Abstract
Objective
The 2004 US Preventative Services Task Force (USPSTF) guidelines do not recommend routinely screening adults for oral cancer given no proven mortality reduction. A large cluster-randomized controlled screening trial in Kerala, India in 2005, however, reported a significant reduction in mortality for screened male tobacco and/or alcohol users. In the United States, office-based screening efforts targeting males of high-risk (regular use of tobacco and/or alcohol) have been unsuccessful due to poor attendance. Given the newfound screening mortality benefit to this high-risk subpopulation, we sought to ascertain the cost-effectiveness threshold of a yearly, community outreach screening program for males>40 years regularly using tobacco and/or alcohol.
Study Design
Markov decision analysis model; societal perspective
Methods
A literature search was performed to determine event probabilities, health utilities and cost parameters to serve as model inputs. Screen versus No Screen strategies were modeled using assumptions and published data. The primary outcome was the difference in costs and quality-adjusted life-years (QALYs) between the two cohorts, representing the potential budget for a screening program. One-way sensitivity analysis was performed for several key parameters.
Results
The No Screen arm was dominated with an incremental cost of $258 and an incremental effectiveness of −0.0414 QALYs. Using the $75,000/QALY metric, the maximum allowable budget for a screening program equals $3,363 ($258+$3,105) per screened person over a 40-year time course.
Conclusion
Given the significant health benefits and financial savings via early detection in the screened cohort, a community-based screening program targeting high-risk males is likely to be cost-effective.
Introduction
Cancers of the oral cavity include neoplasms of the lips, buccal mucosa, the upper and lower alveolar ridges, the retromolar trigone, the anterior two-thirds of the tongue, the floor of the mouth and the hard palate. Based on national data from 1997-2001, oral cancer accounts for 1.6% of all new cancers in males and 0.9% of all new cancers in women in the United States.1 More than 95% of oral and pharyngeal cancers occurs in individuals over 40 years age with average at diagnosis of 60 years.2 While overall 5-year survival from lip cancers is roughly 93%, the other oral cancer subsites evince a much poorer prognosis.1 In a 2004 U.S. report, the 5-year survival for Stage I/II and III/IV oral cancer (including oropharyngeal) was 80.4% and 46.3%, respectively. Nearly 60% of these cancers were discovered in advanced stages.3 The intensity of treatment required for oral cancer --- surgery, radiation therapy or chemotherapy --- generally increases in accordance with the stage of disease. Thus, discovery of late stage cancers significantly hampers patient quality of life and adds to the financial burden of treatment.
At present, oral cancer screening via direct palpation and visual inspection of the oral cavity is not routinely performed for any subset of the United States population. The 2004 US Preventative Services Task Force (USPSTF) guidelines state that “The USPSTF found no new good quality evidence that screening for oral cancer leads to improved health outcomes for either high-risk adults (ie, those over the age of 50 who use tobacco) or for average risk adults in the general population. It is unlikely that controlled trials of screening for oral cancer will ever be conducted in the general population because of the very low incidence of oral cancer in the United States…As a result, the USPSTF could not determine the balance between benefits and harms of screening for oral cancer.” 4 Since 2004, no controlled studies examining the United States population have taken place. However, a large cluster-randomized controlled screening trial in Kerala, India reported in 2005, a significant reduction in mortality for screened male tobacco and/or alcohol users undergoing three rounds of screening at 3-year intervals from 1996–2004. 5 The mortality rate of unscreened high risk males was 42.9 per 100,000 compared to 24.6 per 100,000 in the screened arm yielding a mortality rate ratio of 0.57 (95% CI 0.35–0.93) Based on the data from the Kerala study, it is possible that screening high-risk males at regular intervals could prove beneficial in reducing oral cancer-related mortality in the United States. Historically, patients at high-risk for oral cancer demonstrate limited awareness of oral cancer, knowledge of risk factors for oral cancer, knowledge of other conditions associated with alcohol and tobacco use, and perceived risk for oral cancer.6 Efforts to screen this high-risk demographic in the United States have proven unsuccessful due to poor attendance of high-risk males in free oral and head and neck cancer screening programs. 7,8 As a result, multiple study authors have proposed a shift away from office-based screening to a community-based approach in which trained health workers actively seek out high-risk males to undergo screening.7,9,10
Understanding that community-based programs require considerable financial capital, we sought to determine the cost-effectiveness threshold (i.e. maximum budget of a screening program) for yearly screening of high-risk males using Markov decision analysis modeling.
Materials and Methods
Model Overview
Markov modeling is a powerful tool for depicting clinical events that occur over time. In contrast to decision tree modeling, Markov models enable the modeling of disease progression over time which is particularly helpful in oncologic disease processes.11 We developed a state-transition Markov model to simulate the progression from normal oral mucosa to premalignant lesions to stage I/II oral cancer to stage III/IV oral cancer. The oral tongue represents the highest proportion of oral cancers among cancer sites and all lesions were presumed to be on the oral tongue for purposes of standardization.12 The simulated cohorts begin at age 40 in one of the health states with the distribution based on prevalence data. Members of the cohort move among health states with transition probabilities during a 1-year cycle (Figure 1). All events occurred at the beginning of the cycle. Each cycle attributes age-specific death rates to the cohort in addition to added risk from oral cancer. The simulation runs for 40 cycles, during which time members of the cohort transition to the dead state or successfully reach the age of 80. We have chosen an end age of 80 years based on the presumption that screening yields diminishing returns after this age given average life expectancies of American males.13
Figure 1.
