Abstract
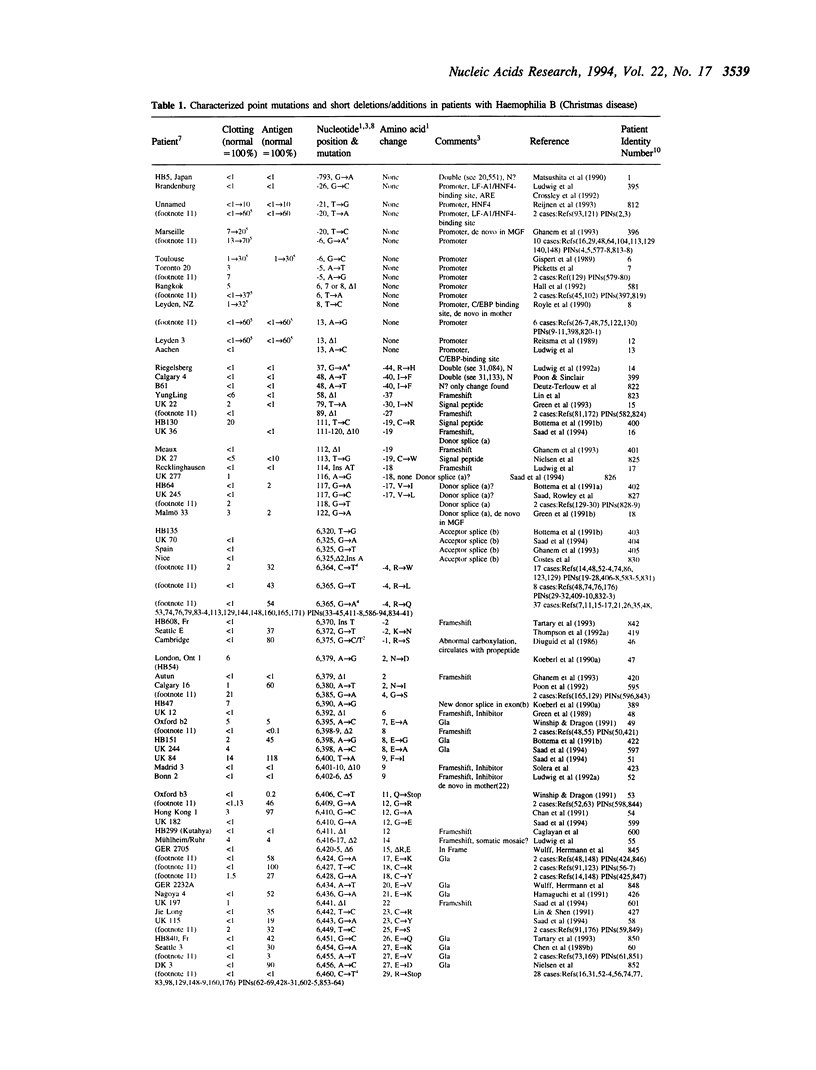
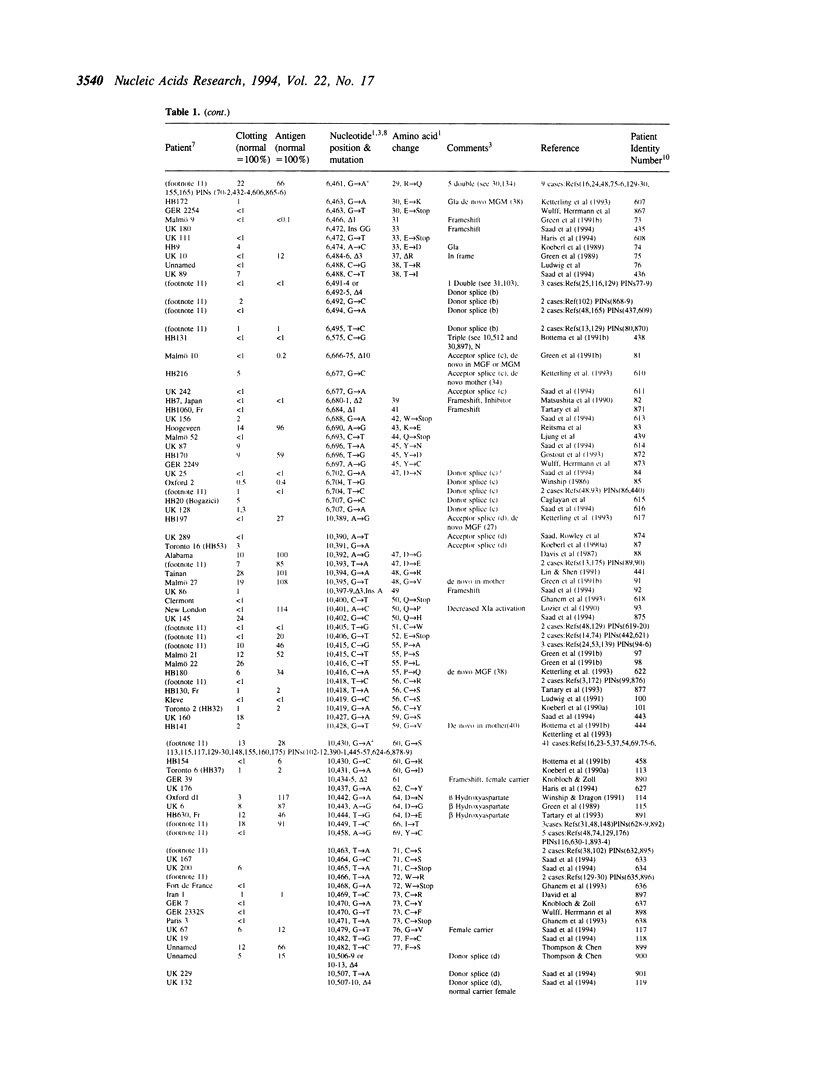
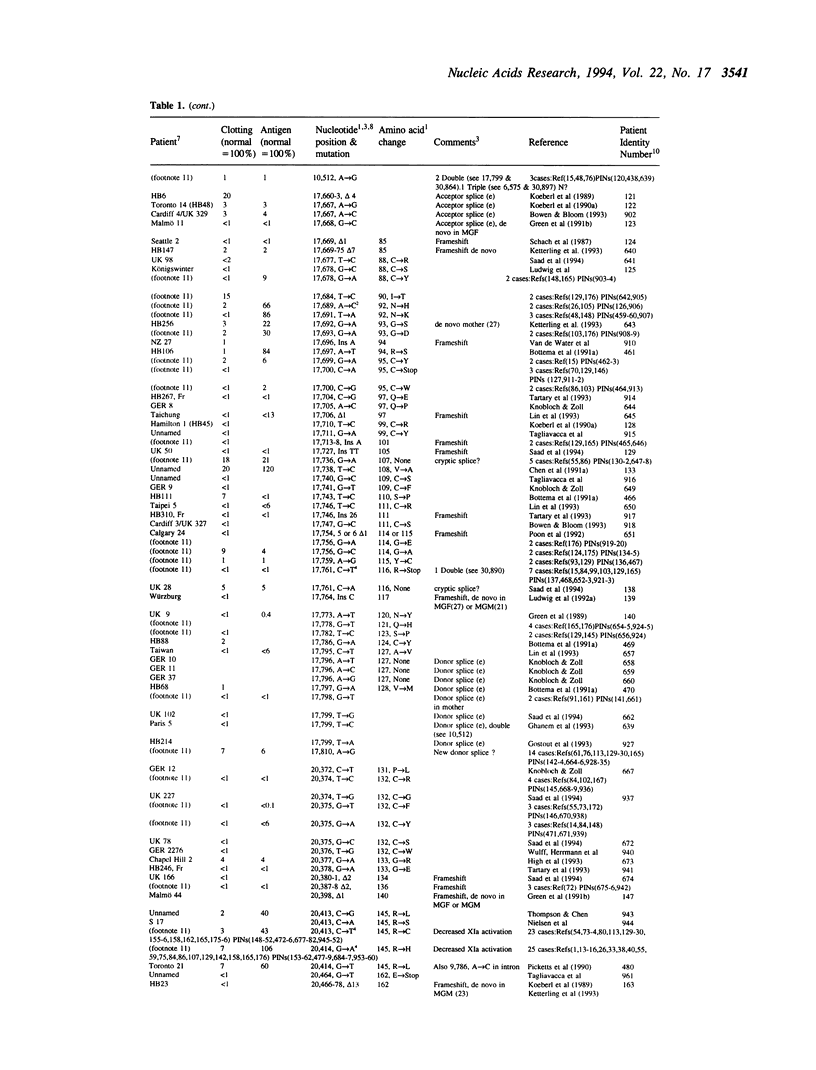
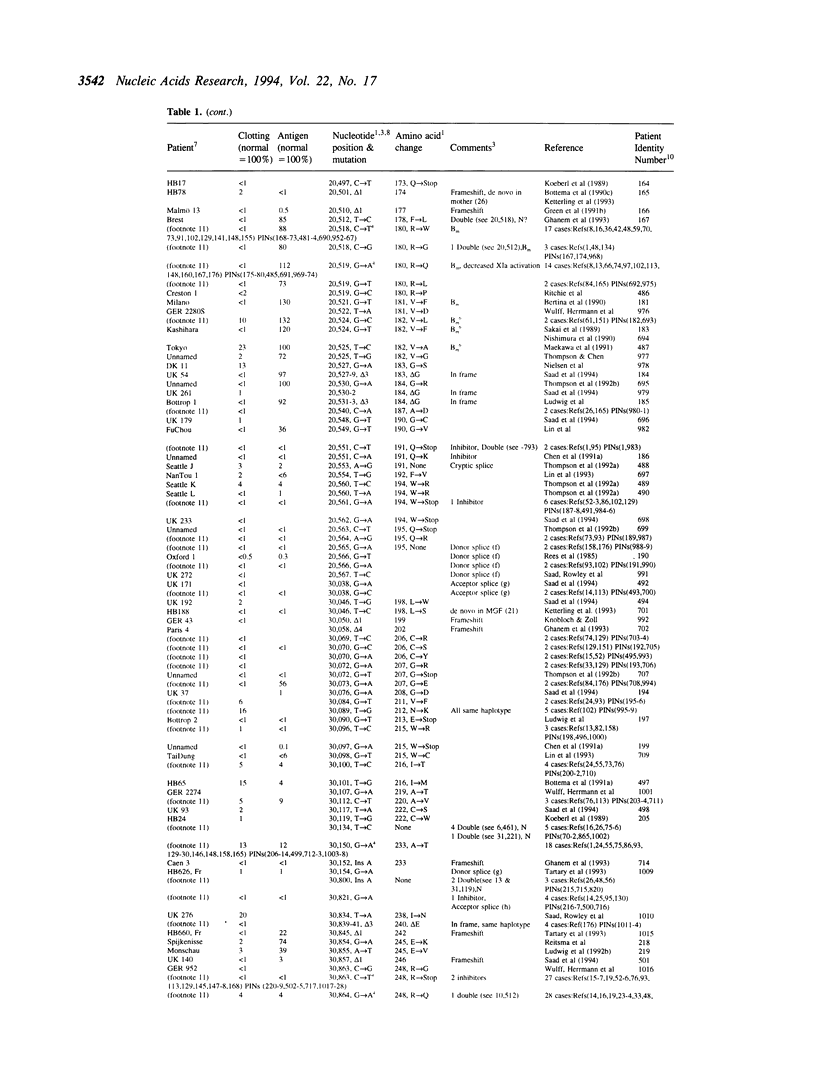
The fifth edition of the haemophilia B database lists in easily accessible form all known factor IX mutations due to small changes (base substitutions and short additions and/or deletions of < 30bp) identified in haemophilia B patients. The 1,142 patient entries are ordered by the nucleotide number of their mutation. Where known, details are given on: factor IX activity, factor IX antigen in circulation, and origin of mutation. References to published mutations are given and the laboratories generating the data are indicated.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M., Rodriguez Ponte M., Malik N. J., Hofmann S., Bösch-Al Jadooa N., Müller H., Bühler E. M. Factor IXBasel: a Swiss family with severe haemophilia B having a point mutation in EGF type B domain. Nucleic Acids Res. 1991 Jan 25;19(2):409–409. doi: 10.1093/nar/19.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley A. K., Rees D. J., Rizza C., Brownlee G. G. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position -4. Cell. 1986 May 9;45(3):343–348. doi: 10.1016/0092-8674(86)90319-3. [DOI] [PubMed] [Google Scholar]
- Bertina R. M., van der Linden I. K., Mannucci P. M., Reinalda-Poot H. H., Cupers R., Poort S. R., Reitsma P. H. Mutations in hemophilia Bm occur at the Arg180-Val activation site or in the catalytic domain of factor IX. J Biol Chem. 1990 Jul 5;265(19):10876–10883. [PubMed] [Google Scholar]
- Bottema C. D., Bottema M. J., Ketterling R. P., Yoon H. S., Janco R. L., Phillips J. A., 3rd, Sommer S. S. Why does the human factor IX gene have a G + C content of 40%? Am J Hum Genet. 1991 Oct;49(4):839–850. [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Cho H. I., Sommer S. S. Hemophilia B in a male with a four-base insertion that arose in the germline of his mother. Nucleic Acids Res. 1989 Dec 11;17(23):10139–10139. doi: 10.1093/nar/17.23.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Ii S., Yoon H. S., Phillips J. A., 3rd, Sommer S. S. Missense mutations and evolutionary conservation of amino acids: evidence that many of the amino acids in factor IX function as "spacer" elements. Am J Hum Genet. 1991 Oct;49(4):820–838. [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Koeberl D. D., Taylor S. A., Sommer S. S. Mutations at arginine residues in two Asian hemophilia B patients. Nucleic Acids Res. 1990 Apr 11;18(7):1924–1924. doi: 10.1093/nar/18.7.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Vielhaber E., Yoon H. S., Gostout B., Jacobson D. P., Shapiro A., Sommer S. S. The pattern of spontaneous germ-line mutation: relative rates of mutation at or near CpG dinucleotides in the factor IX gene. Hum Genet. 1993 Jun;91(5):496–503. doi: 10.1007/BF00217779. [DOI] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Yoon H. S., Sommer S. S. The pattern of factor IX germ-line mutation in Asians is similar to that of Caucasians. Am J Hum Genet. 1990 Nov;47(5):835–841. [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Koeberl D. D., Ketterling R. P., Bowie E. J., Taylor S. A., Lillicrap D., Shapiro A., Gilchrist G., Sommer S. S. A past mutation at isoleucine 397 is now a common cause of moderate/mild haemophilia B. Br J Haematol. 1990 Jun;75(2):212–216. doi: 10.1111/j.1365-2141.1990.tb02651.x. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Scott C. R., Schoof J., Lovrien E. W., Kurachi K. Factor IXPortland: a nonsense mutation (CGA to TGA) resulting in hemophilia B. Am J Hum Genet. 