Abstract
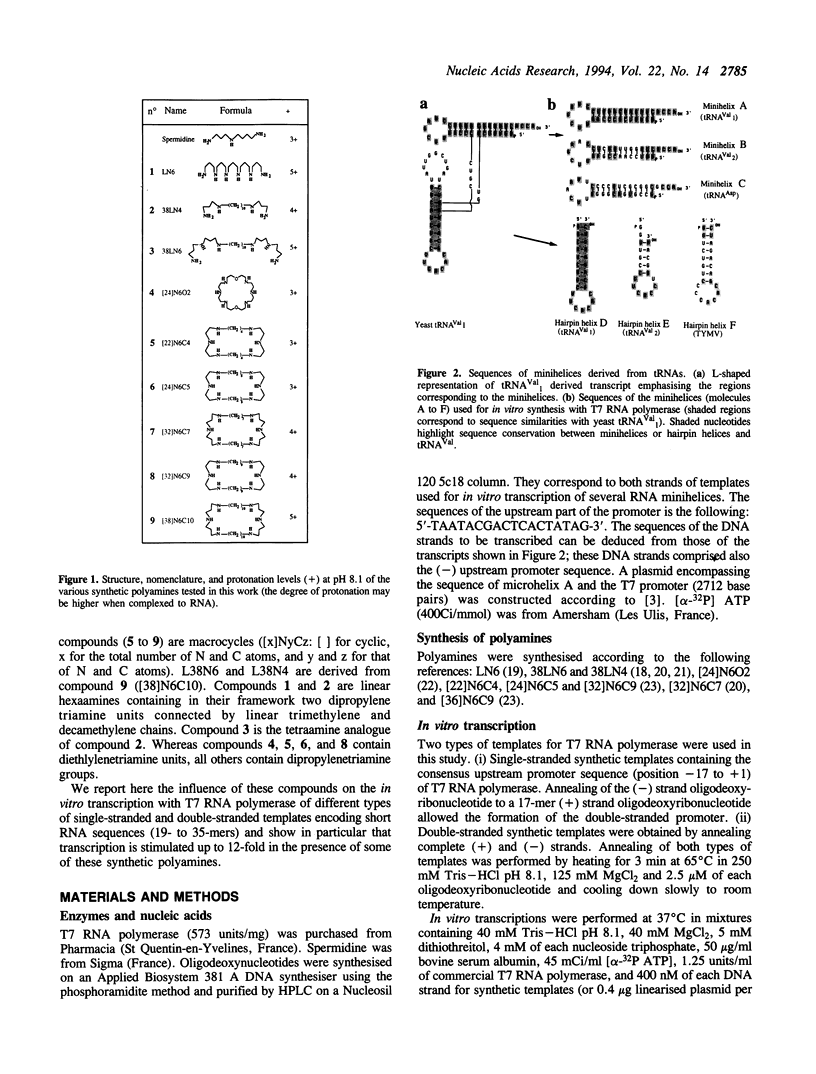
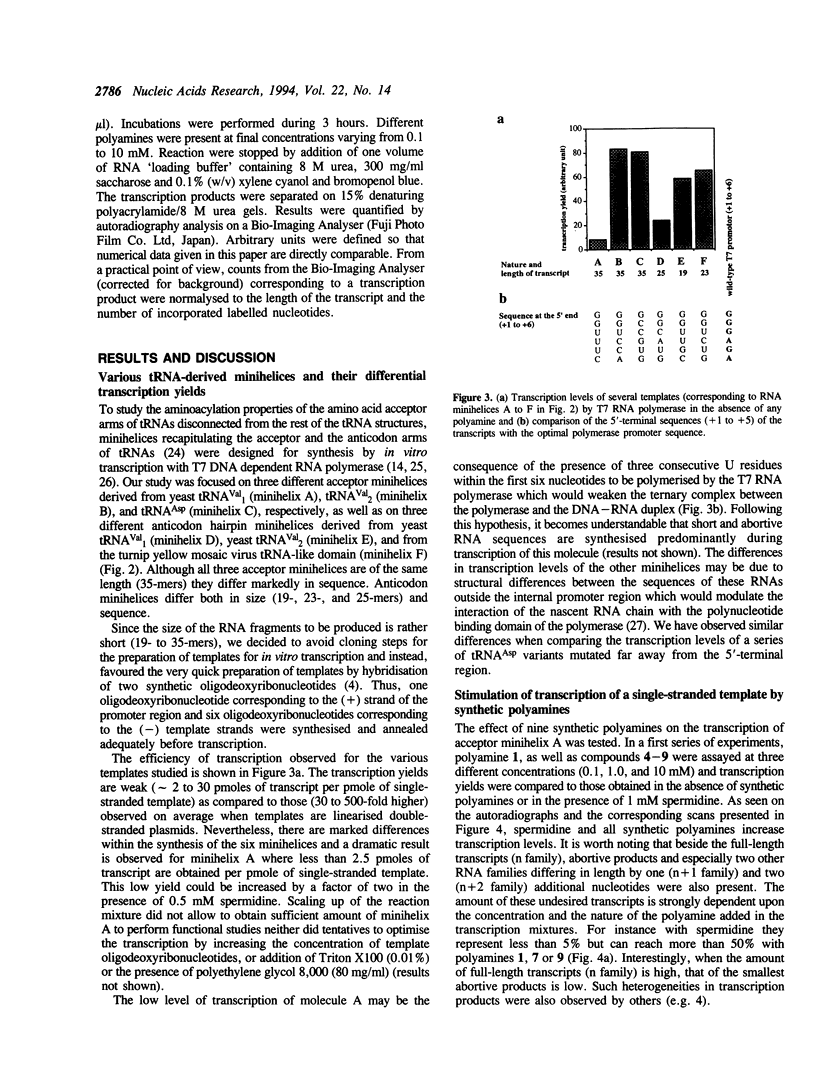
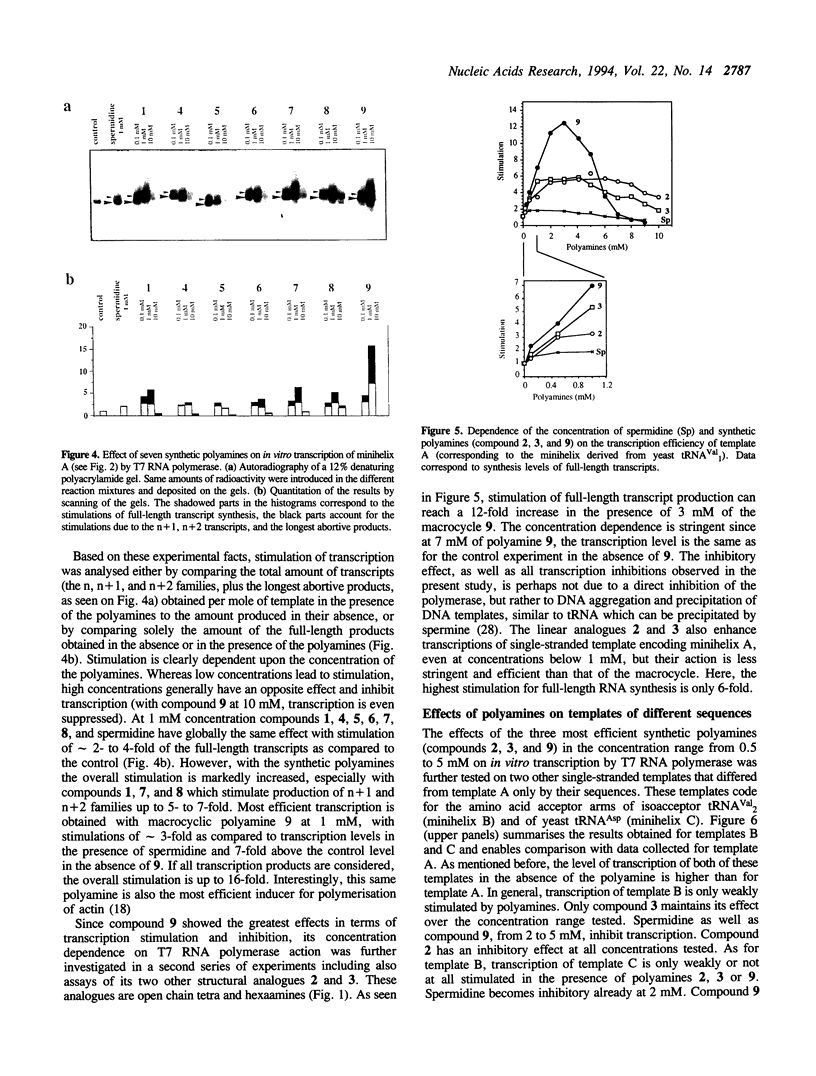
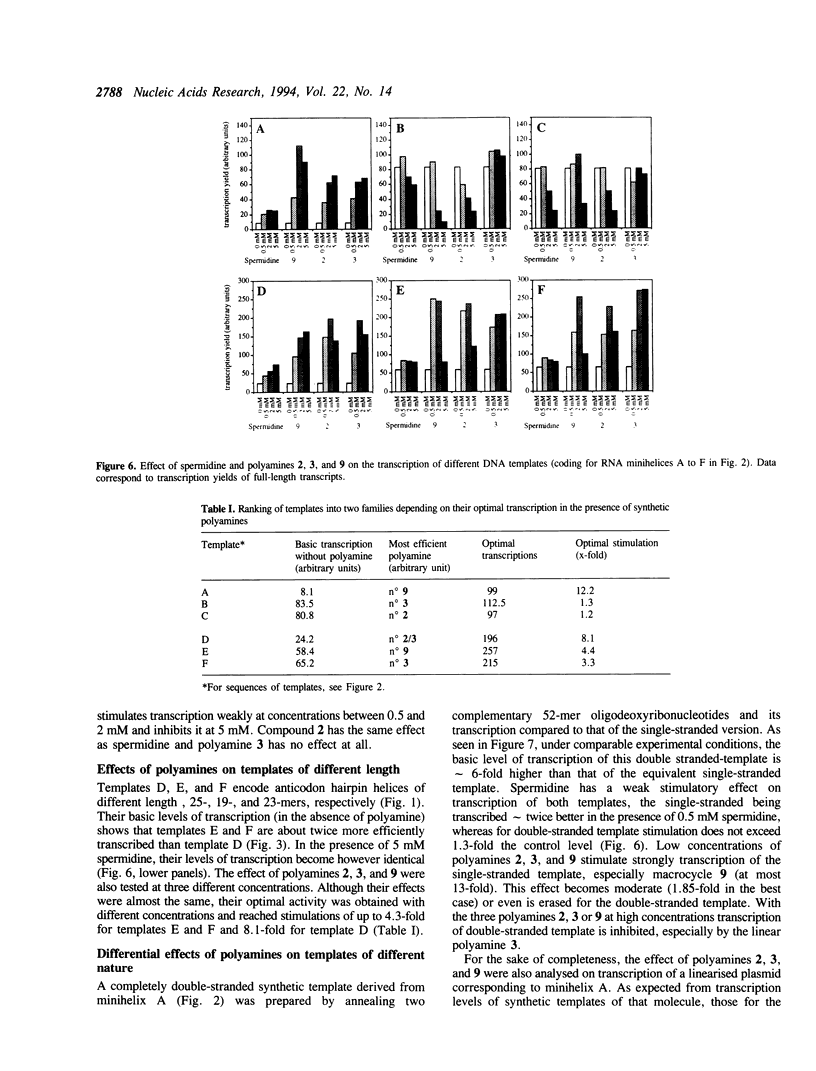
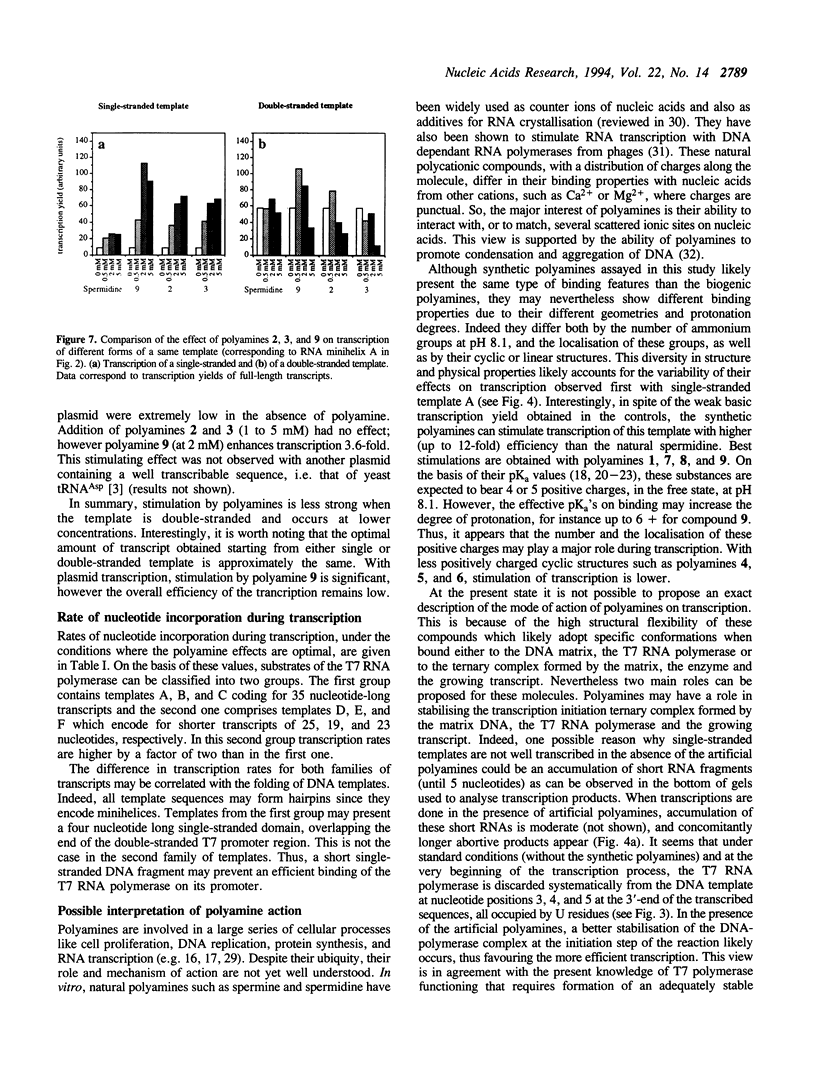
The influence of nine synthetic polyamines on in vitro transcription with T7 RNA polymerase has been studied. The compounds used were linear or macrocyclic tetra- and hexaamine, varying in their size, shape and number of protonated groups. Their effect was tested on different types of templates, all presenting the T7 RNA promoter in a double-stranded form followed by sequences encoding short transcripts (25 to 35-mers) either on single- or double-stranded synthetic oligodeoxyribonucleotides. All polyamines used stimulate transcription of both types of templates at levels dependent on their size, shape, protonation degree, and concentration. For each compound, an optimal concentration could be defined; above this concentration, transcription inhibition occurred. Highest stimulation (up to 12-fold) was obtained by the largest cyclic compound called [38]N6C10.