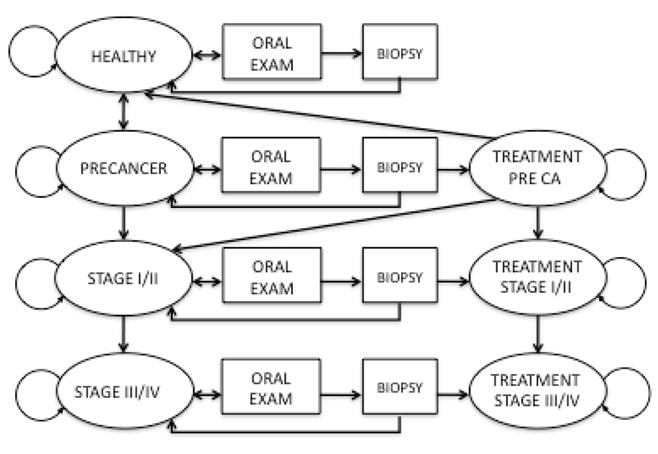
Simplified Model
For each health state, a utility value is designated. Utilities are a measure of a person’s satisfaction and serve as a proxy for quality of life. The amount of time spent in each health state multiplied by the utility value enables the calculation of quality-adjusted life years (QALYs). Costs are assigned to each health state as well as to transitions within health states. For example, the cost of biopsy occurs after oral examination within a health state. At the end of the simulation, quality-adjusted life expectancy (QALE) and costs can be calculated for each strategy for direct comparison. All costs and utilities were discounted annually at 3% per the recommendation of the U.S. Panel on Cost-Effectiveness in Health and Medicine.14
Our Markov model examined two screening strategies: no community-based screening (No Screen) and yearly, community-based screening (Screen) for all high-risk males, defined as age over 40 years with recent, regular use of tobacco and/or alcohol. Costs exclusively associated with the development and management of the screening program (cost of training and hiring health workers, cost of space and equipment, administrative costs) were intentionally not included in the analysis. The purpose of this study is to determine the maximum amount of dollars per capita that should be allocated, from a societal perspective, toward a community-based high-risk oral cancer screening program. The actual design of such a program is beyond the scope of this study.
Clinical Data
Natural History of Oral Cancer
Table 1 lists all relevant clinical probabilities. While abundant prevalence data exists for oral premalignant lesions, little incidence data exists for the United States population. Incidence rates in our study were based on a Japanese study showing an incidence in males of 409 per 100,000 person-years15. Given that the relative risk of our population is roughly 2,16 we chose an incidence of 0.08 per year. Silverman et al showed that at 7 years of follow-up, 30% of the lesions not having undergone malignant transformation regress to normal mucosa.17 A 7-year array of temporary states (tunnel state) was included to model this aspect of the disease. For patients receiving treatment for precancer, a 28% probability of regression in the first year was assigned based on literature demonstrating a 56% level of reduction among patients reducing their tobacco use by 50% or abstaining for 3 months. We assumed a 50% success rate in curbing tobacco habits and thus used one-half the 56% value.
Table 1.
Clinical Parameters
| Parameter | Base Case Value | Range Examined | Reference |
|---|---|---|---|
| Natural History | |||
| Incidence of precancer | 0.008 | 0.002–0.02 | 12,15,16,18 |
| Probability of spontaneous regression of precancerous lesion at 7 years | 0.3 | 0.1–0.5 | 17 |
| Probability of regression of precancer upon treatment and counseling | 0.28 | 0.1–0.8 | 35 |
| Probability of developing Stage I/II cancer from precancer | 0.008 | 0.006–0.05 | 12,17,18,38 |
| Probability of developing Stage III/IV cancer from undiagnosed Stage I/II cancer | 0.5 | 0.25–0.75 | Assumption |
| Treatment, Recurrence and Survival | |||
| Probability of occult neck disease found on neck dissection for Stage I/II treatment | 0.25 | 0.06–0.46 | 21 |
| 5 year local control failure rate for Stage I/II disease | 0.42** | 0.3–0.5 | 22 |
| 5 year local control failure rate for Stage III/IV disease | 0.67** | 0.5–0.8 | 3,22,24 |
| Probability of Stage I/II presenting with local cancer recurrence | 0.18 | 0.1–0.3 | 23 |
| 5 year survival for Stage I/II | 0.80** | 0.6–0.9 | 3 |
| 5 year survival for Stage III/IV | 0.46** | 0.3–0.6 | 3 |
| 5 year survival for Stage I/II recurrence | 0.46** | 0.3–0.6 | 25 |
| 2 year survival for Stage III/IV recurrence | 0.16** | 0.05–0.25 | 25 |
| Screening and Compliance | |||
| Sensitivity of oral exam | 0.74 | 0.6–0.9 | 27 |
| Specificity of oral exam | 0.99 | 0.95–0.99 | 5,27 |
| Probability of oral exam among healthy and precancer patients – No Screen | 0.13 | 0.05–0.25 | 2 |
| Probability of oral exam among healthy and precancer patients – Screen | 0.5 | 0.1–0.9 | Assumption |
| Probability of oral exam among patients with undiagnosed Stage I/II cancer | 0.6 | 0.2–0.9 | Assumption |
| Probability of oral exam among patients with undiagnosed Stage III/IV cancer | 0.9 | 0.6–0.9 | Assumption |
| Probability of compliance with biopsy – No Screen | 0.67 | 0.39–1 | 5,8,9 |
| Probability of compliance with biopsy – Screen | 0.8 | 0.5–1 | Assumption |
All probabilities are based on a 1-year cycle unless otherwise noted
Yearly rates derived from probability using Declining Exponential Approximation of Life Expectancy (DEALE)
The annual rate of malignant transformation has been shown to be between 0.06%-5%;12,15,17–19 thus, the yearly rate of developing Stage I/II disease from precancer was estimated at 0.8%. Given the absence of data, expert opinion by way of the senior author (JTJ) was used to estimate the annual rate of progression from Stage I/II to III/IV at 50%.