1989 Apr;44(4):567–569. [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Thompson A. R., Zhang M., Scott C. R. Three point mutations in the factor IX genes of five hemophilia B patients. Identification strategy using localization by altered epitopes in their hemophilic proteins. J Clin Invest. 1989 Jul;84(1):113–118. doi: 10.1172/JCI114130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Zhang M., Lovrien E. W., Scott C. R., Thompson A. R. CG dinucleotide transitions in the factor IX gene account for about half of the point mutations in hemophilia B patients: a Seattle series. Hum Genet. 1991 Jun;87(2):177–182. doi: 10.1007/BF00204177. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Zhang M., Thompson A. R., Bray G. L., Scott C. R. Splice junction mutations in factor IX gene resulting in severe hemophilia B. Nucleic Acids Res. 1991 Mar 11;19(5):1172–1172. doi: 10.1093/nar/19.5.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley M., Brownlee G. G. Disruption of a C/EBP binding site in the factor IX promoter is associated with haemophilia B. Nature. 1990 May 31;345(6274):444–446. doi: 10.1038/345444a0. [DOI] [PubMed] [Google Scholar]
- Crossley M., Winship P. R., Austen D. E., Rizza C. R., Brownlee G. G. A less severe form of Haemophilia B Leyden. Nucleic Acids Res. 1990 Aug 11;18(15):4633–4633. doi: 10.1093/nar/18.15.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. M., McGraw R. A., Ware J. L., Roberts H. R., Stafford D. W. Factor IXAlabama: a point mutation in a clotting protein results in hemophilia B. Blood. 1987 Jan;69(1):140–143. [PubMed] [Google Scholar]
- Denton P. H., Fowlkes D. M., Lord S. T., Reisner H. M. Hemophilia B Durham: a mutation in the first EGF-like domain of factor IX that is characterized by polymerase chain reaction. Blood. 1988 Oct;72(4):1407–1411. [PubMed] [Google Scholar]
- Diuguid D. L., Rabiet M. J., Furie B. C., Furie B. Molecular defects of factor IX Chicago-2 (Arg 145----His) and prothrombin Madrid (Arg 271----cys): arginine mutations that preclude zymogen activation. Blood. 1989 Jul;74(1):193–200. [PubMed] [Google Scholar]
- Diuguid D. L., Rabiet M. J., Furie B. C., Liebman H. A., Furie B. Molecular basis of hemophilia B: a defective enzyme due to an unprocessed propeptide is caused by a point mutation in the factor IX precursor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5803–5807. doi: 10.1073/pnas.83.16.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M. C., Bouhassira E., Aledort L. M. A codon 338 nonsense mutation in the factor IX gene in unrelated hemophilia B patients: factor IX338 New York. Blood. 1989 Aug 1;74(2):737–742. [PubMed] [Google Scholar]
- Emre M., Rinne U. K., Rascol A., Lees A., Agid Y., Lataste X. Effects of a selective partial D1 agonist, CY 208-243, in de novo patients with Parkinson disease. Mov Disord. 1992;7(3):239–243. doi: 10.1002/mds.870070309. [DOI] [PubMed] [Google Scholar]
- Fernández Gomez A., Ruz Polonio J., Luque de Castro M. D., Valcárcel Cases M. Automatic enzymatic-fluorimetric determination of ethanol in blood by flow injection analysis. Clin Chim Acta. 1985 May 30;148(2):131–137. doi: 10.1016/0009-8981(85)90223-2. [DOI] [PubMed] [Google Scholar]
- Frame B. Hypocalcemia and osteomalacia associated with anticonvulsant therapy. Ann Intern Med. 1971 Feb;74(2):294–295. doi: 10.7326/0003-4819-74-2-294. [DOI] [PubMed] [Google Scholar]
- Fraser B. M., Poon M. C., Hoar D. I. Identification of factor IX mutations in haemophilia B: application of polymerase chain reaction and single strand conformation analysis. Hum Genet. 1992 Feb;88(4):426–430. doi: 10.1007/BF00215677. [DOI] [PubMed] [Google Scholar]
