Abstract
The concept that activation of cellular pathways of programmed cell death (PCD) may lead to the death of neurons has been an important hypothesis for adult neurodegenerative diseases. For Parkinson's disease (PD), up until now, the evidence for this hypothesis has largely been of two types: clear evidence of a role for PCD in neurotoxin models of the disease, and somewhat controversial evidence from human postmortem studies. With the rapid pace of discoveries in recent years of the genetic basis of PD, a new form of evidence has emerged. The prevailing concept of the role for PCD in PD has been that its mediators are ‘downstream’ effectors of more proximate and specific causes related to genetic or environmental factors. However, recent studies of three genes which cause autosomal recessive forms of parkinsonism, parkin, PTEN-induced kinase, and DJ-1, suggest that they may have more intimate relationships with the mediators of PCD and that loss-of-function mutations may result in an increased propensity for neurons to die. Intriguingly, independent studies of the function of these genes have suggested that they may share roles in regulating survival signaling pathways, such as those mediated by the survival signaling kinase Akt. Further elucidation of these relationships will have implications for the pathogenesis and neuroprotective treatment of PD.
Keywords: Akt, apoptosis, DJ-1, parkin, PINK-1
The rise in the past 15 years or so of the concept that programmed cell death (PCD) may play a role in the pathogenesis of neurodegenerative disease has led to a paradigm shift in thinking about approaches to neuroprotection in these disorders. With the recognition of a possible role for PCD came the realization that even in the absence of a specific understanding of proximate causes and even in the presence of diverse proximate causes, it may be possible to abrogate the neuron death that is the core pathology of these diseases by targeting the shared pathways of PCD.
The evidence of a role for PCD among these diseases, most notably Alzheimer's disease, Parkinson's disease (PD), motor neuron disease, and Huntington's disease, varies in its strength and is in all cases incomplete. There has been a concern that targeting PCD for any of these disorders may be aimed too far ‘downstream’ to retain sufficient cellular health and function to offer clinical benefit. Furthermore, although the pathways of PCD are widely shared among neuron death scenarios of various causes, they are also highly diverse and redundant, such that blocking any one of them alone may be futile. Given all of these caveats, it is perhaps not surprising that there has yet to emerge for any of these diseases a clinically effective neuroprotection based on targeting the pathways of PCD. Nevertheless, for some of these disorders, very impressive neuroprotective capabilities have been demonstrated in a variety of animal models. Therefore, the inability thus far to derive effective neuroprotective approaches may be as much a failure of effective implementation as a failure of the fundamental strategy. There remains good reason to hope that with increased knowledge of PCD pathways, neuroprotection may be possible.
For PD in particular, the evidence that PCD plays a role in pathogenesis has, up until recently, largely been of two types. First, in diverse animal models of PD, there is abundant evidence for the occurrence of apoptotic morphology and expression of PCD mediators. Among these models, very substantial neuroprotection has been achieved by either pharmacologic or genetic blockade of the pathways of PCD. Secondly, there is evidence from examination of human postmortem PD brain that apoptotic morphology may occur and that PCD mediators are expressed. These types of evidence have been reviewed elsewhere (Burke 2007) and will not be further considered here; it will suffice to say that these forms of evidence, while suggestive, are not definitive.
Over the past 10 years, there has been a remarkable growth in our knowledge of the genetic basis of parkinsonism (see reviews by (Moore et al. 2005; Cookson 2005). These discoveries have provided a third form of evidence that suggests a role for PCD in PD, because a number of the genes discovered appear to regulate these pathways. While a role for PCD ‘downstream’ of proximate genetic causes would not be unexpected, there is emerging evidence that this scheme may be an oversimplification; there may, in some cases, be more intimate relationships between the genetic abnormalities and the mediators of PCD, such that these mediators act in a proximate role.
The purpose of this review will therefore be twofold. We will consider the evidence that PCD mediators are operative ‘downstream’ of known genetic causes, and in this respect play a role like that postulated for non-genetic forms of PD, in which environmental factors have been postulated to participate. More importantly, we will consider evidence that some of the genetic causes of parkinsonism directly and primarily result in dysregulation of the pathways of PCD. This latter type of evidence is best developed for the autosomal recessive forms of parkinsonism, so they will be our focus. For the purposes of this review, we will reserve use of the term ‘Parkinson's disease’ for those conditions in which substantia nigra dopamine neuron loss is accompanied by Lewy body pathology (Gibb and Lees 1988), although this requirement is controversial (Calne and Mizuno 2004). Nevertheless, as Lewy body pathology is rare in cases associated with parkin mutations, and the brain pathology remains unknown in cases associated with PTEN (phosphatase and tensin homolog deleted on chromosome ten)-induced kinase (PINK1) and DJ-1 mutations, the clinical phenotype of these conditions will herein be referred to as ‘parkinsonism.’
The molecular pathways of PCD: an overview
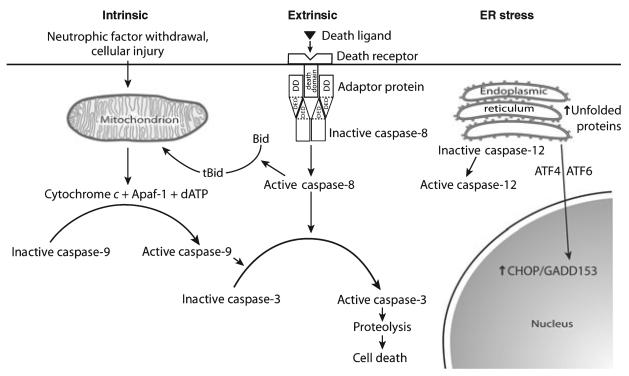
We will herein refer to many of the molecular mediators of PCD, so we will initially review briefly the principal mechanisms. There are three principal pathways by which the molecular events of PCD can be initiated: by the intrinsic and extrinsic pathways and by endoplasmic reticulum (ER) stress (Fig. 1). The schematic depicted in Fig. 1 is an oversimplification, as many possible interactions between these three pathways are not shown, but it serves as a useful organizational framework. The main focus of attention in this review will be on the intrinsic pathway, which has been more extensively investigated in PD and models thereof, but some observations relevant to the extrinsic pathway and ER stress will be touched upon briefly.
Fig. 1.
The three principal pathways for the activation of programmed cell death. DD, death domain; DED, death effector domain.
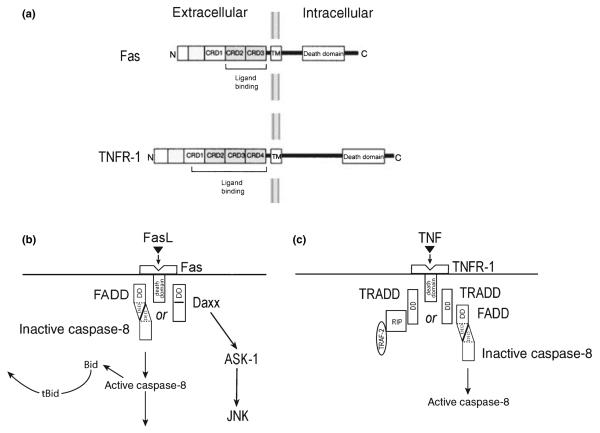
Programmed cell death is initiated in the extrinsic pathway by the binding of extracellular ligands to any of a number of receptors (death receptors) which belong to the tumor necrosis factor (TNF) superfamily (Locksley et al. 2001). Members of this family are characterized by a homotrimeric structure and extracellular cysteine-rich domains which interact with ligands that also form homotrimeric assemblies (Fig. 2). For the purposes of this review, we will restrict our attention to just two members of the TNF superfamily: Fas (also known as Apo-1 or CD95) and tumor necrosis factor receptor-1 (TNFR-1), as they have both been implicated in the pathogenesis of PD (Burke 2007). These two members of the TNF superfamily are characterized by the presence of intracellular death domains (DD), which mediate homotypic interactions with other proteins containing DDs, which, in turn, mediate PCD signaling (Fig. 2). Following the binding of the Fas ligand to the Fas receptor, signaling is mediated by a variety of pathways (Choi and Benveniste 2004), but two in particular have been implicated in cell death. In the first, a homotoypic interaction occurs between the intracellular DD of Fas and the DD of Fas-associated protein with DD (FADD). FADD contains at its N-terminus a death effector domain (DED) (Tibbetts et al. 2003), which mediates an interaction with a similar DED in pro-caspase 8 (Fig. 2). This interaction permits autocleavage, and activation, of caspase 8 by induced proximity. This complex, comprised of ligand, receptor, FADD and pro-caspase 8 is referred to as the death-inducing signaling complex (Wajant 2003). The cleavage and activation of caspase 8 mediates death by two distinct mechanisms. In some cells (Type I), the induced caspase 8 activation is sufficient to mediate cleavage of downstream caspases, such as caspase 3, leading to cell death (Fig. 2). In other cells (Type II) caspase 8 cleaves a Bcl-2 homology (BH3) domain-only member of the Bcl-2 family, Bid. This truncated form (tBid) can induce mitochondrial release of cytochrome c and other mitochondrial mediators of cell death (Figs 1 and 2). An alternate mechanism for mediation of cell death by Fas is initiated by an interaction between the DD of Fas and that of the protein Daxx (Yang et al. 1997). Unlike FADD, Daxx does not contain a DED. This interaction between Fas and Daxx results in the activation of apoptosis signaling kinase (ASK1), a mitogen-activated protein kinase (MAPK) kinase kinase (Ichijo et al. 1997), which, in turn, activates c-jun N-terminal kinase (JNK). Daxx has been suggested to interact with DJ-1 (Junn et al. 2005), which is mutated in some autosomal recessive forms of parkinsonism, although the interaction has yet to be identified in vivo.