Treatment, Recurrence and Survival
In our model, biopsy represents the gold standard for suspicious lesions and all patients have 100% compliance once diagnosed with biopsy-proven cancer. Treatment for precancer in this simulation was excisional biopsy with twice yearly evaluation.20 Stage I/II lesions were treated with partial glossectomy with primary closure and unilateral supraomohyoid neck dissection. Twenty-five percent of patients were upstaged to Stage III/IV disease after positive nodes on neck dissection.21 These patients received adjuvant chemotherapy and intensity-modulated radiation therapy (CRT). Stage III/IV lesions were treated with radical surgery (glossectomy, mandibulectomy, unilateral neck dissection, tracheostomy, free flap reconstruction) followed by CRT. After the completion of radiation therapy treatment, all patients were followed with PET scan at 2 months, 4 months and 7 months within the first year of followup per protocol at our institution. If there was no evidence of recurrence after the third scan, surveillance PET scans were no longer performed. After receiving surgery and/or CRT, all patients were seen monthly for the first year, every 2 months for the second year, every 3 months for the third and fourth year and once yearly during the fifth year and beyond.
Yearly recurrence rates for Stage I/II disease during the first five years was estimated at 10%,22 with the majority (82%) of recurrent disease demonstrating regional and/or distant disease.23 Yearly recurrence rates for Stage III/IV disease was estimated at 20%.3,22,24 After 5 years without recurrence, subjects were assumed to be cured.
The mortality rates for healthy and precancer states were obtained from 2004 U.S. Life Tables for males.13 The 5-year survival estimates for Stage I/II and III/IV disease were 80.4% and 46.3%, respectively, based on Surveillance, Epidemiology, and End Results (SEER) data for oral and oropharyngeal cancer.3 Patients who recurred with Stage I/II disease were assigned a 5-year survival of 46%, while members of the cohort who recurred after treatment for Stage III/IV disease had particularly poor prognosis with a 2-year survival of 16%.25 Patients with untreated Stage III/IV disease were assigned the same survival rates. Survival curves were converted to yearly death rates using Declining Exponential Approximation of Life Expectancy (DEALE).26
Screening and Compliance
The yearly probability of an oral exam for healthy and precancer patients by a health care provider in the No Screen cohort was estimated at 13% based on data from American smokers over 40.2 Subjects with Stage I/II and III/IV disease were assumed to have increased yearly rates of oral examination due to self-referral at 60% and 90%, respectively. In the Screen arm, we have assumed 50% penetrance with 50% of subjects with no lesion or precancer lesion receiving a yearly oral exam.
Sensitivity and specificity of the oral exam for premalignant and malignant lesions by practicing physicians and dentists demonstrates a sensitivity and specificity of 74% and 99%, respectively.5,27 Given our assumption that trained health workers will be performing the exams in the Screen group, the sensitivity dropped to 70% while the specificity remained at 99% in the Screen group.5,28
Compliance with initial biopsy in the No Screen arm was estimated at 67%.5,8 Based on experience from community-based screenings,9 biopsy will occur at the time of the screening to increase compliance which we estimated at 80% in the Screen group. This difference in compliance rate was one of the variables challenged in the sensitivity analysis. We assumed biopsy compliance for Stage III/IV lesions to be 100% in both arms given the dramatic presentation of disease. Once treatment for Stage I/II or III/IV cancer was initiated, compliance with followup was assumed to be 100%.
Costs
Table 2 lists all model cost inputs. Only costs related to workup and treatment of oral cancer were included in this analysis. Costs for outpatient evaluation and office biopsies were based on Medicare-based Current Procedural Terminology (CPT) provider reimbursement. Given our assumption that only patients with Stage I/II or III/IV disease self-refer to their PCP, visits to the PCP for healthy and precancer patients were assumed to be unrelated to any oral lesions and thus excluded from the cost analysis. All surgical interventions included a blanket pre-operative fee of $140 to include the cost of level 4 return office visit, pre-op bloodwork, EKG, chest x-ray. Stage I/II and III/IV pre-operative workup included a CT neck with contrast and PET/CT, respectively. Hospital costs for inpatient treatment were estimated from the Agency for Healthcare Research and Quality’s (AHRQ) Healthcare Cost and Utilization Project (HCUP) on-line query system, HCUPnet. Stage I/II inpatient treatment costs carried a principle procedure coding of partial glossectomy with costs of $10,101; Stage III/IV inpatient treatment costs bore a pricipal procedure coding of partial mandibulectomy with costs of $24,842.29 Professional surgical fees were based on Medicare reimbursement for the appropriate CPT codes. Professional fees related to other fields (anesthesiology, pathology) were not included for purposes of model simplification.
Table 2.
