal caps but can form dorsal mesoderm in response to ventral mesoderm induction, have been reported for LiCl (Slack et al., 1988), noggin (Smith et al., 1993; Cunliffe and Smith, 1994), and Xwnt-8 mRNA (Christian et al., 1992). Since the expression of chordin is zygotic and localized specifically in the organizer, this molecule, like noggin (Smith et al., 1993), is a candidate for the horizontal dorsalizing signal of the model of Slack (1991).
A Molecule Shared by the Head, Trunk, and Tail Organizers
The amphibian organizer consists of several cell populations with region-specific inducing activities (Hamburger, 1988; Gont et al., 1993). On the basis of morphogenetic movements, three very different cell populations can be distinguished in the organizer. First, cells with crawling migration movements involute, fanning out to form the prechordal plate (Keller, 1976). Second, cells involute through the dorsal lip, driven by convergence and extension movements, giving rise to the notochord of the trunk (Keller, 1991). Third, involution ceases, and the continuation of mediolateral intercalation movements leads to posterior extension movements and to the formation of the tail notochord and of the chordoneural hinge (Gont et al., 1993). The three cell populations correspond to the head, trunk, and tail organizers, respectively. Despite their different behaviors from the cell biological point of view, the three organizer regions share a common axis-forming molecule, chordin, which is expressed at the right time and in the right place to participate in cell signaling by Spemann’s organizer and is activated by organizer-specific homeobox genes.
Experimental Procedures
Embryo Manipulation
UV treatment was performed 30 min after fertilization for 60 s with an UVG-11 lamp (UV-Products, Incorporated). LiCl treatment was carried out in 0.12 M LiCl in 0.1× modified Barth solution (MBS; Gurdon, 1976) for 40 min starting at the 32-cell stage. For ventral marginal zone (VMZ) experiments, an explant comprising 60° of the VMZs opposite to the dorsal lip was excised from stage 10.25–10.5 embryos and cultured in 0.3× MBS until the stage indicated.
Differential Screening
RNA was isolated from LiCl-treated or UV-treated Xenopus gastrula embryos at stage 10.5 as described (Cho et al., 1991). The poly(A)+ fraction was purified with oligo(dT) latex (QIAGEN) and used to synthesize first-strand cDNA. After alkaline lysis of template RNA, the single-stranded cDNA was hybridized with a 5-fold excess of biotin-labeled maternal RNA (8-cell stage), followed by addition of streptavidin and phenol extraction as described (Sive and St John, 1988). Single-stranded cDNA enriched in zygotic genes was recovered from the aqueous phase. After a second identical subtraction, the cDNA was labeled with [α-32P]dCTP by the random primer labeling method (Prime-It II kit, Stratagene) and was used as a probe (LiCl or UV probe).
Unamplified Xenopus dorsal lip cDNA (2.5 × 104 pfu at stage 10.5) ZAP phage library (Blumberg et al., 1991) was plated and lifted onto two replica nitrocellulose filters. Duplicate filters were hybridized with the LiCl or UV probes. Those plaques that gave a much stronger signal with the LiCl probe than with the UV probe were taken as positive clones. Twenty-two clones were positive after the second purification. These clones were classified into six groups by cross-hybridization using dot blot analysis. Whole-mount in situ hybridization analyses were performed using the longest insert in each group as a probe.
From the differential screening, three chordin cDNAs were isolated, the longest of which was 2.3 kb (clone 59). Preliminary sequencing, the size of mature transcript in Northern blots (about 4 kb), and the lack of biological activity of microinjected synthetic RNA indicated that these clones were not full length. After rescreening the dorsal lip library with clone 59 as a probe, 44 additional chordin cDNAs were isolated, of which 5 contained 3.8 kb inserts that were full length and showed biological activity.
In Situ Hybridization and Immunohistochemistry
Whole-mount in situ hybridization was performed as described previously (Harland, 1991), except for the use of a new substrate for alkaline phosphatase (BM purple AP substrate, Boehringer). Much better signals were obtained with this substrate than with the conventional BCIP/NBT. The probe was synthesized using T7 RNA polymerase in the presence of digoxygenin–UTP using as a template a partial chordin clone, clone 59 (in pBluescript SK(−)) linearized with EcoRI. The probes for gsc and Xnot2 RNA were prepared as described previously (Cho et al., 1991; Gont et al., 1993). A fragment spanning the coding region of noggin cDNA was obtained by RT–PCR using stage 11 Xenopus embryo mRNA as a template and subcloned into the HindIII–Xbal site of pBluescript KS(−). Sequencing analysis showed no base changes to the previously reported nucleotide sequence (Smith and Harland, 1992). The noggin antisense probe was synthesized with T7 polymerase using this plasmid linearized with Ncol (the Ncol site was introduced at the initial Met site by PCR).