Fig. 2.
Mediators of the extrinsic pathway of PCD by Fas and TNFR-1 signaling. (a) The protein domain structures of Fas and TNFR-1. Each receptor has cysteine-rich domains (CRD) in the ligand binding region, characteristic of members of the TNF superfamily of receptors. There is a death domain in the intracellular portion of each receptor. (b) Fas death signaling patheways. (c) TNFR-1 signaling pathways. See text for details.
Cell death signaling through TNFR-1 can also proceed by alternate pathways, initiated by interaction with TNFR-1-associated death domain protein (TRADD). Unlike FADD, TRADD does not contain a DED. Through its DD, it interacts with FADD, resulting in the activation of caspase 8, as described for Fas. An alternate interaction may occur, however, between TRADD, receptor-interacting protein kinase and TNFR-associated factor-2 (TRAF2). This interaction results in the activation of the nuclear factor κB (NF-κB) pathway and suppression of apoptosis (Wajant 2003).
Another pathway for the activation of PCD is the ER stress pathway (Fig. 1) (Ron and Walter 2007). The ER provides a cellular compartment for the post-translational modification and folding of membrane and secretory proteins. If prompt protein folding cannot be achieved within the ER, as a result of protein mutations, or cellular stresses (such as ER Ca2+ depletion or inhibition of glycosylation), or protein overload, then there will be a prolonged exposure of internal protein hydrophobic domains, with potential toxicity to the cell. The cell has developed multiple mechanisms to deal with this problem, including increased production of protein-folding chaperones, suppression of protein translation, and ER-associated protein degeneration by the proteasome (Mori 2000). These diverse cellular mechanisms are named the unfolded protein response (Ron and Walter 2007). If, however, these efforts to maintain cellular homeostasis fail, the ER transmits molecular signals to initiate PCD. The molecular mechanisms by which ER stress mediates cell death are not well understood (Ron and Walter 2007). Two transcription factors, ATF4 and ATF6 (Fig. 1) induce expression of another transcription factor, CHOP/GADD153, which, in turn, mediates PCD (Oyadomari and Mori 2004), in part by increasing oxidation within the ER (Marciniak et al. 2004). There is also evidence that CHOP decreases expression of the anti-apoptotic protein Bcl-2 (McCullough et al. 2001). ER stress also leads to PCD by the activation of caspase 12 (Nakagawa et al. 2000; Rao et al. 2001) which is localized to the ER. The downstream effects of caspase 12 activation are not fully known, but may result in a cytochrome c-independent activation of caspase 9 (Momoi 2004). While caspase 12 has been clearly demonstrated to be a mediator of ER stress-induced apoptosis in rodent cells, it is questionable whether it plays such a role in human cells, because the human gene contains a frame shift mutation with a premature stop codon (Fischer et al. 2002). However, recent evidence suggests that human caspase 4 may function like rodent caspase 12 to mediate ER stress-related apoptosis (Hitomi et al. 2004). A possible role for ER stress has been suggested for inherited parkinsonism as a result of loss-of-function mutations in the parkin gene (Ishikawa and Tsuji 1996; Kitada et al. 1998), as discussed below.
In the intrinsic pathway of PCD, the pivotal event that commits the cell to death is the release of cytochrome c and other protein mediators of PCD from the mitochondrion (Figs 1 and 3). This event is controlled by members of the Bcl-2 family, of which there are now over 20 members identified (Scorrano and Korsmeyer 2003). Among the members of the Bcl-2 family, there are competing relationships among anti- and pro-apoptotic members, and the fate of the cell is determined by which is predominant. All members of the Bcl-2 family contain at least one of four conserved domains called BH domains (Adams and Cory 1998). Most anti-apoptotic Bcl-2 proteins contain at least BH1 and BH2; the two prototypic examples, Bcl-2 and Bcl-XL, contain all four. There are two classes of pro-apoptotic Bcl-2 family members. The ‘multidomain’ members, such as Bax and Bak, contain BH1, 2, and 3 domains. The ‘BH3 only’ members, such as Bid, Bim, and Bad, contain only BH3. The anti-apoptotic proteins Bcl-2 and Bcl-XL both contain C-terminal membrane anchor domains, which localize them to the outer mitochondrial membrane as well as the ER and the nuclear membrane. At the mitochondrial outer membrane, these proteins protect against cytochrome c release and the initiation of the downstream cell death cascade.
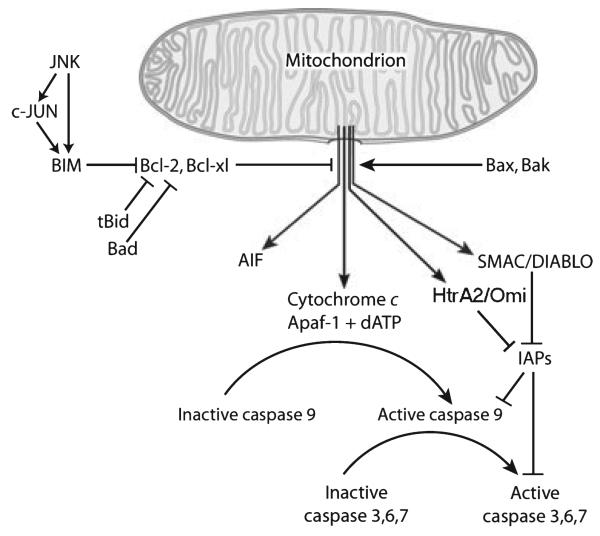
Fig. 3.
The intrinsic pathway of programmed cell death.
This protective effect of the anti-apoptotic Bcl-2 proteins is antagonized by interaction with Bax or Bak (Fig. 3). This interaction allows the release of cytochrome c and other pro-apoptotic proteins from the mitochondrion, and initiation of the cell death cascade. Bax is normally located in the cytoplasm; with the onset of apoptotic stimulation it translocates to the mitochondrion to initiate cytochrome c release. Bak is normally expressed on the outer membrane of the mitochondrion. Activation of Bax or Bak is absolutely required for apoptosis to occur, because cells which carry a double homozygous deletion of both are resistant to a wide variety of inducers of apoptosis (Wei et al. 2001), Bax and Bak are activated by BH3 only pro-apoptotic members of the Bcl-2 family. These proteins are, in turn, activated by death-inducing signals by a variety of post-translational modifications. Bid, for example, is activated by cleavage by caspase 8, as previously described. Bad is activated by alteration of its phosphorylation status (Datta et al. 2000). Activation of Bax or Bak by BH3 only proteins results in their homo-oligomerization, and by mechanisms which are not completely understood, permeabilization of the outer mitochondrial membrane (Scorrano and Korsmeyer 2003).