Cost and Utility Parameters
| Parameter | Base Case Value | Range Examined | Reference |
|---|---|---|---|
| Costs | |||
| Cost of Level IV ENT Outpatient Consult | $152 | - | ∞ |
| Cost of Level IV established visit to primary care provider or otolaryngologist | $68 | - | ∞ |
| Cost of office biopsy | $92 | $0–$200 | ∞ |
| Cost of excisional tongue biopsy | $110 | - | ∞ |
| Cost of pre-operative labs, chest x-ray and EKG | $72 | - | ∞ |
| Cost of surveillance PET scan (skull base to mid-thigh) | $950 | - | Ω |
| Cost of pre-operative PET/CT scan (skull base to mid-thigh) | $1,068 | - | Ω |
| Cost of pre-operative CT neck with contrast | $351 | - | Ω |
| Surgeon fee for unilateral suprahyoid neck dissection | $1,274 | - | ∞ |
| Surgeon fee for excision of Stage I/II lesion | $219 | - | ∞ |
| Surgeon fee for composite resection for Stage III/IV disease | $1,935 | - | ∞ |
| Surgeon fee for free flap reconstruction | $2,586 | - | ∞ |
| Surgeon fee for tracheotomy | $377 | - | ∞ |
| Inpatient costs for Stage I/II surgery (partial glossectomy with unilateral neck dissection) | $10,101 | $5,000–$15,000 | 2007 median cost, HCUPnet 29 |
| Inpatient costs for Stage III/IV surgery (mandibulectomy with reconstruction) | $24,842 | $10,000–$40,000 | 2007 median cost, HCUPnet 29 |
| Chemotherapy | $5,769 | $2,000–$10,000 | 30 |
| Intensity-modulated radiation therapy | $22,219 | $5,000–$30,000 | 31 |
| Total Stage I/II Treatment Costs Year 1 | $11,600 | - | ∞ 29 |
| Total Stage III/IV Treatment Costs Year 1 | $61,900 | $30,000–120,000 | ∞ 29 |
| Willingess-to-pay Threshold | $75,000 per QALY | $50,000–150,000 per QALY | 39 |
| Utilities | |||
| Baseline utility | 0.84 | 0.7–1 | 32 |
| Precancer | 0.92 | 0.9–1 | 33 |
| Stage I/II cancer | 0.88 | 0.7–1 | 33 |
| Stage III/IV cancer | 0.68 | 0.5–1 | 33 |
= Medicare payment to Pennsylvania Area 99 based on 2009 Current Procedural Terminology Codes
= Federal Register, Volume 71, No. 226, November 24, 2006
Chemotherapy costs were based on a published report for outpatient chemotherapy for advanced head and neck cancer.30 Radiation therapy costs were derived from 2004 Medicare reimbursement for intensity-modulated radiation therapy (IMRT).31 Total one-year costs of treatment for Stage I/II and III/IV disease were $11,600 and $61,900, respectively.
Utilities
Table 2 contains all utility values used in the model. Age-specific utility data was obtained from data developed by the National Center for Health Statistics.32 Given the health behaviors of this cohort, we assume a baseline disutility of 0.16 which corresponds to ‘good’ self-rated health with no physical limitation. 32 All utility data for premalignant and malignant states emanates from one study which established utility values of 0.92, 0.88, 0.68 for premalignant disease, small cancers and large cancers, respectively.33 Study participants provided one utility value for the pre-operative and post-operative management. For our study, we used these utility values for both the pre-operative and post-operative course. Once patients reached the 5-year disease-free interval, the disutility from the cancer was halved given their presumed increased quality of life. We used the cost/QALY threshold of $75,000/QALY.
Analysis
The Markov decision tree was constructed using TreeAge 2009 Software (TreeAge Software Inc., Williamstown, MA) The decision analysis was run for 40 consecutive cycles with a starting age of 40. Ninety-three percent of the cohort began in the healthy state and the remaining 7% were distributed according to actual prevalence of oral precancer and cancer states. Costs and QALYs were generated for the No Screen and Screen strategies.
Only six inputs were altered in the Screen vs. No Screen arm (Table 3): probability of compliance with biopsy; probability of yearly oral exam; sensitivity of oral exam; cost ENT consult visit; cost of pre-operative visit (considered new patient for Screen group); cost of office biopsy.
Table 3.
Input Differences between No Screen and Screen
| Variable | No Screen | Screen |
|---|---|---|
| P(compliance with biopsy) | 0.67 | 0.8 |
| P(yearly oral exam) | 0.13 | 0.5 |
| Sensitivity of oral exam | 0.74 | 0.70 |
| Cost ENT Consult Visit | $151 | $0 |
| Cost of pre-operative visit | $68 | $151 |
| Cost of office biopsy | $92 | $0 |
One-way sensitivity analyses were performed on all variables.
Results
Model Validation
The No Screen arm was developed first to reflect the current state of oral cancer diagnosis and treatment in the American health system. In order to test the model’s validity, two key parameters in the model at cycle 20 (mid-way through simulation) were compared to published literature values (Table 4).
Table 4.
Comparison of Parameters for Model Validation
| Parameter | Model Value | Reference Value | Source |
|---|---|---|---|
| Prevalence of Precancer | 7.1% | 7.0% | 16,18 |
| Yearly Death Rate from Oral Cancer | 0.0402% | 0.0235 – 0.0588%* | 10 |
Includes oropharyngeal cancer
With the prevalence of precancer in the general U.S. population at 2.8-2.9%,18 we applied the relative risk for past smokers consuming alcohol of 2-2.5 to yield an expected prevalence rate of 7.0% in our cohort.16 Our model demonstrated 7.1% of the cohort in either a treated or untreated precancer state.