- Giannelli F., Green P. M., High K. A., Sommer S., Lillicrap D. P., Ludwig M., Olek K., Reitsma P. H., Goossens M., Yoshioka A. Haemophilia B: database of point mutations and short additions and deletions--second edition. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2193–2219. doi: 10.1093/nar/19.suppl.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli F., Green P. M., High K. A., Sommer S., Poon M. C., Ludwig M., Schwaab R., Reitsma P. H., Goossens M., Yoshioka A. Haemophilia B: database of point mutations and short additions and deletions--fourth edition, 1993. Nucleic Acids Res. 1993 Jul 1;21(13):3075–3087. doi: 10.1093/nar/21.13.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostout B., Vielhaber E., Ketterling R. P., Yoon H. S., Bottema C. D., Kasper C. K., Koerper M. A., Sommer S. S. Germline mutations in the factor IX gene: a comparison of the pattern in Caucasians and non-Caucasians. Hum Mol Genet. 1993 Mar;2(3):293–298. doi: 10.1093/hmg/2.3.293. [DOI] [PubMed] [Google Scholar]
- Green P. M., Bentley D. R., Mibashan R. S., Nilsson I. M., Giannelli F. Molecular pathology of haemophilia B. EMBO J. 1989 Apr;8(4):1067–1072. doi: 10.1002/j.1460-2075.1989.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. M., Montandon A. J., Bentley D. R., Ljung R., Nilsson I. M., Giannelli F. The incidence and distribution of CpG----TpG transitions in the coagulation factor IX gene. A fresh look at CpG mutational hotspots. Nucleic Acids Res. 1990 Jun 11;18(11):3227–3231. doi: 10.1093/nar/18.11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. M., Montandon A. J., Ljung R., Bentley D. R., Nilsson I. M., Kling S., Giannelli F. Haemophilia B mutations in a complete Swedish population sample: a test of new strategy for the genetic counselling of diseases with high mutational heterogeneity. Br J Haematol. 1991 Jul;78(3):390–397. doi: 10.1111/j.1365-2141.1991.tb04453.x. [DOI] [PubMed] [Google Scholar]
- Green P. M., Montandon A. J., Ljung R., Nilsson I. M., Giannelli F. Haplotype analysis of identical factor IX mutants using PCR. Thromb Haemost. 1992 Jan 23;67(1):66–69. [PubMed] [Google Scholar]
- Hamaguchi M., Matsushita T., Tanimoto M., Takahashi I., Yamamoto K., Sugiura I., Takamatsu J., Ogata K., Kamiya T., Saito H. Three distinct point mutations in the factor IX gene of three Japanese CRM+ hemophilia B patients (factor IX BMNagoya 2, factor IX Nagoya 3 and 4). Thromb Haemost. 1991 May 6;65(5):514–520. [PubMed] [Google Scholar]
- Handford P. A., Mayhew M., Baron M., Winship P. R., Campbell I. D., Brownlee G. G. Key residues involved in calcium-binding motifs in EGF-like domains. Nature. 1991 May 9;351(6322):164–167. doi: 10.1038/351164a0. [DOI] [PubMed] [Google Scholar]
- Haris I. I., Green P. M., Bentley D. R., Giannelli F. Mutation detection by fluorescent chemical cleavage: application to hemophilia B. PCR Methods Appl. 1994 Apr;3(5):268–271. doi: 10.1101/gr.3.5.268. [DOI] [PubMed] [Google Scholar]
- Hase S., Kawabata S., Nishimura H., Takeya H., Sueyoshi T., Miyata T., Iwanaga S., Takao T., Shimonishi Y., Ikenaka T. A new trisaccharide sugar chain linked to a serine residue in bovine blood coagulation factors VII and IX. J Biochem. 1988 Dec;104(6):867–868. doi: 10.1093/oxfordjournals.jbchem.a122571. [DOI] [PubMed] [Google Scholar]