In the intrinsic pathway, the release of cytochrome c results in the activation of a caspase cascade (Fig. 3). In mammalian cells, there are now 14 identified caspases (Thornberry and Lazebnik 1998). All of these proteases contain a cysteine at their active site, and they all cleave on the carboxyl side of an aspartate (thus, ‘caspases’). Among the caspases which play a role in PCD, there are two groups: ‘initiator’ caspases (2, 8, 9, and 10) and ‘effector’ caspases (3, 6, and 7). These two groups are distinguished by their N-terminals. Initiator caspases contain long N-terminal regions which are involved in the regulation of their activation; for example, the DED in caspase 8 (Fig. 2). The effector caspases contain only short (20–30 amino acids) N-terminal prodomains. All caspases are produced by cells as inactive zymogens. All caspases are activated by proteolytic cleavage (by an initiator caspase) to produce a large (~20 kDa) and a small (~10 kDa) subunit, which then associate to form a heterodimer. These heterodimers, in turn, associate to form a heterotetramer consisting of two p20/p10 heterodimers, which comprises the active form of the enzyme.
Following the release of cytochrome c from mitochondria, caspase 9 becomes activated upon association with a cytoplasmic protein apoptosis-protease-activating factor-1, in the presence of dATP, to form a ~1.4 MDa complex called ‘the apoptosome’ (Riedl and Shi 2004) (Fig. 3). Activated caspase 9 then cleaves and activates caspase 3 and other effector caspases. Effector caspases then systematically cleave select cellular proteins to either eliminate their function, or, alternatively, to activate proteins which then become pro-apoptotic.
The activity of caspases is regulated by inhibitor-of-apoptosis proteins (IAPs), of which there are eight known in mammalian cells (Riedl and Shi 2004) (Fig. 3). These proteins all contain regions (baculoviral IAP repeats) which bind to and inhibit select caspases. In mammalian cells, caspase 3, 7, and 9 are all subject to IAP inhibition. This inhibition of IAPs can, in turn, be blocked by a family of proteins that contain a tetrapetide motif which binds to, and blocks, the baculoviral repeats of IAPs. In mammals, the founding member of this family was found to be released by mitochondria and termed the ‘second mitochondria-derived activator of caspases’ (SMAC) or direct IAP-binding protein (Chai et al. 2000; Shi 2002) (Fig. 3). Another protein that is released from mitochondria upon activation of cell death pathways is HtrA2 (or (Omi) (Suzuki et al. 2001). The mature form of HtrA2 contains at its N-terminal a tetrapeptide IAP binding motif, homologous to that of SMAC, and, like SMAC, HtrA2 binds to and inhibits the caspase-blocking properties of x-chromosome-linked inhibitor of apoptosis. In addition to its ability to activate caspases by x-chromosome-linked inhibitor of apoptosis inhibition, HtrA2 has serine protease activity (Suzuki et al. 2001). Interestingly, loss-of-function mutations in HtrA2/Omi have been identified in some apparently sporadic cases of PD (Strauss et al. 2005). It would seem paradoxical that loss-of-function mutations in a molecule which activates caspases would be implicated in neurode-generation; the paradox is explained by the observation that loss-of-function affects the serine protease activity, which, in turn, causes mitochondrial dysfunction, as discussed below in relation to PINK1.
In addition to cytochrome c and SMAC/direct IAP-binding protein, mitochondria release a protein apoptosis-inducing factor (AIF) in response to cell death stimuli (Susin et al. 1999). When first identified, it was shown that this protein is capable of causing nuclear condensation and DNA fragmentation, as occurs in apoptosis, and that none of these effects are blocked by caspase inhibitors (Susin et al. 1999). More recently, it has been shown that, by a mechanism not yet understood, AIF release from the mitochondrion is mediated by the DNA repair enzyme poly (ADP-ribose) polymerase I; poly (ADP-ribose) polymerase inhibition or genetic deletion prevent mitochondrial AIF release, translocation to the nucleus, and cell death (Yu et al. 2002). The demonstration of this pathway indicates that there are important non-caspase-dependent pathways to cell death.
While there are likely to be multiple upstream pathways acting upon the pro-apoptotic BH3 only proteins to initiate mitochondrial release of death mediators, one such pathway which has been the focus of extensive investigation involves the activation of the transcription factor c-jun by phosphorylation (reviewed in Silva et al. 2005a). Phosphorylation and activation of c-jun is mediated by JNK, which, in turn, is activated by phosphorylation by a complex kinase cascade which includes the mixed lineage kinases (MLKs) (Silva et al. 2005a). There is abundant evidence that this kinase cascade plays an important role in initiating PCD (Wang et al. 2004). In relation to PD specifically there are now numerous experiments utilizing either pharmacologic or genetic approaches to blocking the JNK/c-jun kinase cascade which have demonstrated efficacy in preventing dopamine neuron death in animal models of parkinsonism (Silva et al. 2005a). This signaling pathway acts upon BH3 only mediators in multiple ways to initiate death. There is evidence that c-jun induces the expression of Bim (Putcha et al. 2001; Whitfield et al. 2001) (Fig. 3). In addition, JNK not only phosphorylates and activates c-jun, but it also phosphorylates and activates Bim directly (Lei and Davis 2003) (Fig. 3). In addition, JNK phosphorylates and directly activates Bad (Donovan et al. 2002).
Akt: a multi-faceted inhibitor of PCD
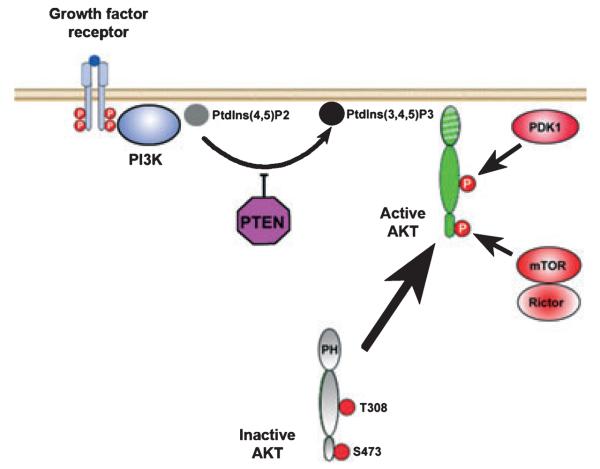
Akt (AKR/J mouse; T-8 strain) is a serine/threonine kinase with diverse roles related to the regulation of cell growth, proliferation, migration, glucose metabolism, transcription, protein synthesis, angiogenesis, and cell survival (Brazil and Hemmings 2001). For the purpose of this review, we will focus only on its role as a mediator of cell survival by inhibition of apoptosis; for more general treatments, the reader is referred to excellent reviews (Brazil and Hemmings 2001; Vivanco and Sawyers 2002; Brazil et al. 2004). Activation of Akt occurs following the binding of a protein growth factor to its receptor on the surface of the cell (See Fig. 4). Ligand binding induces autophosphorylation of tyrosine residues in the cytoplasmic portion of the receptor, resulting in the recruitment and activation of phosphatidylinositol 3-kinase (PI3K). PI3K phosphorylates phosphatidylinositol 4,5 biphosphate to phosphatidylinositol 3,4,5 triphosphate [PtdIns (3,4,5)P3], which mediates localization of Akt to the inner surface of the cell membrane by interaction with its pleckstrin homology domain. PtdIns(3,4,5)P3 can be de-phosphorylated by PTEN, which thus serves as a negative regulator of Akt activation. Once localized to the inner surface of the cell membrane, Akt is activated by phosphorylation at two critical residues: Thr308 in the kinase domain and Ser473 in the hydrophobic motif. The Thr308 kinase is 3-phosphoinsitide-dependent kinase 1; like Akt it is localized to the inner surface of the cell membrane by an interaction between PtdIns(3,4,5)P3 and its pleckstrin homology domain (Hanada et al. 2004). The kinase for the Ser473 residue in the hydrophobic motif had been elusive and the subject of debate for many years, but recently Sarbassov et al. (2005) have provided compelling evidence that it is a complex consisting of mammalian target of rapamycin, G protein β-subunit-like protein, and rictor (Bayascas and Alessi 2005).
Fig. 4.
Akt signaling pathways. Ligand binding induces autophosphorylation of the cytoplasmic portion of the receptor, resulting in the recruitment and activation of PI3K. PI3K phosphorylates phosphatidylinositol 4,5 biphosphate [PtdIns(4,5)P2] to phosphatidylinositol 3,4,5 triphosphate [PtdIns (3,4,5)P3], which mediates localization of Akt to the inner surface of the cell membrane by interaction with its pleckstrin homology (PH) domain. PTEN acts as a negative regulator of Akt activation. Once localized to the inner surface of the cell membrane, Akt is activated by phosphorylation at two critical residues: Thr308 in the kinase domain and Ser473 in the hydrophobic motif. The Thr308 kinase is 3-phosphoinsitide-dependent kinase 1 (PDK1) (Hanada et al. 2004). The kinase for the Ser473 residue is a complex consisting of mammalian target of rapamycin (mTOR), G protein β-subunit-like protein (GβL), and rictor (Bayascas and Alessi 2005; Sarbassov et al. 2005).