The second parameter examined was yearly death rate from oral cancer. During cycle twenty, 40.2 of 100,000 (0.0402%) of the cohort died from either Stage I/II or III/IV disease. A study examining death rates from the SEER database for oral cancer (including pharynx) in greater Detroit and Michigan from demonstrated a death rate of 23.5 per 100,000 for White-American males living in Detroit between the ages of 50–74. African-Americans had a slightly lower mortality rate at 22.2 per 100,000.10 Risk factors for this population are not known; therefore a range from a relative risk of 1 to 2.5 yields expected mortality rates from 23.5 to 58.8 per 100,000 (0.0235% to 0.0588%).
Base Case
The No Screen arm was dominated, that is more expensive and less effective than screening (Table 5). This strategy had an incremental cost of $258 and an incremental effectiveness of −0.0414 QALYs. Using the $75,000/QALY metric, the QALYs translate to an incremental effectiveness of −$3,105. Therefore, the maximum allowable budget - from a societal perspective - for a community-based screening program equals $3,363 ($258 + $3,105) per screened person over a 40-year time course.
Table 5.
Baseline Results Demonstrating Differences in Cost-Effectiveness Between Strategies
| Strategy | Cost | Incremental Cost | Effectiveness | Incremental Effectiveness | Cost /Effectiveness | Incr C/E (ICER) |
|---|---|---|---|---|---|---|
| Screen | $640.20 | 15.467 | $41 | |||
| No Screen | $898.50 | $258.30 | 15.4257 | −0.0414 | $58 | (Dominated) |
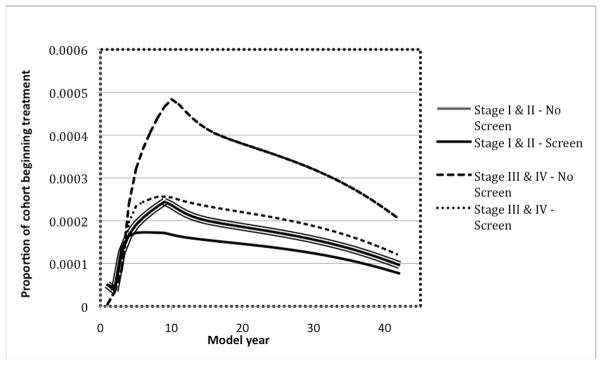
Figure 2 demonstrates the proportion of the cohort beginning treatment for Stage I/II and III/IV disease in both Screen and No Screen cohorts. Both Stage I/II and III/IV disease were reduced in the Screen arm. The most significant decrease occurred in the number of patients with Stage III/IV disease. In the Screen arm the yearly death rate from oral cancer at cycle 20 was 0.0239%, a 40% reduction in death rate compared to the No Screen strategy.
Figure 2.
Disease Stages Within Each Cohort
Sensitivity Analysis
One-way sensitivity analysis was performed for all variables. Table 6 shows the variables with the greatest impact on the maximum budget for an oral cancer screening program. The budget was most affected by probability of malignant transformation from precancer, willingness-to-pay threshold, probability of compliance among screen cohort, incidence of precancer and probability of oral exam among healthy and precancer patients. Treatment costs associated with advanced disease had the smallest impact on the budget in the sensitivity analysis. Of note, when the yearly probability of developing precancer dropped to that of the general population at 0.003, the value of screening (i.e. the budget) decreased by roughly one-third to $2,217. Conversely, if the incidence of precancer reached values concordant with ultra-high risk men at 0.02, the value of screening increased by two-thirds to $5,665.
Table 6.
Sensitivity Analysis Key Variables
| Variable | Base-Case Values | Sensitivity Analysis Values | Cost Savings with Screening | Increased QALYs with Screening | Maximum Budget for Screening Program (per patient screened)* |
|---|---|---|---|---|---|
| Base Case | $258.30 | 0.0414 | $3,363 | ||
| Incidence of precancer | 0.008 | ||||
| 0.003 | $162.20 | 0.0274 | $2,217 | ||
| 0.02 | $452.90 | 0.0695 | $5,665 | ||
| Probability of developing Stage I/II cancer from precancer | 0.008 | ||||
| 0.0006 | −$46.80 | 0.0151 | $1,086 | ||
| 0.05 | $1,529.40 | 0.1540 | $13,079 | ||
| Probability of oral exam among healthy and precancer patients – Screen Cohort | 0.5 | ||||
| 0.13 | $97.00 | 0.0117 | $975 | ||
| 0.8 | $367.70 | 0.0629 | $5,085 | ||
| Probability of compliance with biopsy – Screen Cohort | 0.8 | ||||
| 0.67 | $90.90 | 0.0113 | $938 | ||
| 0.95 | $436.60 | 0.0759 | $6,129 | ||
| Total costs (Yr 1) for Stage III/IV Tx | $61,900 | ||||
| $30,000 | $121.00 | 0.0414 | $3,226 | ||
| $120,000 | $519.00 | 0.0414 | $3,624 | ||
| Willingness-to-pay Threshold | $75,000/QALY | ||||
| $0/QALY | $258.30 | 0.0414 | $258 | ||
| $50,000/QALY | $258.30 | 0.0414 | $2,328 | ||
| $100,000/QALY | $258.30 | 0.0414 | $4,398 | ||
| $150,000/QALY | $258.30 | 0.0414 | $6,468 |
using a $75,000/QALY willingness-to-pay threshold, unless as noted
The Screen strategy remained superior in all variable ranges in terms of QALYs gained. Screen also showed a benefit from medical costs savings in all variables ranges except at low rates of malignant transformation. When the probability of developing Stage I/II disease from precancer equals 0.0006, the Screen arm leads to increased costs of $46.80. Given that the Screen cohort gains an average of 0.0151 QALYs, the budget of a screening program remains positive at $1,086 over the 40 year cycle.