- Hirosawa S., Fahner J. B., Salier J. P., Wu C. T., Lovrien E. W., Kurachi K. Structural and functional basis of the developmental regulation of human coagulation factor IX gene: factor IX Leyden. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4421–4425. doi: 10.1073/pnas.87.12.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. N., Kasper C. K., Roberts H. R., Stafford D. W., High K. A. Molecular defect in factor IXHilo, a hemophilia Bm variant: Arg----Gln at the carboxyterminal cleavage site of the activation peptide. Blood. 1989 Feb 15;73(3):718–721. [PubMed] [Google Scholar]
- Ketterling R. P., Bottema C. D., Koeberl D. D., Ii S., Sommer S. S. T296----M, a common mutation causing mild hemophilia B in the Amish and others: founder effect, variability in factor IX activity assays, and rapid carrier detection. Hum Genet. 1991 Jul;87(3):333–337. doi: 10.1007/BF00200915. [DOI] [PubMed] [Google Scholar]
- Ketterling R. P., Bottema C. D., Phillips J. A., 3rd, Sommer S. S. Evidence that descendants of three founders constitute about 25% of hemophilia B in the United States. Genomics. 1991 Aug;10(4):1093–1096. doi: 10.1016/0888-7543(91)90207-u. [DOI] [PubMed] [Google Scholar]
- Ketterling R. P., Vielhaber E. L., Lind T. J., Thorland E. C., Sommer S. S. The rates and patterns of deletions in the human factor IX gene. Am J Hum Genet. 1994 Feb;54(2):201–213. [PMC free article] [PubMed] [Google Scholar]
- Ketterling R. P., Vielhaber E., Sommer S. S. The rates of G:C-->T:A and G:C-->C:G transversions at CpG dinucleotides in the human factor IX gene. Am J Hum Genet. 1994 May;54(5):831–835. [PMC free article] [PubMed] [Google Scholar]
- Knobloch O., Zoll B., Zerres K., Brackmann H. H., Olek K., Ludwig M. Recurrent mutations in the factor IX gene: founder effect or repeat de novo events. Investigation of the German haemophilia B population and review of de novo mutations. Hum Genet. 1993 Aug;92(1):40–48. doi: 10.1007/BF00216143. [DOI] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Buerstedde J. M., Sommer S. S. Functionally important regions of the factor IX gene have a low rate of polymorphism and a high rate of mutation in the dinucleotide CpG. Am J Hum Genet. 1989 Sep;45(3):448–457. [PMC free article] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Ketterling R. P., Bridge P. J., Lillicrap D. P., Sommer S. S. Mutations causing hemophilia B: direct estimate of the underlying rates of spontaneous germ-line transitions, transversions, and deletions in a human gene. Am J Hum Genet. 1990 Aug;47(2):202–217. [PMC free article] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Sarkar G., Ketterling R. P., Chen S. H., Sommer S. S. Recurrent nonsense mutations at arginine residues cause severe hemophilia B in unrelated hemophiliacs. Hum Genet. 1990 Apr;84(5):387–390. doi: 10.1007/BF00195805. [DOI] [PubMed] [Google Scholar]
- Liddell M. B., Lillicrap D. P., Peake I. R., Bloom A. L. Defective propeptide processing and abnormal activation underlie the molecular pathology of factor IX Troed-y-Rhiw. Br J Haematol. 1989 Jun;72(2):208–215. doi: 10.1111/j.1365-2141.1989.tb07684.x. [DOI] [PubMed] [Google Scholar]
- Liddell M. B., Peake I. R., Taylor S. A., Lillicrap D. P., Giddings J. C., Bloom A. L. Factor IX Cardiff: a variant factor IX protein that shows abnormal activation is caused by an arginine to cysteine substitution at position 145. Br J Haematol. 1989 Aug;72(4):556–560. doi: 10.1111/j.1365-2141.1989.tb04323.x. [DOI] [PubMed] [Google Scholar]
- Lin S. W., Shen M. C. Characterization of genetic defects of hemophilia B of Chinese origin. Thromb Haemost. 1991 Oct 1;66(4):459–463. [PubMed] [Google Scholar]
- Lin S. W., Shen M. C. Genetic basis and carrier detection of hemophilia B of Chinese origin. Thromb Haemost. 1993 Mar 1;69(3):247–252. [PubMed] [Google Scholar]