The first evidence that PI3K/AKT signaling plays a role in supporting the survival of neurons was obtained in studies of nerve growth factor-treated PC12 cells (Yao and Cooper 1995). Subsequently, other investigators confirmed that PI3K signaling could prevent cell death in a variety of other tissue culture models utilizing cerebellar, sympathetic (Crowder and Freeman 1998), sensory, cortical, and motor neurons (reviewed in Kaplan and Miller 2000). A role for Akt in mediating neuronal survival was first demonstrated by Dudek et al. (1997) in a primary postnatal cerebellar granule cell culture model, in which apoptosis is induced by either low potassium or growth factor withdrawal (D'Mello et al. 1993). Since these initial observations, a large number of studies have demonstrated that Akt protects from apoptosis as a result of wide variety of death-inducing stimuli, including the withdrawal of growth factors, UV irradiation, matrix detachment, cell cycle disturbance, DNA damage, and treatment of cells with anti-Fas antibody (reviewed in Datta et al. 1999). Conversely, Luo et al. (2003) have shown that in a variety of tissue culture models, deactivation of Akt accompanies cell death induced by many different agents.
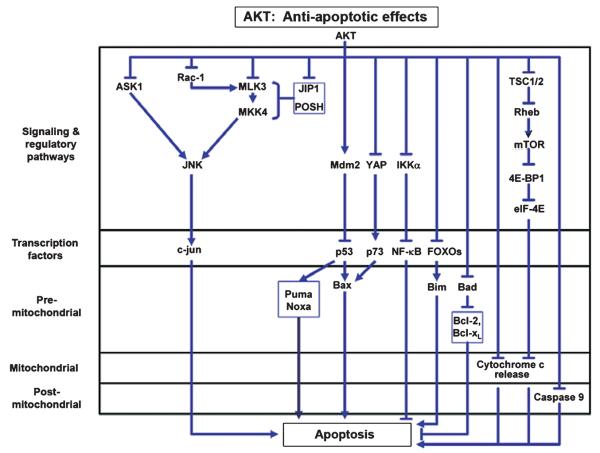
Akt has been demonstrated to inhibit apoptosis by many mechanisms affecting diverse apoptotic pathways at multiple levels, from upstream signaling pathways which regulate transcriptional activity, to downstream targets, such as caspase 9 (Fig. 5). It is beyond the scope of the present review to consider all of these mechanisms in detail. For a more thorough overview, the reader is referred to several comprehensive reviews (Datta et al. 1999; Brunet et al. 2001; Downward 2004). For the purposes of this review, we will illustrate some of the mechanisms by which Akt acts to inhibit pro-apoptotic transcriptional activation by c-jun. In addition, we will briefly outline the mechanism by which Akt activates eIF-4E in a recently defined, novel role as an inhibitor of apoptosis by regulating mitochondrial cytochrome c release.
Fig. 5.
Anti-apoptotic mechanisms of Akt. Akt has been described to inhibit apoptosis at multiple levels: signaling pathways, transcription factors, and, in the intrinsic pathway of PCD, at pre-mitochondrial, mitochondrial, and post-mitochondrial levels. Detailed mechanisms for some of these pathways are presented in the text. For more complete reviews, see (Datta et al. 1999; Brunet et al. 2001; Downward 2004).
Akt negatively regulates the phosphorylation and activation of c-jun by a number of mechanisms (Fig. 5). In some cellular contexts, the c-jun phosphorylation cascade is activated by the small GTP binding proteins Rac1 or Cdc42. Both of these signaling proteins have been shown to participate in nerve growth factor withdrawal induced apoptosis in sympathetic neurons (Bazenet et al. 1998) and in PC12 cells (Xu et al. 2001). Akt has been shown to phosphorylate Rac1 at Ser71, and thereby reduce its ability to bind GTP, as required for activation (Kwon et al. 2000). Downstream of Rac1 and Cdc42, Akt also negatively regulates the MLK3. Barthwal and colleagues have demonstrated that Akt phosphorylates MLK3, resulting in diminished JNK activation, and decreased cell death. ASK1, like MLK3, is a MAPK kinase kinase; it activates both JNK and p38 (Ichijo et al. 1997). Kim et al. (2001) have demonstrated that Akt binds to ASK1, phosphorylates it, and thereby reduces its kinase activity. This modification of ASK1 results in reduced activation of JNK, and a reduction of apoptosis in cell lines. The scaffold proteins JNK interacting protein 1 and POSH (plenty of Src homology 3) are also Akt targets. Akt1 binds to JNK interacting protein 1 in primary neurons and inhibits its ability to potentiate JNK activation (Kim et al. 2002). Similarly, Figueroa et al. (2003) have shown that Akt2 binds to POSH and negatively regulates its ability to activate JNK. This inhibition appears to be mediated by phosphorylation of MLK3, resulting in its dissociation from the POSH signaling complex.
Akt also inhibits apoptosis at the post-transcriptional level by phosphorylation of pro-apoptotic proteins, including premitochondrial mediators, such as Bad (Datta et al. 1997) and post-mitochondrial mediators such as caspase 9 (Zhou et al. 2000). More recently, an anti-apoptotic effect of Akt has been demonstrated to be mediated through activation of mTor (McCormick 2004; Wendel et al. 2004). Wendel et al. (2004) have demonstrated in a murine lymphoma model that the anti-apoptotic effects of Akt can be blocked by rapamycin, an inhibitor of mTor. The anti-apoptotic effect of Akt could be restored by eIF-4E, and this effect was not blocked by rapamycin, indicating that eIF-4E is downstream. While eIF-4E is best known for its role as an elongation initiation factor for the translation of protein from mRNA, it has more recently been shown to play a role in blocking apoptosis as well. Li et al. (2003) have demonstrated that eIF-4E is capable of blocking apoptosis induced by c-myc. They show that eIF-4E increases both the abundance and translation of the mRNA of the anti-apoptotic Bcl-XL, and thereby decreases mitochondrial cytochrome c release (Li et al. 2003). Activation of eIF-4E by mTor is mediated by phosphorylation and inhibition of translation initiation factor eIF-4E binding protein (4E-BP1), a negative regulator of eIF-4E (reviewed in Schmelzle and Hall 2000; Manning and Cantley 2003). Activation of mTor by Akt is achieved indirectly by Akt phosphorylation and inhibition of tuberin [tuberous sclerosis complex 2 (TSC2)]. Tuberin/TSC2 functions in a complex with hamartin/TSC1 as a GTPase-activating protein to inhibit a Ras-related small GTPase Rheb (Manning and Cantley 2003). Rheb is positive regulator of Tor signaling. Thus, Akt ultimately activates mTor by blocking negative regulation of Rheb (Manning and Cantley 2003).
PCD and new discoveries in the genetics of parkinsonism
Parkin
Mutations in the gene parkin (PARK2) were first identified in Japanese families with an autosomal recessive juvenile onset form of PD (ARJP) and they are the most common cause of ARJP worldwide (Jain et al. 2005). ARJP patients show all the motor features of parkinsonism, and they respond to treatment with levodopa. At postmortem, there is loss of dopamine neurons of the substantia nigra, and noradrenergic neurons of the locus ceruleus. The first mutations identified were deletion mutations (Kitada et al. 1998) and subsequently a large variety of mutations were discovered, including frame shift and point mutations. These various mutations result in loss of parkin function, which has been shown to be that of an E3 ubiquitin ligase (Shimura et al. 2000). Thus, parkin has been postulated to participate in the ubiquitination, and subsequent degradation by the proteasome, of specific protein substrates. A number of these substrates have been identified, including CDCrel-1 (Zhang et al. 2000), parkin-associated endothelin receptor-like receptor (Pael-R) (Imai et al. 2001), a glycosylated form of synuclein (Shimura et al. 2001), synphilin (Chung et al. 2001), cyclin E (Staropoli et al. 2003), α/β tubulin (Ren et al. 2003), and p38 subunit of aminoacyl-tRNA synthetase complex (Ko et al. 2005). It has been hypothesized that the accumulation, or loss of regulation, of such substrates because of loss of parkin function is responsible for dopamine neuron death. However, among these substrates, only p38 accumulates in the ventral midbrain of parkin null mice (Ko et al. 2005). The possible role of p38 in dopamine neuron death is therefore of considerable interest.