Discussion
The primary purpose of this study was to provide an acceptable budget for an oral cancer screening program based on decreased medical costs and increases QALYs gained.
We have shown that yearly oral cancer screening via visual inspection and manual palpation in the community-based setting for high-risk American males over 40 years age would be considered cost-effective with a budget of $3363 per screened person over the 40 year cycle. This figure is derived from both savings in costs of management ($258) and increase in quality-adjusted life years ($3105) of the Screen group.
Sensitivity analysis illustrates the impact of key variables on the value of a screening program. First, the level of penetrance into this high-risk group via screening greatly affects the effectiveness of the program. Put simply, the greater the number of mouths that can be examined the greater the opportunity to catch disease in its early stages. In addition, compliance with biopsy bears a tremendous impact on the cost-effectiveness of a screening program. This represents potential missed opportunities of early cancer treatment. Finally, by varying the incidence of precancer in this model, we were able to simulate both a general population cohort and an ultra high-risk cohort. The findings demonstrate the per capita dollar value of screening each of these cohorts.
We tested our model with a validation step to ensure real-world applicability. Direct values from the literature were not available for either validation reference value. Prevalence of leukoplakia was estimated by assuming a relative risk of 2.5 for our high-risk population. Leukoplakia prevalence within the model at mid-cycle appeared to closely follow this estimated reference value of leukoplakia prevalence. The second validation criterion, death rates from oral cancer was derived from SEER data for oral cavity & pharynx cancer death rates from 1993–2002 in Detroit males aged 50–74. According to a 2009 American Cancer Society report, 30% of deaths from oral cavity and oropharynx result from oropharynx primary lesions.34 Removing this fraction, our model validation shows a model value of 0.0402% and a new reference value range from 0.0165%–0.0412%. While the model value remains within the range, it remains at the upper extreme suggesting that the model may have mildly overestimated death rates in this population.
We sought to develop a conservative model of the burden and costs of oral cancer to strengthen our findings. The utility of the cohort in the healthy state was chosen to be 0.84. We incorporated a disutility of 0.16 in the healthy population to bias against an intervention which decreases a relative disutility such as screening. We also defined high-risk as regular, recent (within last year) users of tobacco or alcohol. The relative risk for development of premalignant lesions increases from 1.9 for past tobacco and current light drinkers (<15g/day) to 10.2 for current tobacco users and heavy drinkers. We selected a low relative risk of roughly 2.5 which represents past tobacco users and moderate alcohol users.16 We did not model the potential reduction in prevalence in the screening arm as a result of patient education.6 It has been shown that cessation of tobacco can lead to a reduction or disappearance of lesions in 44-80% of subjects17,35
With respect to diagnosis, treatment and workup costs, only direct costs were modeled in our analysis. Head and neck cancer, particularly advanced disease, bears enormous indirect costs including lost wages and the time of caregivers.36 We also did not model indirect costs of biopsy including anxiety for biopsies as a result of false positive oral examinations. With specificity of oral exam near 99%, this represents a relatively small proportion of the population. Costs of complications were not included in this analysis. Recent literature demonstrates that 17% of costs for chemotherapy for advanced head and neck carcinoma are ascribed to complication-related treatment.37
We intentionally did not include costs of the program in our analysis. We felt that the modeling of program costs would require major assumptions and potentially undermine the credibility of our study. Our primary objective was to provide financial parameters to direct further research for screening program development.
Our analysis has several limitations. In regards to uncertainty about the natural history of oral cancer, we chose one year as our cycle length and effectively applied a 3-year minimum cycle to oral cancer. In our model, the lesion starts as normal tissue, progresses to leukoplakia at 0.8% per year and then to Stage I/II cancer at 0.8% per year and to Stage III/IV at 50% per year. It is likely that aggressive variants of squamous cell carcinoma exhibit aggressive behavior with a shorter time course. Similarly, we categorized all lesions as oral tongue for purposes of standardization. Floor of mouth and buccal mucosa lesions present different treatment challenges which were not entertained in this study.
We attempted to utilize data from the United States for oral cancer but encountered difficulty in obtaining specific data. For example, we used data from national SEER reports which combine oral and oropharyngeal carcinoma into a single category. As a result, 5-year survival rates used in this study represented a composite of oral and oropharyngeal carcinoma survival data. While the National Cancer Database (NCDB) separately records oral cancer data, it is not a population-based registry and does not provide incidence and prevalence figures. Hence, we were not able to use the NCDB oral cancer figures in our study. Similarly, incidence and utility data were sparse. Incidence data in a large prospective study for premalignant lesions in the United States was not available. While India has produced much literature on this topic given the high prevalence of disease, we used incidence data from a large Japanese study to more closely resemble the risk factors of males in the United States.19 Data regarding utilities for oral precancer and cancer states emanates from a small British study in which lesions were classified as premalignant, small (<2cm) and large (>2cm). While Stage II lesions are >2cm, we applied utilities for small (<2cm) to Stage I and II given similar treatment options.