- Lozier J. N., Monroe D. M., Stanfield-Oakley S., Lin S. W., Smith K. J., Roberts H. R., High K. A. Factor IX New London: substitution of proline for glutamine at position 50 causes severe hemophilia B. Blood. 1990 Mar 1;75(5):1097–1104. [PubMed] [Google Scholar]
- Ludwig M., Brackmann H. H., Olek K. Prenatal diagnosis of haemophilia B by the use of polymerase chain reaction and direct sequencing. Klin Wochenschr. 1991 Mar 18;69(5):196–200. doi: 10.1007/BF01646940. [DOI] [PubMed] [Google Scholar]
- Ludwig M., Grimm T., Brackmann H. H., Olek K. Parental origin of factor IX gene mutations, and their distribution in the gene. Am J Hum Genet. 1992 Jan;50(1):164–173. [PMC free article] [PubMed] [Google Scholar]
- Ludwig M., Sabharwal A. K., Brackmann H. H., Olek K., Smith K. J., Birktoft J. J., Bajaj S. P. Hemophilia B caused by five different nondeletion mutations in the protease domain of factor IX. Blood. 1992 Mar 1;79(5):1225–1232. [PubMed] [Google Scholar]
- Ludwig M., Schwaab R., Eigel A., Horst J., Egli H., Brackmann H. H., Olek K. Identification of a single nucleotide C-to-T transition and five different deletions in patients with severe hemophilia B. Am J Hum Genet. 1989 Jul;45(1):115–122. [PMC free article] [PubMed] [Google Scholar]
- Ludwig M., Schwaab R., Olek K., Brackmann H. H., Egli H. Haemophilia B+ with inhibitor. Thromb Haemost. 1988 Apr 8;59(2):340–340. [PubMed] [Google Scholar]
- Matsushita T., Tanimoto M., Yamamoto K., Sugiura I., Hamaguchi M., Takamatsu J., Kamiya T., Saito H. DNA sequence analysis of three inhibitor-positive hemophilia B patients without gross gene deletion: identification of four novel mutations in factor IX gene. J Lab Clin Med. 1990 Oct;116(4):492–497. [PubMed] [Google Scholar]
- Miyata T., Sakai T., Sugimoto M., Naka H., Yamamoto K., Yoshioka A., Fukui H., Mitsui K., Kamiya K., Umeyama H. Factor IX Amagasaki: a new mutation in the catalytic domain resulting in the loss of both coagulant and esterase activities. Biochemistry. 1991 Nov 26;30(47):11286–11291. doi: 10.1021/bi00111a014. [DOI] [PubMed] [Google Scholar]
- Monroe D. M., McCord D. M., Huang M. N., High K. A., Lundblad R. L., Kasper C. K., Roberts H. R. Functional consequences of an arginine180 to glutamine mutation in factor IX Hilo. Blood. 1989 May 1;73(6):1540–1544. [PubMed] [Google Scholar]
- Montandon A. J., Green P. M., Bentley D. R., Ljung R., Nilsson I. M., Giannelli F. Two factor IX mutations in the family of an isolated haemophilia B patient: direct carrier diagnosis by amplification mismatch detection (AMD). Hum Genet. 1990 Jul;85(2):200–204. doi: 10.1007/BF00193196. [DOI] [PubMed] [Google Scholar]
- Montandon A. J., Green P. M., Giannelli F., Bentley D. R. Direct detection of point mutations by mismatch analysis: application to haemophilia B. Nucleic Acids Res. 1989 May 11;17(9):3347–3358. doi: 10.1093/nar/17.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. R., Schwartz M., Scheibel E. Screening for mutations in the gene encoding factor IX. J Inherit Metab Dis. 1992;15(3):339–341. doi: 10.1007/BF02435971. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Takao T., Hase S., Shimonishi Y., Iwanaga S. Human factor IX has a tetrasaccharide O-glycosidically linked to serine 61 through the fucose residue. J Biol Chem. 1992 Sep 5;267(25):17520–17525. [PubMed] [Google Scholar]
- Nishimura K., Harada K., Masuda S., Sugii A. High-performance liquid chromatography of serum albumins on an N-methylpyridinium polymer-based column. J Chromatogr. 1990 Jan 26;525(1):176–182. doi: 10.1016/s0378-4347(00)83391-5. [DOI] [PubMed] [Google Scholar]