Although the precise role of the ubiquitin ligase activity of parkin in the maintenance of dopamine neuron viability remains to be defined, there is abundant evidence that parkin can provide neuroprotection by blocking PCD. A number of in vitro studies have demonstrated protection against cellular toxins. Darios et al. (2003) have demonstrated that PC12 cells stably transfected with parkin are resistant to apoptosis induced by ceramide. This protective ability was dependent on the E3 ubiquitin ligase activity of parkin, as it was abrogated by disease-causing mutations. Jiang et al. (2004) showed that human neuroblastoma cells (SH-SY5Y) stably over-expressing parkin are resistant to apoptosis induced by either dopamine or 6-hydroxydopamine. This protective effect was accompanied by an attenuated induction of JNK and caspase 3, two apoptotic mediators. As in the study by Darios et al. (2003), the anti-apoptotic effect was abrogated by mutations which disrupt the E3 ubiquitin ligase activity of parkin. Machida et al. (2005) demonstrated that even in the absence of neurotoxin exposure, anti-sense knockdown of parkin in SH-SY5Y cells results in apoptotic death, with characteristic morphologic changes and activation of the caspases. The potential clinical relevance of these studies is suggested by the investigations of Del Rio and colleagues who showed that lymphocytes derived from patients homozygous for loss-of-function parkin mutations have increased sensitivity to dopamine, 6-hydroxydopamine, and iron-mediated apoptosis (Jimenez et al. 2004).
These in vitro observations, indicating an anti-apoptotic function of parkin, have been supported by in vivo observations made in Drosophila. Drosophila null for the parkin homolog demonstrate a diminished lifespan and locomotor deficits which are due to the apoptotic death of select muscle groups (Greene et al. 2003; Pesah et al. 2004). In addition, Pesah et al. (2004) have noted an increased sensitivity of the parkin homolog-null flies to the herbicide paraquat. A possible mechanism for this increased susceptibility to apoptosis has been suggested by studies of Cha et al. (2005) who determined that in the absence of the parkin homolog, there is an up-regulation of JNK. In these flies dopamine neurons are shrunken, show decreased tyrosine hydroxylase expression, and increased expression of phosphorylated JNK. The reduced size of the dopamine neurons could be reversed by expression of a dominant negative form of JNK. Further experiments demonstrated that parkin negatively regulates the JNK signaling pathway in an E3 ligase-dependent manner (Cha et al. 2005).
Some of the putative substrates of parkin have been implicated in specific pathways of PCD. As discussed above, one important pathway is that induced by ER stress. Imai et al. (2000) observed that unfolded protein stress within the ER, such as that induced by tunicamycin, results in up-regulation of parkin, and that parkin can protect cells from this death stimulus in tissue culture. This protective ability was abrogated by ARJP-causing mutations. These investigators further showed that one parkin substrate, Pael-R is a difficult to fold protein, and its over-expression results in the formation of aggregates and cell death. Parkin ubiquitinates Pael-R, suppresses the formation of Pael-R aggregates, and protects from cell death (Imai et al. 2001). Thus, one hypothesis of parkin function is that it protects dopamine neurons from ER stress-mediated apoptosis. The ability of Pael-R to induce selective death of dopamine neurons has received support from studies of its expression in Drosophila (Yang et al. 2003). Co-expression of parkin-induced degradation of Pael-R, and mitigated its cellular toxicity. Conversely, reduction of expression of the endogenous Drosophila parkin homolog by RNA interference accelerated the neurodegeneration induced by Pael-R. Furthermore, in the fly model, over-expression of parkin suppressed toxicity as a result of α-synuclein. In these Drosophila studies, however, direct evidence that ER stress was induced by Pael-R or that cell death was mediated by ER stress was not presented. Evidence of the ability of Pael-R to induce dopamine neuron degeneration by PCD in vivo in mice has been presented by Kitao et al. (2007), utilizing simultaneous co-injection of multiple adenovirus vectors. In this study, increased expression of Pael-R by vector transfer resulted in the death of substantia nigra pars compacta dopamine neurons in parkin null, but not wildtype mice. Neuron death was blocked by increased expression of the ER chaperone ORP150. In this study, however, a direct role for CHOP/GADD153, a principal mediator of ER stress-induced PCD (Matsumoto et al. 1996; Zinszner et al. 1998) was not shown, nor was expression of the splice variant of x-box binding protein-1, a definitive marker for ER stress (Yoshida et al. 2001; Calfon et al. 2002). In addition, the known propensity of adenoviral vectors to induce neuroinflammation makes simultaneous injection of three vectors difficult to interpret. Thus, the role of Pael-R in the pathogenesis of ARJP awaits further confirmation. The possible role of ER stress-mediated cell death in dopamine neurons has received independent support from both in vitro (Ryu et al. 2002; Holtz and O'Malley 2003) and in vivo studies (Silva et al. 2005b) indicating that dopamine neuron death induced by 6-hydroxydopamine is due, at least in part, to the activity of the transcription factor CHOP/GADD153.
Another pathway in which parkin may protect from apoptosis is that mediated by cell cycle regulators. There is now abundant evidence that proteins which mediate passage through the cell cycle can be re-expressed in post-mitotic neurons and induce apoptosis (reviewed in Greene et al. 2004). Staropoli et al. (2003) have demonstrated that parkin functions in a protein complex which includes proteins hSel-10 and Cullin-1 to ubiquitinate and degrade the cell cycle regulator cyclin E. Cyclin E, like other cyclins, serves to regulate cyclin-dependent kinase-2 kinase, which regulates the G1 to S cell cycle transition. Staropoli et al. (2003) have found that down-regulation of parkin by use of short interfering RNAs (siRNAs) resulted in an accumulation of cyclin E in both embryonic cortical and dopaminergic mesencephalic neurons and an increased susceptibility to apoptosis induced by excitotoxicity. Conversely, increased parkin expression in cerebellar granule cells attenuated cyclin E accumulation and protected cells from kainic acid-induced apoptosis. The potential clinical relevance of their observations was supported by their finding that abnormal accumulation of cyclin E is detectable in human ARJP brains (Staropoli et al. 2003). These observations await confirmation by other investigators.
The effect of polyubiquitination on cellular target proteins depends on the nature of the ubiquitin chain formed (reviewed in Pickart and Fushman 2004). There are seven lysine residues in ubiquitin. K48-linked polyubiquitin chains target substrate protein for proteasomal degradation. K63-linked chains, however, do not act as proteolytic signals, but have diverse effects, related to DNA repair, kinase activity and cellular trafficking.
Particularly intriguing observations demonstrating a role for parkin in a proteasome-independent ubiquitination pathway have been made by Fallon et al. (2006) in relation to Eps15, a protein involved in endocytosis and trafficking of the epidermal growth factor receptor (EGFR). These investigators show that the ubiquitin-like domain (Ubl) of parkin does not interact with the S5a subunit of the proteasome. Instead, they find that the Ubl of parkin interacts with the ubiquitin-interacting motif of Eps15, and that wildtype parkin ubiquitinates Eps15. Both the interaction and the ubiquitination are abrogated by ARJP-associated mutations. Eps15 had previously been identified as an EGFR phosphorylation target; in conjunction with phosphorylation, it is also ubiquitinated. Fallon et al. (2006) find that epidermal growth factor treatment of cells increases the interaction of parkin and Eps15. This interaction, and the ubiquitination of Eps15, are associated with a delayed internalization of the EGFR, and a slower rate of its degradation. These investigators further demonstrate that downstream signaling of EGFR, the phosphorylation of Akt, is diminished in parkin-deficient cells. These authors therefore propose that loss of parkin may predispose SN dopamine neurons to degeneration as a result of down-regulation of PI3K/Akt signaling. This suggestion is especially intriguing, given the recent evidence that PD-causing mutations in another gene, DJ-1, as further discussed below, have also been proposed to negatively affect Akt signaling (Kim et al. 2005a; Yang et al. 2005). We have previously shown that mRNA expression of Akt1 in the ventral mesencephalon, unlike that in striatum and cortex, is sustained into adulthood, suggesting a physiologic role not only in development, as expected, but also in the maintenance of mature neurons (Ries et al. 2006). In addition, AAV vector-mediated expression of a constitutively active form of Akt has marked trophic effects on SN dopamine neurons of adult and aged mice, inducing neuronal enlargement, sprouting, and increased expression of tyrosine hydroxylase (Ries et al. 2006). The observations of Fallon et al. (2006) are of interest for the additional reason that epidermal growth factor has previously been shown to be neuroprotective in Parkinson models (Pezzoli et al. 1991; Iwakura et al. 2005). Thus, the interaction of parkin with Eps15 appears to be an important avenue for further study.