All cancers in this model were presumed to be oral tongue for purposes of standardization. Given that this high-risk population is at risk for head and neck squamous cell carcinomas including other regions of oral cavity, oropharynx, and larynx, consideration should be given to include direct and indirect examination of these areas during screening. While not explicitly included in this model, screening for multiple sites during the head and neck examination could increase the overall cost-effectiveness of screening.
The United Kingdom National Health Service’s (NHS) Health Technology Assessment Programme published a report in 2006 on the cost-effectiveness of screening for oral cancer in primary care. Similar to our analysis, a Markov analysis was undertaken to identify subpopulations benefiting from such an intervention. The results demonstrated that opportunistic high-risk screening, particularly men, by general dental practitioners to be a ‘practical proposition’ with an incremental cost-effectiveness ratio below our presumed $75,000 per QALY threshold. While this report examined a national health system and an office-based screening setting, the identification of high-risk males as unique opportunities bears mention.
While the design and execution of a community-based screening program is beyond the scope of this paper as previously stated, the ‘next steps’ warrant some discussion. For a particular cohort of 40-year old men, the budget for a screening program amounts to roughly $84 ($3363 divided by 40 year cycle) per individual per year. Further research needs to clarify the feasibility of such a screening endeavor. Factors determining feasibility include: number of high-risk males in a community, costs of training and employment of health workers, costs of space, ability to perform biopsy during screening, costs of biopsy materials and pathologist reading fees, tobacco and alcohol prevention education materials. There appears to be a strong benefit to develop such a program in affiliation with a university assuming government funds are not available. The costs for training and hiring health workers can be substantially reduced through the use of medical and dental students. Trained health workers have proven nearly as accurate as practicing generalists and dentists in the identification of suspicious lesions.28
Though the universities may assist in defraying costs of personnel and other resources, recruitment of the desired patient population remains the limiting factor. As previous studies have shown, free, office-based head and neck cancer screenings attract low-risk women as opposed to high-risk men. The cohort examined in this model is exclusively composed of men with recent, regular use of alcohol and/or tobacco. This high-risk cohort often does not seek regular medical care and access, therefore, to this population remains paramount. Significant capital investment will be required to seek out, incentivize and educate high-risk individuals to participate in an oral cancer screening.
With regards to community-based screening, one North American study has reported remarkable success with screenings. Poh et al9 reported a 98% participation in screenings held at a free dental clinic in an impoverished, high-risk area in downtown Vancouver. Incidentally, 2 out of 200 patients screened had biopsy-positive oral cancer and 8 patients had biopsy-proved precancer. Despite the small sample size, the number of subjects afflicted with oral cancer illustrates the extraordinary burden of disease within this community. The authors attributed much of the success of their program to their community-based approach. Participants were found to be reluctant to move outside very small boundaries; proximity to the screening site played a critical role in their participation.
Conclusion
The 2005 study from Kerala, India provided evidence of a screening mortality benefit in high-risk males. Assuming that this mortality benefit applies to the high-risk American male population, the significant preservation of quality-adjusted life and medical cost savings generates a maximum allowable budget for the screening program of $3363 per screened individual over the 40-year cycle.
Given the substantial morbidity, mortality and financial burden to society that results when oral cancer is diagnosed in advanced stages, the development of community-based screening programs targeting high-risk adult males in the United States above the age of 40 years need to be highly considered.
Acknowledgments
The primary author has the following grant support: National Institutes of Health T32 CA60397 and SPORE P50 CA097190.
Footnotes
The authors have no financial relationships or conflicts of interest to disclose.
References
- 1.Jameson MJLP. Neoplasms of the Oral Cavity. In: Johnson BJBJT, editor. Head & Neck Surgery -Otolaryngology. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1551–1556. [Google Scholar]
- 2.Ling H, Gadalla S, Israel E, et al. Oral cancer exams among cigarette smokers in Maryland. Cancer Detect Prev. 2006;30:499–506. doi: 10.1016/j.cdp.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 4.Scattoloni J. Screening for Oral Cancer: Brief Evidence Update [Google Scholar]
- 5.Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365:1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 6.Cruz GD, Le Geros RZ, Ostroff JS, Hay JL, Kenigsberg H, Franklin DM. Oral cancer knowledge, risk factors and characteristics of subjects in a large oral cancer screening program. J Am Dent Assoc. 2002;133:1064–1071. doi: 10.14219/jada.archive.2002.0330. quiz 1094. [DOI] [PubMed] [Google Scholar]
- 7.Gourin CG, Kaboli KC, Blume EJ, Nance MA, Koch WM. Characteristics of participants in a free oral, head and neck cancer screening program. Laryngoscope. 2009;119:679–682. doi: 10.1002/lary.20093. [DOI] [PubMed] [Google Scholar]