- Noyes C. M., Griffith M. J., Roberts H. R., Lundblad R. L. Identification of the molecular defect in factor IX Chapel Hill: substitution of histidine for arginine at position 145. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4200–4202. doi: 10.1073/pnas.80.14.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang C. P., Crossley M., Kent G., Brownlee G. G. Comparative sequence analysis of mammalian factor IX promoters. Nucleic Acids Res. 1990 Nov 25;18(22):6731–6732. doi: 10.1093/nar/18.22.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picketts D. J., Lillicrap D. P., Mueller C. R. Synergy between transcription factors DBP and C/EBP compensates for a haemophilia B Leyden factor IX mutation. Nat Genet. 1993 Feb;3(2):175–179. doi: 10.1038/ng0293-175. [DOI] [PubMed] [Google Scholar]
- Poon M. C., Anand S., Fraser B. M., Hoar D. I., Sinclair G. D. Hemophilia B carrier determination based on family-specific mutation detection by DNA single-strand conformation analysis. J Lab Clin Med. 1993 Jul;122(1):55–63. [PubMed] [Google Scholar]
- Poon M. C., Chui D. H., Patterson M., Starozik D. M., Dimnik L. S., Hoar D. I. Hemophilia B (Christmas disease) variants and carrier detection analyzed by DNA probes. J Clin Invest. 1987 Apr;79(4):1204–1209. doi: 10.1172/JCI112938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poort S. R., Briët E., Bertina R. M., Reitsma P. H. A Dutch family with moderately severe hemophilia B (factor IXHeerde) has a missense mutation identical to that of factor IX London 2. Nucleic Acids Res. 1989 May 11;17(9):3614–3614. doi: 10.1093/nar/17.9.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poort S. R., Briët E., Bertina R. M., Reitsma P. H. A Dutch pedigree with mild hemophilia B with a missense mutation in the first EGF domain (factor IXOud en Nieuw Gastel). Nucleic Acids Res. 1989 Jul 25;17(14):5869–5869. doi: 10.1093/nar/17.14.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poort S. R., Briët E., Bertina R. M., Reitsma P. H. Two mutations of the factor IX gene including a donor splice consensus deletion and a point mutation in a Dutch patient with severe hemophilia B. Thromb Haemost. 1990 Nov 30;64(3):379–384. [PubMed] [Google Scholar]
- Rao K. J., Lyman G., Hamsbhushanam K., Scott J. P., Jagadeeswaran P. Human factor IXLincoln Park: a molecular characterization. Mol Cell Probes. 1990 Oct;4(5):335–340. doi: 10.1016/0890-8508(90)90024-t. [DOI] [PubMed] [Google Scholar]
- Reijnen M. J., Peerlinck K., Maasdam D., Bertina R. M., Reitsma P. H. Hemophilia B Leyden: substitution of thymine for guanine at position -21 results in a disruption of a hepatocyte nuclear factor 4 binding site in the factor IX promoter. Blood. 1993 Jul 1;82(1):151–158. [PubMed] [Google Scholar]
- Reitsma P. H., Bertina R. M., Ploos van Amstel J. K., Riemens A., Briët E. The putative factor IX gene promoter in hemophilia B Leyden. Blood. 1988 Sep;72(3):1074–1076. [PubMed] [Google Scholar]
- Reitsma P. H., Mandalaki T., Kasper C. K., Bertina R. M., Briët E. Two novel point mutations correlate with an altered developmental expression of blood coagulation factor IX (hemophilia B Leyden phenotype). Blood. 1989 Feb 15;73(3):743–746. [PubMed] [Google Scholar]
- Royle G., Van de Water N. S., Berry E., Ockelford P. A., Browett P. J. Haemophilia B Leyden arising de novo by point mutation in the putative factor IX promoter region. Br J Haematol. 1991 Feb;77(2):191–194. doi: 10.1111/j.1365-2141.1991.tb07976.x. [DOI] [PubMed] [Google Scholar]