Immunohistochemistry for notochord staining was carried out with MZ-15 antibody (Smith and Watts, 1985). β-galactosidase staining was performed at pH 6.8.
Synthetic mRNAs
The protein coding region of chordin was amplified by PCR, and the amplified fragment was subcloned into pSP35T (Amaya et al., 1991) and designated pSP35-chd.
mRNA was synthesized in vitro in the presence of cap analog and GTP (ratio 5:1) using the Megascript kit (Ambion) from pSP35-chd linearized with Xbal. The mRNAs for gsc, Xnot, and XIHbox-1 were synthesized as described previously (Niehrs et al., 1994; Gont et al.. 1993; Wright et al., 1989). For control experiments, sense β-galactosidase RNA (from pCDM8-β-gal; Sasai et al 1992) or prolactin RNA (from pSP35T; Amaya et al., 1991) was injected. Microinjection into Xenopus blastomeres was as described (Cho et al., 1991).
Activin Treatment of Animal Cap Explants and RNA Blotting Analysis
Activin and CHX treatments of animal cap explants were performed as described previously (Rosa, 1989; Cho et al., 1991). Animal caps were excised at stage 8, preincubated in 1× MBS with or without 5 µg/ml CHX for 30 min, and treated with 30 ng/ml recombinant human activin A (Genentech) for 150 min in the presence or absence of CHX. Total RNA was isolated from these explants and embryos of several early stages with RNA-STAT 60 kit (Tel-Test “B,” lnc). Total RNA (10 µg) was separated by formaldehyde–agarose gel electrophoresis, transferred to Gene Screen Plus (DuPont), and hybridized with full-length chordin, gsc, or noggin probes in 5× SSPE, 1% SDS, 150 µg/ml heat-denatured salmon sperm DNA and 50% formamide at 42°C as recommended by the manufacturer.
Acknowledgments
We thank Dr. F. Watts and Genentech for the gifts of MZ-15 antibody and human recombinant activin A, respectively. We are indebted to Drs. S. Cramton, A. Fainsod, L. Leyns, and D. Bachiller for critical reading of this manuscript and to A. Cuellar for technical assistance. Y. S. was supported by a Sankyo Life Science fellowship and a Human Frontier Science Project Organization long-term fellowship. H. S. was supported by a Deutsche Forschungsgemeinschaft fellowship. This work was supported by grants of the National Institutes of Health (HD21502-09) and the Human Frontier Science Project Organization.
Footnotes
GenBank Accession Number
The accession number for the sequence reported in this paper is L35764.
References
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Wright CVE, De Robertis EM, Cho KWY. Organizer-specific homeogenes in Xenopus laevis embryos. Science. 1991;253:194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992;6:3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- Cho KWY, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Christian JL, Olson DJ, Moon RT. Xwnt-8 modifies the character of mesoderm induced by bFGF in isolated Xenopus ectoderm. EMBO J. 1992;11:33–41. doi: 10.1002/j.1460-2075.1992.tb05024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe V, Smith JC. Specification of mesodermal pattern in Xenopus laevis by interactions between Brachyury, noggin and Xwnt-8. EMBO J. 1994;13:349–359. doi: 10.1002/j.1460-2075.1994.tb06268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen ML, Jamrich M. A novel, activin-inducible, blastopore lip–specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev. 1992;6:599–608. doi: 10.1101/gad.6.4.599. [DOI] [PubMed] [Google Scholar]
- Gerhart J, Doniach T, Stewart R. Organizing the Xenopus organizer. In: Keller R, Clark WH, Griffin F, editors. Gastrulation: Movements, Patterns, and Molecules. New York: Plenum Press; 1991. pp. 57–76. [Google Scholar]
- Gilbert SF. Developmental Biology. Fourth Edition. Sunderland, Massachusetts: Sinauer Associates, Inc.; 1994. pp. 597–615. [Google Scholar]
- Gont LK, Steinbeisser H, Blumberg B, De Robertis EM. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993;119:991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Gurdon J. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. I. Embryol. Exp. Morphol. 1976;36:523–540. [PubMed] [Google Scholar]
- Hamburger V. The Heritage of Experimental Embryology: Hans Spemann and the Organizer. Oxford: Oxford University Press; 1988. [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Meth. Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hunt LT, Barker WC. von Willebrand factor shares a distinctive cysteine-rich domain with thrombospondin and procollagen. Biochem. Biophys. Res. Commun. 1987;144:876–882. doi: 10.1016/s0006-291x(87)80046-3. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as a Spemann’s Organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Keller RE. Vital dye mapping of the gastrula and neurula of Xenopus II. Prospective area and morphogenic movements of the deep layer. Dev. Biol. 1976;51:118–137. doi: 10.1016/0012-1606(76)90127-5. [DOI] [PubMed] [Google Scholar]