- 8.Jullien JA, Zakrzewska JM, Downer MC, Speight PM. Attendance and compliance at an oral cancer screening programme in a general medical practice. Eur J Cancer B Oral Oncol. 1995;31B:202–206. doi: 10.1016/0964-1955(94)00048-9. [DOI] [PubMed] [Google Scholar]
- 9.Poh CF, Hislop G, Currie B, et al. Oral cancer screening in a high-risk underserved community--Vancouver Downtown Eastside. J Health Care Poor Underserved. 2007;18:767–778. doi: 10.1353/hpu.2007.0106. [DOI] [PubMed] [Google Scholar]
- 10.Kolker JL, Ismail AI, Sohn W, Ramaswami N. Trends in the incidence, mortality, and survival rates of oral and pharyngeal cancer in a high-risk area in Michigan, USA. Community Dent Oral Epidemiol. 2007;35:489–499. doi: 10.1111/j.1600-0528.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- 11.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–458. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 12.Shah JP, Johnson NW, Batsakis JG. Oral cancer. London New York: Martin Dunitz; 2003. p. 496. Distributed in the US by Thieme New York. [Google Scholar]
- 13.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56:1–39. [PubMed] [Google Scholar]
- 14.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. p. xxiii.p. 425. [Google Scholar]
- 15.Nagao T, Ikeda N, Fukano H, Hashimoto S, Shimozato K, Warnakulasuriya S. Incidence rates for oral leukoplakia and lichen planus in a Japanese population. J Oral Pathol Med. 2005;34:532–539. doi: 10.1111/j.1600-0714.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 16.Maserejian NN, Joshipura KJ, Rosner BA, Giovannucci E, Zavras AI. Prospective study of alcohol consumption and risk of oral premalignant lesions in men. Cancer Epidemiol Biomarkers Prev. 2006;15:774–781. doi: 10.1158/1055-9965.EPI-05-0842. [DOI] [PubMed] [Google Scholar]
- 17.Silverman S, Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 1984;53:563–568. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Werning JW. Oral cancer : diagnosis, management, and rehabilitation. New York: Thieme Medical Publishers; 2007. p. xiii.p. 354. [Google Scholar]
- 19.Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37:1–10. doi: 10.1111/j.1600-0714.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 20.van der Waal I, Schepman KP, van der Meij EH, Smeele LE. Oral leukoplakia: a clinicopathological review. Oral Oncol. 1997;33:291–301. doi: 10.1016/s1368-8375(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 21.Ferlito A, Silver CE, Rinaldo A. Elective management of the neck in oral cavity squamous carcinoma: current concepts supported by prospective studies. Br J Oral Maxillofac Surg. 2009;47:5–9. doi: 10.1016/j.bjoms.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu SY, Lu CL, Chiou CT, et al. Surgical outcomes and prognostic factors of oral cancer associated with betel quid chewing and tobacco smoking in Taiwan. Oral Oncol. doi: 10.1016/j.oraloncology.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Shingaki S, Kobayashi T, Suzuki I, Kohno M, Nakajima T. Surgical treatment of stage I and II oral squamous cell carcinomas: analysis of causes of failure. Br J Oral Maxillofac Surg. 1995;33:304–308. doi: 10.1016/0266-4356(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 24.Hinerman RW, Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head Neck. 2004;26:984–994. doi: 10.1002/hed.20091. [DOI] [PubMed] [Google Scholar]
- 25.Sun GW, Tang EY, Yang XD, Hu QG. Salvage treatment for recurrent oral squamous cell carcinoma. J Craniofac Surg. 2009;20:1093–1096. doi: 10.1097/SCS.0b013e3181abb307. [DOI] [PubMed] [Google Scholar]
- 26.Sox HC. Medical decision making. Boston: Butterworths; 1988. p. 406. [Google Scholar]
- 27.Jullien JA, Downer MC, Zakrzewska JM, Speight PM. Evaluation of a screening test for the early detection of oral cancer and precancer. Community Dent Health. 1995;12:3–7. [PubMed] [Google Scholar]
- 28.Mathew B, Sankaranarayanan R, Sunilkumar KB, Kuruvila B, Pisani P, Nair MK. Reproducibility and validity of oral visual inspection by trained health workers in the detection of oral precancer and cancer. Br J Cancer. 1997;76:390–394. doi: 10.1038/bjc.1997.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Healthcare Cost and Utilization Project (HCUP) HCUP Overview. Agency for Healthcare Research and Quality; Rockville, MD: Nov, 2009. www.hcup-us.ahrq.gov/overview.jsp. [PubMed] [Google Scholar]
- 30.Vokes EE, Schilsky RL, Choi KE, et al. A randomized study of inpatient versus outpatient continuous infusion chemotherapy for patients with locally advanced head and neck cancer. Cancer. 1989;63:30–36. doi: 10.1002/1097-0142(19890101)63:1<30::aid-cncr2820630105>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Mundt AJ, Roeske JC. Intensity modulated radiation therapy : a clinical perspective. Hamilton, Ont. ; Lewiston, N.Y: BC Decker; 2005. p. xxiv.p. 648. [Google Scholar]
- 32.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Downer MC, Jullien JA, Speight PM. An interim determination of health gain from oral cancer and precancer screening: 1. Obtaining health state utilities. Community Dent Health. 1997;14:139–142. [PubMed] [Google Scholar]
- 34.American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 35.Pindborg JJ, Roed-Peterson B, Renstrup G. Role of smoking in floor of the mouth leukoplakias. J Oral Pathol. 1972;1:22–29. [PubMed] [Google Scholar]
- 36.Menzin J, Lines LM, Manning LN. The economics of squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2007;15:68–73. doi: 10.1097/MOO.0b013e328017f669. [DOI] [PubMed] [Google Scholar]
- 37.Lang K, Sussman M, Friedman M, et al. Incidence and costs of treatment-related complications among patients with advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2009;135:582–588. doi: 10.1001/archoto.2009.46. [DOI] [PubMed] [Google Scholar]
- 38.Schepman KP, van der Meij EH, Smeele LE, van der Waal I. Malignant transformation of oral leukoplakia: a follow-up study of a hospital-based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncol. 1998;34:270–275. [PubMed] [Google Scholar]
- 39.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]