- Saad S., Rowley G., Tagliavacca L., Green P. M., Giannelli F. First report on UK database of haemophilia B mutations and pedigrees. UK Haemophilia Centres. Thromb Haemost. 1994 May;71(5):563–570. [PubMed] [Google Scholar]
- Sakai T., Yoshioka A., Yamamoto K., Niinomi K., Fujimura Y., Fukui H., Miyata T., Iwanaga S. Blood clotting factor IX Kashihara: amino acid substitution of valine-182 by phenylalanine. J Biochem. 1989 May;105(5):756–759. doi: 10.1093/oxfordjournals.jbchem.a122740. [DOI] [PubMed] [Google Scholar]
- Schach B. G., Yoshitake S., Davie E. W. Hemophilia B (factor IXSeattle 2) due to a single nucleotide deletion in the gene for factor IX. J Clin Invest. 1987 Oct;80(4):1023–1028. doi: 10.1172/JCI113155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siguret V., Amselem S., Vidaud M., Assouline Z., Kerbiriou-Nabias D., Piétu G., Goossens M., Larrieu M. J., Bahnak B., Meyer D. Identification of a CpG mutation in the coagulation factor-IX gene by analysis of amplified DNA sequences. Br J Haematol. 1988 Dec;70(4):411–416. doi: 10.1111/j.1365-2141.1988.tb02509.x. [DOI] [PubMed] [Google Scholar]
- Solera J., Magallón M., Martin-Villar J., Coloma A. Identification of a new haemophilia BM case produced by a mutation located at the carboxy terminal cleavage site of activation peptide. Br J Haematol. 1991 Jul;78(3):385–389. doi: 10.1111/j.1365-2141.1991.tb04452.x. [DOI] [PubMed] [Google Scholar]
- Spitzer S. G., Kuppuswamy M. N., Saini R., Kasper C. K., Birktoft J. J., Bajaj S. P. Factor IXHollywood: substitution of Pro55 by Ala in the first epidermal growth factor-like domain. Blood. 1990 Oct 15;76(8):1530–1537. [PubMed] [Google Scholar]
- Spitzer S. G., Warn-Cramer B. J., Kasper C. K., Bajaj S. P. Replacement of isoleucine-397 by threonine in the clotting proteinase factor IXa (Los Angeles and Long Beach variants) affects macromolecular catalysis but not L-tosylarginine methyl ester hydrolysis. Lack of correlation between the ox brain prothrombin time and the mutation site in the variant proteins. Biochem J. 1990 Jan 1;265(1):219–225. doi: 10.1042/bj2650219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell K. M., Figueiredo M. S., Brownlee G. G., Jones P., Bolton-Maggs P. H. Haemophilia B Liverpool: a new British family with mild haemophilia B associated with a -6 G to A mutation in the factor IX promoter. Br J Haematol. 1993 Sep;85(1):188–190. doi: 10.1111/j.1365-2141.1993.tb08667.x. [DOI] [PubMed] [Google Scholar]
- Suehiro K., Kawabata S., Miyata T., Takeya H., Takamatsu J., Ogata K., Kamiya T., Saito H., Niho Y., Iwanaga S. Blood clotting factor IX BM Nagoya. Substitution of arginine 180 by tryptophan and its activation by alpha-chymotrypsin and rat mast cell chymase. J Biol Chem. 1989 Dec 15;264(35):21257–21265. [PubMed] [Google Scholar]
- Suehiro K., Miyata T., Takeya H., Takamatsu J., Saito H., Murakawa M., Okamura T., Niho Y., Iwanaga S. Blood clotting factor IX Nagoya 3: the molecular defect of zymogen activation caused by an arginine-145 to histidine substitution. Thromb Res. 1990 Nov 15;60(4):311–320. doi: 10.1016/0049-3848(90)90109-p. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Miyata T., Kawabata S., Yoshioka A., Fukui H., Iwanaga S. Factor IX Kawachinagano: impaired function of the Gla-domain caused by attached propeptide region due to substitution of arginine by glutamine at position -4. Br J Haematol. 1989 Jun;72(2):216–221. doi: 10.1111/j.1365-2141.1989.tb07685.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Miyata T., Kawabata S., Yoshioka A., Fukui H., Takahashi H., Iwanaga S. Blood clotting factor IX Niigata: substitution of alanine-390 by valine in the catalytic domain. J Biochem. 1988 Dec;104(6):878–880. doi: 10.1093/oxfordjournals.jbchem.a122575. [DOI] [PubMed] [Google Scholar]