Full text
PDF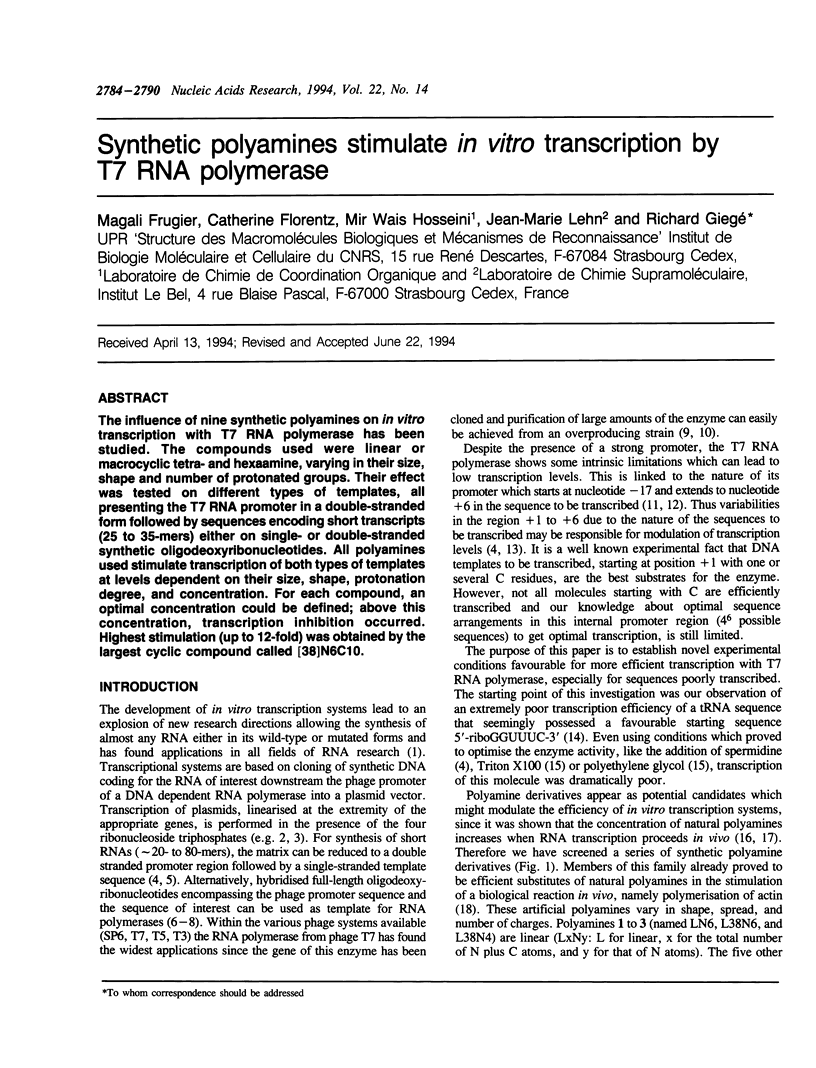

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dock A. C., Lorber B., Moras D., Pixa G., Thierry J. C., Giégé R. Crystallization of transfer ribonucleic acids. Biochimie. 1984 Mar;66(3):179–201. doi: 10.1016/0300-9084(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Musier-Forsyth K., Schimmel P. Small RNA helices as substrates for aminoacylation and their relationship to charging of transfer RNAs. Eur J Biochem. 1992 Jun 1;206(2):315–321. doi: 10.1111/j.1432-1033.1992.tb16929.x. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Schimmel P. Aminoacylation of RNA minihelices with alanine. Nature. 1989 Feb 2;337(6206):478–481. doi: 10.1038/337478a0. [DOI] [PubMed] [Google Scholar]
- Frugier M., Florentz C., Giegé R. Anticodon-independent aminoacylation of an RNA minihelix with valine. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3990–3994. doi: 10.1073/pnas.89.9.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugier M., Florentz C., Giegé R. Efficient aminoacylation of resected RNA helices by class II aspartyl-tRNA synthetase dependent on a single nucleotide. EMBO J. 1994 May 1;13(9):2218–2226. doi: 10.1002/j.1460-2075.1994.tb06499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giege R., Moras D., Thierry J. C. Yeast transfer RNAasp: a new high-resolution x-ray diffracting crystal form of a transfer RNA. J Mol Biol. 1977 Sep;115(1):91–96. doi: 10.1016/0022-2836(77)90248-0. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Ikeda R. A., Ligman C. M., Warshamana S. T7 promoter contacts essential for promoter activity in vivo. Nucleic Acids Res. 1992 May 25;20(10):2517–2524. doi: 10.1093/nar/20.10.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen E. D., Durbin R. K., Risman S. S., McAllister W. T. Specific contacts between the bacteriophage T3, T7, and SP6 RNA polymerases and their promoters. J Biol Chem. 1991 Jan 5;266(1):645–651. [PubMed] [Google Scholar]
- Lehman N., Joyce G. F. Evolution in vitro: analysis of a lineage of ribozymes. Curr Biol. 1993;3(11):723–734. doi: 10.1016/0960-9822(93)90019-k. [DOI] [PubMed] [Google Scholar]
- Martin C. T., Muller D. K., Coleman J. E. Processivity in early stages of transcription by T7 RNA polymerase. Biochemistry. 1988 May 31;27(11):3966–3974. doi: 10.1021/bi00411a012. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatché N. Polyamines stimulate DNA-dependent RNA synthesis catalyzed by vaccinia virus. Biochim Biophys Acta. 1985 Nov 13;826(2-3):113–120. doi: 10.1016/0167-4781(85)90116-2. [DOI] [PubMed] [Google Scholar]