- Keller RE. Early embryonic development of Xenopus laevis. Meth. Cell Biol. 1991;36:62–109. doi: 10.1016/s0091-679x(08)60273-3. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Moon RT, Christian JL. Competence modifiers synergize with growth factors during mesoderm induction and patterning in Xenopus. Cell. 1992;71:709–712. doi: 10.1016/0092-8674(92)90545-n. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Keller R, Cho KWY, De Robertis E. The homeobox gene goosecoid controls cell migration in Xenopus embryos. Cell. 1993;72:491–503. doi: 10.1016/0092-8674(93)90069-3. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Steinbeisser H, De Robertis EM. Mesodermal patterning by a gradient of the vertebrate homeobox gene goosecoid. Science. 1994;263:817–820. doi: 10.1126/science.7905664. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North Holland Publishing Company; 1967. [Google Scholar]
- Rebagliati MR, Weeks DL, Harvey RP, Melton DA. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985;42:769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Rosa FM. Mix. 1, a homeobox mRNA inducible by mesoderm inducers, is expressed mostly in the presumptive endoderm cells of Xenopus embryos. Cell. 1989;57:965–974. doi: 10.1016/0092-8674(89)90335-8. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Jessell T. Pintallavis, a gene expressed in the organizer region and midline cells of frog embryos: involvement in the development of the neural axis. Development. 1992;116:81–93. doi: 10.1242/dev.116.Supplement.81. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992;6:2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan CM, Jessel TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth–promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Sive HL, St John T. A simple subtractive hybridization technique employing photoactivatable biotin and phenol extraction. Nucl. Acids Res. 1988;16:10937. doi: 10.1093/nar/16.22.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JMW. From Egg to Embryo. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Stack JMW, Issacs HV, Darlington BG. Inductive effects of fibroblast growth factor and lithium ion on Xenopus blastula ectoderm. Development. 1988;103:581–590. doi: 10.1242/dev.103.3.581. [DOI] [PubMed] [Google Scholar]
- Smith JC. Mesoderm-inducing factors in early vertebrate development. EMBO J. 1993;12:4463–4470. doi: 10.1002/j.1460-2075.1993.tb06135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Watts FM. Biochemical specificity of Xenopus notochord. Differentiation. 1985;29:109–115. doi: 10.1111/j.1432-0436.1985.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BMJ, Green JBA, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, Knecht AK, Wu M, Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Spemann H. Uber den Anteil von Implantat und Wirtskeim an der Orientierung und Beschaffenheit der induzierten Enbryonanlage. Roux’ Arch. f. Entw. mech. 1931;123:389–517. doi: 10.1007/BF01380646. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Uber Induktion von Embryonalanlagen durch Implantation Artfremder Organisatoren. Roux’ Arch. f. Entw. mech. 1924;100:599–638. [Google Scholar]
- Steinbeisser H, De Robertis EM, Ku M, Kessler DS, Melton DA. Xenopus axis formation: induction of goosecoid by injected Xwnt-8 and activin mRNA. Development. 1993;118:499–507. doi: 10.1242/dev.118.2.499. [DOI] [PubMed] [Google Scholar]
- Stewart RM, Gerhart JC. The anterior extent of dorsal development of the Xenopus embryonic axis depends on the quantity of organizer in the late blastula. Development. 1990;109:363–372. doi: 10.1242/dev.109.2.363. [DOI] [PubMed] [Google Scholar]
- Tadano T, Otani H, Taira M, Dawid I. Differential induction of regulatory genes during mesoderm formation in Xenopus laevis embryos. Dev. Genet. 1993;14:204–211. doi: 10.1002/dvg.1020140307. [DOI] [PubMed] [Google Scholar]
- Taira M, Jamrich M, Good PJ, Dawid IB. The LIM domain-containing homeobox gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992;6:356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Thomsen GH, Melton DA. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell. 1993;74:433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- von Dassow G, Schmidt JE, Kimelman D. Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeobox gene. Genes Dev. 1993;7:355–366. doi: 10.1101/gad.7.3.355. [DOI] [PubMed] [Google Scholar]
- Wright CVE, Cho KWY, Hardwicke J, Collins RH, De Robertis EM. Interference with function of a homeobox gene in Xenopus embryos produces malformations of anterior spinal cord. Cell. 1989;59:81–93. doi: 10.1016/0092-8674(89)90871-4. [DOI] [PubMed] [Google Scholar]