Another recently identified non-degradative ubiquitination by parkin regulates NF-κB signaling. Given the broad spectrum of cellular insults for which parkin provides neuroprotection, Henn et al. (2007) sought to identify general cell stress pathways regulated by parkin, and they found that parkin significantly increases the transcriptional activity of NF-κB. This NF-κB activating ability of parkin was abrogated by disease-causing mutations. They further demonstrated that the ability of parkin to provide neuroprotection was dependent on NF-κB activation. Activation was associated with ubiquitination of inhibitor of κB kinase γ and TRAF2, but did not lead to enhancement of their degradation. Instead, parkin ubiquitination was dependent on the ubiquitin K63 residue. These investigators therefore postulate that parkin mediates regulatory ubiquitination of inhibitor of κB kinase γ and TRAF2, resulting in the release of inhibitor of κB inhibition of NF-κB, and the up-regulation of NF-κB-regulated pro-survival genes (Henn et al. 2007).
In summary, while the anti-apoptotic effects of parkin well established, the precise mechanisms(s) remain unknown, and both degradative and non-degradative ubiquitination pathways remain under investigation.
PTEN-induced kinase 1
The second most common cause of ARJP are mutations in PINK1 (PARK6) (Valente et al. 2004). Given the evidence cited above that parkin may regulate Akt signaling, it is of interest that PINK1 was first identified as a gene up-regulated by exogenous expression of PTEN, a negative regulator of Akt signaling (Unoki and Nakamura 2001). Valente et al. (2001) first mapped PARK6 in a Sicilian family to chromosome 1p35-p36. Sequence analysis of candidate genes led to the identification in a Spanish family of a G309D substitution mutation at a highly conserved position in a putative kinase domain, and in two Italian families another substitution resulting in truncation of 145 C-terminal amino acids (Valente et al. 2004). These investigators showed that the putative kinase is localized to mitochondria and protects SH-SY5Y cells from proteasome inhibitor-induced apoptosis (Valente et al. 2004). This protective effect was abrogated by the G309D mutation. Beilina et al. (2005) demonstrated the kinase activity of PINK1 by use of an autophosphorylation assay, and they showed that the activity is diminished by disease-causing mutations.
The ability of wildtype PINK1 to protect from apoptotic stimuli has been confirmed by Petit et al. (2005) who showed that both basal and staurosporine-induced apoptosis in SH-SY5Y cells are reduced by wildtype PINK1. Over-expression of PINK1 also diminished both basal and staurosporine-induced activation of caspase 3. These anti-apoptotic effects of PINK1 were abrogated by disease-causing mutations (Petit et al. 2005). Deng et al. (2005) used the converse approach, to down-regulate PINK1 expression by siRNA, and they demonstrated that diminished expression of PINK1 in SH-SY5Y cells decreased their viability as a result of an increased amount of apoptosis. Insight into the anti-apoptotic mechanisms of PINK1 was provided by the studies of Pridgeon et al. (2007) who showed that the TNFR-associated protein 1 (TRAP1) is an endogenous substrate for PINK1. PINK1 and TRAP1 are co-localized in mitochondria, and the ability of PINK1 to phosphorylate TRAP1 is abrogated by disease-causing mutations. These investigators further show that PINK1 protects against oxidative stress-induced apoptosis, and this protective effect is dependent on phosphorylation of TRAP1.
Another candidate molecular partner for PINK1 in mediating its ability to protect cells from death is HtrA2/Omi (Plun-Favreau et al. 2007). Plun-Favreau and colleagues used a tandem affinity approach to demonstrate that PINK1 is a binding partner for HtrA2/Omi, and regulates its phosphorylation. The functional consequence of phosphorylation is to increase the serine protease activity of HtrA2/Omi. While the original identification of HtrA2/Omi demonstrated an ability to bind IAPs and induce cell death, its functional role may be context-dependent, because HtrA2/Omi null mice do not have a neuroprotection phenotype; on the contrary, they demonstrate neurodegeneration (Martins et al. 2004). Thus, the ability of PINK1 to enhance neuron survival may be mediated by its ability to regulate phosphorylation of HtrA2/Omi and up-regulate its serine protease activity, which appears to be important for the maintenance of mitochondrial morphology (Strauss et al. 2005).
A role for PINK1 in the regulation of apoptosis in vivo has been demonstrated in Drosophila independently by three groups of investigators. Strikingly, each group has demonstrated a genetic interaction between PINK1 and parkin. Park et al. (2006) demonstrated in PINK1 loss-of-function mutants that they have a diminished lifespan and an abnormal posture of the wings. The abnormal wing posture was associated with degeneration of flight muscles because of apoptosis. In addition, they observed a minor loss of dopamine neurons. In both flight muscles and dopamine neurons, degeneration was associated with morphologic abnormalities of mitochondria. These investigators, as well as Clark et al. (2006) and Yang et al. (2006)) noted the marked similarity between this phenotype and that of parkin loss-of-function mutants in Drosophila. Each group demonstrated, remarkably, that the PINK1 loss-of-function phenotype was reversed by parkin expression. The converse, however, was not true; PINK1 expression did not reverse the parkin loss-of-function phenotype, indicating that these two genes are likely to function in a shared pathway, with parkin downstream. As noted above, Cha et al. (2005) had previously observed that loss of parkin function in Drosophila results in increased expression of JNK, an important mediator of PCD. Interestingly, Park et al. (2006) demonstrated in PINK1 loss-of-function flies that there is increased activation of a JNK gene target, and the phenotype is reversed by loss-of-function of hep, a MAPK kinase 7 homolog.
Thus, there is substantial evidence both from in vitro and in vivo studies in Drosophila, that PINK1 (and parkin) play an important, primary role in regulating pathways of PCD. However, for reasons that remain unknown, null mutants of neither PINK1 (Kitada et al. 2007) nor parkin (Goldberg et al. 2003) result in the loss of dopamine neurons or their axonal projections. While there are many possible explanations for the lack of a phenotype in mice, one obvious possibility is that these pathways are highly redundant in mammals, and subject to compensatory changes.
DJ-1
Mutations in the gene for DJ-1 (PARK7) also cause autosomal recessive early onset familial PD. Bonifati et al. (2003) first localized the gene for PARK7 in families from Italy and the Netherlands to chromosome 1p36. They subsequently determined that in the Dutch family a deletion mutation affects the coding region of DJ-1, and in the Italian family, a L166P mutation is present and likely to result in loss-of-function (Bonifati et al. 2003). Human DJ-1 was first identified as an oncogene (Nagakubo et al. 1997), and later determined to be H2O2-responsive, suggesting that it may function as an antioxidant protein (Mitsumoto and Nakagawa 2001).
Consistent with the possibility that DJ-1 may play a protective role in neurons, a number of investigators have demonstrated anti-apoptotic effects in tissue culture. Yokota et al. (2003) demonstrated in mouse Neuro2a cells and human embryonic kidney cells that down-regulation of DJ-1 by siRNA increased their susceptibility to H2O2-induced apoptosis. Down-regulation of DJ-1 also increased susceptibility to death by ER stress. Canet-Aviles et al. (2004) confirmed the ability of DJ-1 to provide protection against oxidative stress, induced in their study by H2O2, paraquat, and MPP+. This antioxidant capacity was dependent on Cys106, and proposed to be mediated by the formation of cysteine-sulfinic acid. The critical role of this residue is supported by studies in Drosophila, in which mutation of the homologous Cys104 residue to alanine abolishes the ability of DJ-1 to protect against oxidative injury as a result of paraquat exposure (Meulener et al. 2006). Using an affinity purification approach to identify proteins which interact with DJ-1, Xu et al. (2005) demonstrated that DJ-1 interacts with nuclear RNA binding protein p54nrb and pyrimidine tract-binding protein-associated splicing factor (PSF). These proteins are both regulators of transcription and RNA metabolism. These investigators demonstrated that over-expression of PSF induces apoptosis, which can be blocked by co-expression of wildtype DJ-1, but to a lesser extent by mutant forms. Conversely, down-regulation of DJ-1 made cells more susceptible to PSF-induced apoptosis. Like Yokota et al., Xu et al. (2005) demonstrated that DJ-1 protected cells from H2O2-induced apoptosis, and furthermore showed that its protein interactor p54nrb did so as well.