- Oriol-Audit C., Hosseini M. W., Lehn J. M. 'Superpolyamines'. Macrocyclic polyamines induce highly efficient actin polymerization. Eur J Biochem. 1985 Sep 16;151(3):557–559. doi: 10.1111/j.1432-1033.1985.tb09139.x. [DOI] [PubMed] [Google Scholar]
- Patra D., Lafer E. M., Sousa R. Isolation and characterization of mutant bacteriophage T7 RNA polymerases. J Mol Biol. 1992 Mar 20;224(2):307–318. doi: 10.1016/0022-2836(92)90996-w. [DOI] [PubMed] [Google Scholar]
- Perret V., Garcia A., Puglisi J., Grosjean H., Ebel J. P., Florentz C., Giegé R. Conformation in solution of yeast tRNA(Asp) transcripts deprived of modified nucleotides. Biochimie. 1990 Oct;72(10):735–743. doi: 10.1016/0300-9084(90)90158-d. [DOI] [PubMed] [Google Scholar]
- Peterson E. T., Blank J., Sprinzl M., Uhlenbeck O. C. Selection for active E. coli tRNA(Phe) variants from a randomized library using two proteins. EMBO J. 1993 Jul;12(7):2959–2967. doi: 10.1002/j.1460-2075.1993.tb05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudinger J., Florentz C., Dreher T., Giegé R. Efficient mischarging of a viral tRNA-like structure and aminoacylation of a minihelix containing a pseudoknot: histidinylation of turnip yellow mosaic virus RNA. Nucleic Acids Res. 1992 Apr 25;20(8):1865–1870. doi: 10.1093/nar/20.8.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa R., Chung Y. J., Rose J. P., Wang B. C. Crystal structure of bacteriophage T7 RNA polymerase at 3.3 A resolution. Nature. 1993 Aug 12;364(6438):593–599. doi: 10.1038/364593a0. [DOI] [PubMed] [Google Scholar]
- Sousa R., Patra D., Lafer E. M. Model for the mechanism of bacteriophage T7 RNAP transcription initiation and termination. J Mol Biol. 1992 Mar 20;224(2):319–334. doi: 10.1016/0022-2836(92)90997-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Dunn J. J. Organization and expression of bacteriophage T7 DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):999–1007. doi: 10.1101/sqb.1983.047.01.114. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Polyamine requirement for efficient translation of amber codons in vivo. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7087–7091. doi: 10.1073/pnas.79.23.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990 Aug 3;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Wyatt J. R., Chastain M., Puglisi J. D. Synthesis and purification of large amounts of RNA oligonucleotides. Biotechniques. 1991 Dec;11(6):764–769. [PubMed] [Google Scholar]