- Tartary M., Vidaud D., Piao Y., Costa J. M., Bahnak B. R., Fressinaud E., Congard B., Laurian Y., Meyer D., Lavergne J. M. Detection of a molecular defect in 40 of 44 patients with haemophilia B by PCR and denaturing gradient gel electrophoresis. Br J Haematol. 1993 Aug;84(4):662–669. doi: 10.1111/j.1365-2141.1993.tb03143.x. [DOI] [PubMed] [Google Scholar]
- Taylor S. A., Deugau K. V., Lillicrap D. P. Somatic mosaicism and female-to-female transmission in a kindred with hemophilia B (factor IX deficiency). Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):39–42. doi: 10.1073/pnas.88.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. A., Liddell M. B., Peake I. R., Bloom A. L., Lillicrap D. P. A mutation adjacent to the beta cleavage site of factor IX (valine 182 to leucine) results in mild haemophilia Bm. Br J Haematol. 1990 Jun;75(2):217–221. doi: 10.1111/j.1365-2141.1990.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Thompson A. R., Bajaj S. P., Chen S. H., MacGillivray R. T. "Founder" effect in different families with haemophilia B mutation. Lancet. 1990 Feb 17;335(8686):418–418. doi: 10.1016/0140-6736(90)90259-8. [DOI] [PubMed] [Google Scholar]
- Thompson A. R., Chen S. H. Characterization of factor IX defects in hemophilia B patients. Methods Enzymol. 1993;222:143–169. doi: 10.1016/0076-6879(93)22011-4. [DOI] [PubMed] [Google Scholar]
- Thompson A. R. Molecular biology of the hemophilias. Prog Hemost Thromb. 1991;10:175–214. [PubMed] [Google Scholar]
- Thompson A. R., Schoof J. M., Weinmann A. F., Chen S. H. Factor IX mutations: rapid, direct screening methods for 20 new families with hemophilia B. Thromb Res. 1992 Jan 15;65(2):289–295. doi: 10.1016/0049-3848(92)90249-a. [DOI] [PubMed] [Google Scholar]
- Tsang T. C., Bentley D. R., Mibashan R. S., Giannelli F. A factor IX mutation, verified by direct genomic sequencing, causes haemophilia B by a novel mechanism. EMBO J. 1988 Oct;7(10):3009–3015. doi: 10.1002/j.1460-2075.1988.tb03164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhaber E., Jacobson D. P., Ketterling R. P., Liu J. Z., Sommer S. S. A mutation in the 3' untranslated region of the factor IX gene in four families with hemophilia B. Hum Mol Genet. 1993 Aug;2(8):1309–1310. doi: 10.1093/hmg/2.8.1309. [DOI] [PubMed] [Google Scholar]
- Wang N. S., Chen S. H., Thompson A. R. Point mutations in four hemophilia B patients from China. Thromb Haemost. 1990 Oct 22;64(2):302–306. [PubMed] [Google Scholar]
- Wang N. S., Zhang M., Thompson A. R., Chen S. H. Factor IX Chongqing: a new mutation in the calcium-binding domain of factor IX resulting in severe hemophilia B. Thromb Haemost. 1990 Feb 19;63(1):24–26. [PubMed] [Google Scholar]
- Ware J., Davis L., Frazier D., Bajaj S. P., Stafford D. W. Genetic defect responsible for the dysfunctional protein: factor IXLong Beach. Blood. 1988 Aug;72(2):820–822. [PubMed] [Google Scholar]
- Winship P. R., Dragon A. C. Identification of haemophilia B patients with mutations in the two calcium binding domains of factor IX: importance of a beta-OH Asp 64----Asn change. Br J Haematol. 1991 Jan;77(1):102–109. doi: 10.1111/j.1365-2141.1991.tb07955.x. [DOI] [PubMed] [Google Scholar]
- Winship P. R. Haemophilia B caused by mutation of a potential thrombin cleavage site in factor IX. Nucleic Acids Res. 1990 Mar 11;18(5):1310–1310. doi: 10.1093/nar/18.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]