An anti-apoptotic role for DJ-1 has also been demonstrated by Junn et al. (2005), but by a different mechanism. Like other investigators, they demonstrated that DJ-1 protects cells (SH-SY5Y cells in this case) from H2O2, and, in addition, that it is protective against cell death induced by dopamine and MPP+. Using a yeast two-hybrid screen approach, they identified an interaction between DJ-1 and the protein Daxx. Daxx had previously been identified as a protein interactor for the Fas death receptor (Yang et al. 1997), which mediates Fas-induced apoptosis by activating the kinase ASK1, which, in turn, activates JNK (Chang et al. 1998) (reviewed by Choi and Benveniste 2004). Junn et al. (2005) demonstrated that DJ-1 sequesters Daxx in the nucleus, preventing it from activating ASK1, and thereby inhibiting apoptosis. This ability to block apoptosis is lost in the L166P mutant.
Studies in vivo in Drosophila have provided support for these observations made in vitro of an anti-apoptotic function for DJ-1. Yang et al. (2005) have shown that knockdown of the Drosophila DJ-1 homolog in dopaminergic neurons by a transgenic RNAi approach resulted in progressive decline in their number, and diminished dopamine content. As would be predicted from the in vitro studies, DJ-1 knockdown in neurons resulted in increased sensitivity to oxidative stress because of H2O2 exposure. These investigators determined that the neurodegeneration phenotype induced by RNAi knockdown of DJ-1 could by suppressed by co-expression of PI3K. Conversely, the degenerative phenotype was exacerbated by co-expression of PTEN. These results are complimented by those of Kim et al. (2005a) who demonstrated in Drosophila that DJ-1 serves as a suppresser of PTEN. In addition, they demonstrated in mammalian cells that increased expression of DJ-1 results in increased phosphorylation and activation of Akt, with enhanced cell survival (Kim et al. 2005a). Thus, converging lines of evidence suggest that DJ-1 positively regulates the anti-apoptotic Akt kinase pathway.
The mechanism by which DJ-1 may achieve regulation of the Akt signaling pathway is unknown. Clements et al. 2006 have shown that DJ-1 stabilizes Nrf2 protein, a key regulator of antioxidant responses. Protein stabilization is achieved by the ability of DJ-1 to prevent the association of a cytosolic inhibitor, Keap1, with Nrf2, thereby preventing Nrf2 ubiquitination and degradation via the proteasome pathway. The precise mechanism by which DJ-1 prevents Keap1 association with Nrf2, and how, in turn, this effect may be related to activation of the Akt pathway, if at all, is unknown.
Unfortunately, as has been the case for parkin null mutations, homozygous null mutations of DJ-1 in mice have not led to a degenerative loss of SN dopamine neurons, as the loss-of-function mutations do in human patients (Chen et al. 2005; Goldberg et al. 2005). However, as would be predicted based on the tissue culture and Drosophila studies, null mice do show a greater sensitivity to oxidative insults (Kim et al. 2005b). In addition, they show a greater sensitivity to MPTP administered in a chronic regimen that induces apoptosis (Tatton and Kish 1997). Thus, in the mammalian in vivo context, DJ-1 is likely to have an anti-apoptotic function which protects dopamine neurons.
Conclusions
A principal weakness of the hypothesis that PCD plays a role in the pathogenesis of PD has been that most of the evidence supporting it has been obtained in animal models induced by neurotoxins, where the actual relevance to human disease processes is unknown. Thus, the emerging evidence that defined genetic causes of parkinsonism may regulate PCD pathways provides stronger additional support for disease relevance. Of additional interest, as our knowledge of the possible functions of parkin, PINK1, and DJ-1 has grown, certain themes on the pathogenesis of PD have gained further support, and others have emerged. One prevailing theme has been that mitochondrial dysfunction plays a role, and this concept has been supported by investigations of these genes, as reviewed herein and elsewhere (Lin and Beal 2006), particularly in relation to parkin and PINK1. Another long prevailing hypotheses for the pathogenesis of PD is that oxidative injury plays a role (Fahn and Sulzer 2004) and that concept has also been supported by findings reviewed here, particularly those related to the antioxidative function of DJ-1. A new and intriguing theme to emerge from studies of these genetic causes of autosomal recessive parkinsonism is the intimate relationships each of these genes appears to have with Akt survival signaling. These relationships raise a host of questions related to the role of Akt and related signaling pathways in the maintenance of the function and viability of mature dopamine neurons. Although we must use caution in generalizing from these genetic forms of parkinsonism to the more common, sporadic cases of PD, nevertheless, elucidation of the answers to these questions may offer a better understanding of the pathogenesis of sporadic PD and effective neuroprotective therapies. A first step towards the development of neuroprotective approaches based on Akt signaling is the demonstration that a constitutively active form has robust trophic and protective effects on dopamine neurons following their transduction by a viral vector approach (Ries et al. 2006).
Acknowledgements
The author is supported by NS26836, NS38370, DAMD17-03-1-0492, The Parkinson's Disease Foundation, The Lowenstein Foundation, and the Michael J. Fox Foundation.
Abbreviations used
- AIF
apoptosis-inducing factor
- ARJP
autosomal recessive juvenile onset
- ASK1
apoptosis signaling kinase
- BH
Bcl-2 homology
- DD
death domain
- DED
death effector domain
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- FADD
Fas-associated protein with DD
- IAP
inhibitor-of-apoptosis protein
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MLK
mixed lineage kinase
- NF-κB
nuclear factor κB
- Pael-R
parkin-associated endothelin receptor-like receptor
- PCD
programmed cell death
- PD
Parkinson's disease
- PI3K
phosphatidylinositol 3-kinase
- PINK
PTEN-induced kinase
- POSH
plenty of Src homology 3
- PSF
protein-associated splicing factor
- PtdIns (3,4,5)P3
phosphatidylinositol 3,4,5 triphosphate
- PTEN
phosphatase and tensin homolog deleted on chromosome ten
- siRNA
short interfering RNA
- SMAC
second mitochondria-derived activator of caspases
- SN
substantia nigra
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- TRADD
TNFR-1-associated death domain protein
- TRAF
TNFR-associated factor
- TRAP
TNFR-associated protein
- TSC
tuberous sclerosis complex
References
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Bayascas JR, Alessi DR. Regulation of Akt/PKB Ser473 phosphorylation. Mol. Cell. 2005;18:143–145. doi: 10.1016/j.molcel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Bazenet CE, Mota MA, Rubin LL. The small GTP-binding protein Cdc42 is required for nerve growth factor withdrawal-induced neuronal death. Proc. Natl Acad. Sci. USA. 1998;95:3984–3989. doi: 10.1073/pnas.95.7.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilina A, van der BM, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc. Natl Acad. Sci. USA. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Burke RE. In: Programmed cell death, in The Handbook of Clinical Neurology: Parkinson's Disease and Related Disorders. Koller WC, Melamed E, editors. Elsevier Limited; Edinburgh: 2007. [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Calne DB, Mizuno Y. The neuromythology of Parkinson's disease. Parkinsonism Relat. Disord. 2004;10:319–322. doi: 10.1016/j.parkreldis.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl Acad. Sci. USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha GH, Kim S, Park J, Lee E, Kim M, Lee SB, Kim JM, Chung J, Cho KS. Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc. Natl Acad. Sci. USA. 2005;102:10345–10350. doi: 10.1073/pnas.0500346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- Chen L, Cagniard B, Mathews T, Jones S, Koh HC, Ding Y, Carvey PM, Ling Z, Kang UJ, Zhuang X. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J. Biol. Chem. 2005;280:21418–21426. doi: 10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res. Brain Res. Rev. 2004;44:65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Chung KKK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM. Parkin ubiquitinates the α-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat. Med. 2001;7:1144–1150. doi: 10.1038/nm1001-1144. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl Acad. Sci. USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. The biochemistry of Parkinson's disease. Annu. Rev. Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J. Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc. Natl Acad. Sci. USA. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Corti O, Lucking CB, et al. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum. Mol. Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 Proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem. Biophys. Res. Commun. 2005;337:1133–1138. doi: 10.1016/j.bbrc.2005.09.178. [DOI] [PubMed] [Google Scholar]
- Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J. Biol. Chem. 2002;277:40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin. Cell Dev. Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon L, Belanger CM, Corera AT, et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat. Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- Figueroa C, Tarras S, Taylor J, Vojtek AB. Akt2 negatively regulates assembly of the POSH-MLK-JNK signaling complex. J. Biol. Chem. 2003;278:47922–47927. doi: 10.1074/jbc.M307357200. [DOI] [PubMed] [Google Scholar]
- Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem. Biophys. Res. Commun. 2002;293:722–726. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J. Neurol. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, et al. Parkindeficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl Acad. Sci. USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Biswas SC, Liu DX. Cell cycle molecules and vertebrate neuron death: E2F at the hub. Cell Death Differ. 2004;11:49–60. doi: 10.1038/sj.cdd.4401341. [DOI] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT – a major therapeutic target. Biochim. Biophys. Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Henn IH, Bouman L, Schlehe JS, et al. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J. Neurosci. 2007;27:1868–1878. doi: 10.1523/JNEUROSCI.5537-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz WA, O'Malley KL. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J. Biol. Chem. 2003;278:19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J. Biol. Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Tsuji S. Clinical analysis of 17 patients in 12 Japanese families with autosomal-recessive type juvenile parkinsonism. Neurology. 1996;47:160–166. doi: 10.1212/wnl.47.1.160. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Piao YS, Mizuno M, Takei N, Kakita A, Takahashi H, Nawa H. Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson's disease and its model: neurotrophic implication in nigrostriatal neurons. J. Neurochem. 2005;93:974–983. doi: 10.1111/j.1471-4159.2005.03073.x. [DOI] [PubMed] [Google Scholar]
- Jain S, Wood NW, Healy DG. Molecular genetic pathways in Parkinson's disease: a review. Clin. Sci. (Lond.) 2005;109:355–364. doi: 10.1042/CS20050106. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum. Mol. Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- Jimenez DR, Moreno S, Garcia-Ospina G, Buritica O, Uribe CS, Lopera F, Velez-Pardo C. Autosomal recessive juvenile parkinsonism Cys212Tyr mutation in parkin renders lymphocytes susceptible to dopamine- and iron-mediated apoptosis. Mov. Disord. 2004;19:324–330. doi: 10.1002/mds.10670. [DOI] [PubMed] [Google Scholar]
- Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc. Natl Acad. Sci. USA. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum MJ, Chao MV. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- Kim RH, Peters M, Jang Y, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005a;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrin-dine (MPTP) and oxidative stress. Proc. Natl Acad. Sci. USA. 2005b;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. From the Cover: impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc. Natl Acad. Sci. USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao Y, Imai Y, Ozawa K, et al. Pael receptor induces death of dopaminergic neurons in the substantia nigra via endoplasmic reticulum stress and dopamine toxicity, which is enhanced under condition of parkin inactivation. Hum. Mol. Genet. 2007;16:50–60. doi: 10.1093/hmg/ddl439. [DOI] [PubMed] [Google Scholar]
- Ko HS, von Coelln R, Sriram SR, et al. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J. Neurosci. 2005;25:7968–7978. doi: 10.1523/JNEUROSCI.2172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T, Kwon DY, Chun J, Kim JH, Kang SS. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J. Biol. Chem. 2000;275:423–428. doi: 10.1074/jbc.275.1.423. [DOI] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl Acad. Sci. USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Takasu T, Perlman DM, Peterson MS, Burrichter D, Avdulov S, Bitterman PB, Polunovsky VA. Translation factor eIF4E rescues cells from Myc-dependent apoptosis by inhibiting cytochrome c release. J. Biol. Chem. 2003;278:3015–3022. doi: 10.1074/jbc.M208821200. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Luo HR, Hattori H, Hossain MA, Hester L, Huang Y, Lee-Kwon W, Donowitz M, Nagata E, Snyder SH. Akt as a mediator of cell death. Proc. Natl Acad. Sci. USA. 2003;100:11712–11717. doi: 10.1073/pnas.1634990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida Y, Chiba T, Takayanagi A, et al. Common anti-apoptotic roles of parkin and alpha-synuclein in human dopaminergic cells. Biochem. Biophys. Res. Commun. 2005;332:233–240. doi: 10.1016/j.bbrc.2005.04.124. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem. Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins LM, Morrison A, Klupsch K, et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol. Cell. Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Minami M, Takeda K, Sakao Y, Akira S. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 1996;395:143–147. doi: 10.1016/0014-5793(96)01016-2. [DOI] [PubMed] [Google Scholar]
- McCormick F. Cancer: survival pathways meet their end. Nature. 2004;428:267–269. doi: 10.1038/428267a. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. GADD153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc. Natl Acad. Sci. USA. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic. Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- Momoi T. Caspases involved in ER stress-mediated cell death. J. Chem. Neuroanat. 2004;28:101–105. doi: 10.1016/j.jchemneu.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Harding M, Bellen H, Mardon G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, et al. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J. Biol. Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- Pezzoli G, Zecchinelli A, Ricciardi S, Burke RE, Fahn S, Scarlato G, Carenzi A. Intraventricular infusion of epidermal growth factor restores dopaminergic pathway in hemiparkinsonian rats. Mov. Disord. 1991;6:281–287. doi: 10.1002/mds.870060403. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, et al. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat. Cell Biol. 2007;11:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J. Biol. Chem. 2001;276:33869–33874. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhao J, Feng J. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J. Neurosci. 2003;23:3316–3324. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Ries V, Henchcliffe C, Kareva T, Rzhetskaya M, Bland RJ, During MJ, Kholodilov N, Burke RE. Oncoprotein Akt/ PKB: trophic effects in murine models of Parkinson's disease. Proc. Natl Acad. Sci. USA. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J. Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, Mizuno Y, Kosik KS, Selkoe DJ. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson's disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- Silva RM, Kuan CY, Rakic P, Burke RE. Mixed lineage kinase-c-jun N-terminal kinase signaling pathway: a new therapeutic target in Parkinson's disease. Mov. Disord. 2005a;20:653–664. doi: 10.1002/mds.20390. [DOI] [PubMed] [Google Scholar]
- Silva RM, Ries V, Oo TF, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J. Neurochem. 2005b;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E, Abeliovich A. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron. 2003;37:735–749. doi: 10.1016/s0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Strauss KM, Martins LM, Plun-Favreau H, et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum. Mol. Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell. 2001;8:613–621. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine-treated mice using terminal deoxynucleotidyl transferase labeling and acridine orange. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Tibbetts MD, Zheng L, Lenardo MJ. The death effector domain protein family: regulators of cellular homeostasis. Nat. Immunol. 2003;4:404–409. doi: 10.1038/ni0503-404. [DOI] [PubMed] [Google Scholar]
- Unoki M, Nakamura Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene. 2001;20:4457–4465. doi: 10.1038/sj.onc.1204608. [DOI] [PubMed] [Google Scholar]
- Valente EM, Bentivoglio AR, Dixon PH, Ferraris A, Ialongo T, Frontali M, Albanese A, Wood NW. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35-p36. Am. J. Hum. Genet. 2001;68:895–900. doi: 10.1086/319522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wajant H. Death receptors. Essays Biochem. 2003;39:53–71. doi: 10.1042/bse0390053. [DOI] [PubMed] [Google Scholar]
- Wang LH, Besirli CG, Johnson EM., Jr Mixed-lineage kinases: a target for the prevention of neurodegeneration. Annu. Rev. Pharmacol. Toxicol. 2004;44:451–474. doi: 10.1146/annurev.pharmtox.44.101802.121840. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol. Cell. Biol. 2001;21:4713–4724. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Geula C, Yankner BA. The Parkinson's disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum. Mol. Genet. 2005;14:1231–1241. doi: 10.1093/hmg/ddi134. [DOI] [PubMed] [Google Scholar]
- Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron. 2003;37:911–924. doi: 10.1016/s0896-6273(03)00143-0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl Acad. Sci. USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl Acad. Sci. USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- Yokota T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H. Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem. Biophys. Res. Commun. 2003;312:1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc. Natl Acad. Sci. USA. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]