Abstract
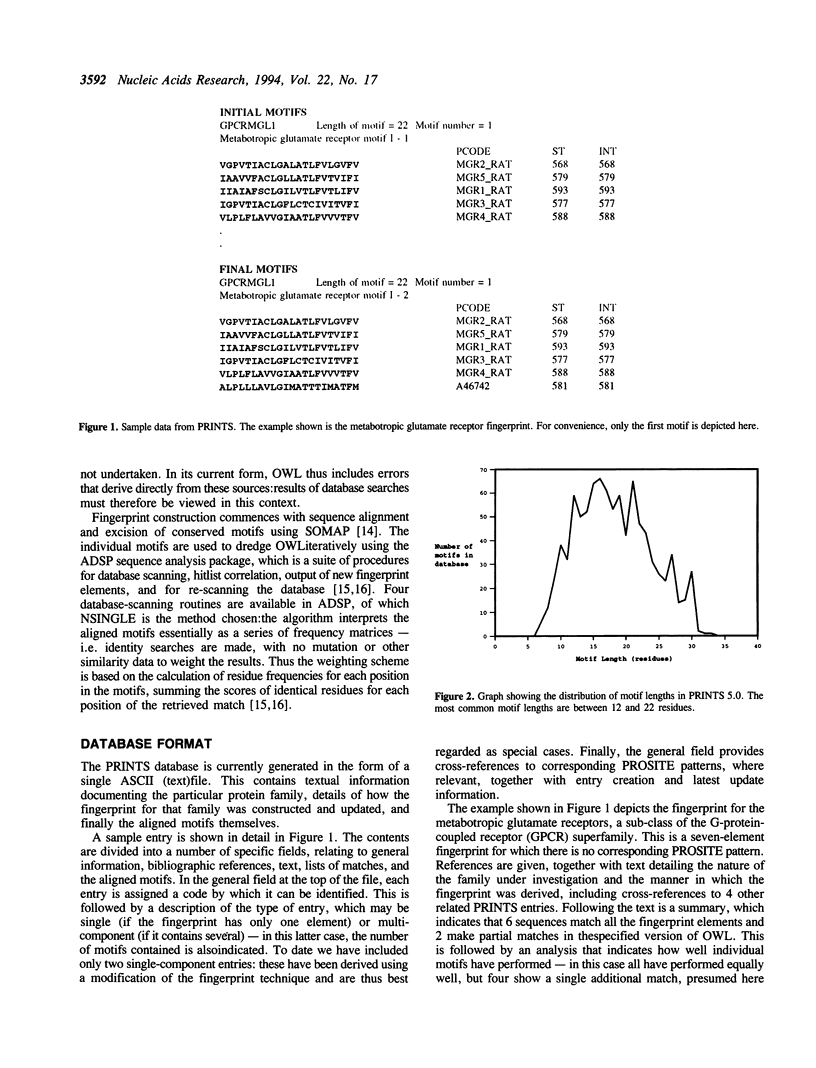
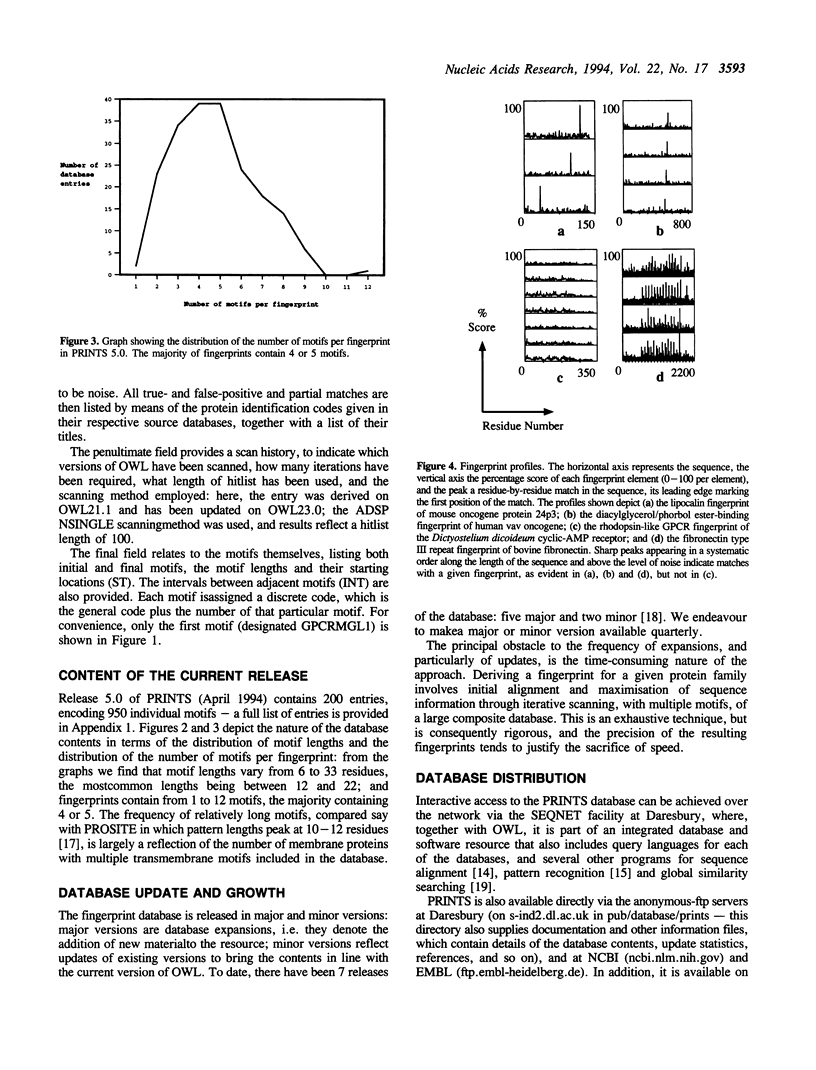
PRINTS is a compendium of protein motif 'fingerprints'. A fingerprint is defined as a group of motifs excised from conserved regions of a sequence alignment, whose diagnostic power or potency is refined by iterative databasescanning (in this case the OWL composite sequence database). Generally, the motifs do not overlap, but are separated along a sequence, though they may be contiguous in 3D-space. The use of groups of independent, linearly- or spatially-distinct motifs allows protein folds and functionalities to be characterised more flexibly and powerfully than conventional single-component patterns or regular expressions. The current version of the database contains 200 entries (encoding 950 motifs), covering a wide range of globular and membrane proteins, modular polypeptides, and so on. The growth of the databaseis influenced by a number of factors; e.g. the use of multiple motifs; the maximisation of sequence information through iterative database scanning; and the fact that the database searched is a large composite. The information contained within PRINTS is distinct from, but complementary to the consensus expressions stored in the widely-used PROSITE dictionary of patterns.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg D., Attwood T. K., Bleasby A. J., Findlay J. B., North A. C., Maughan N. A., Parry-Smith D. J., Perkins D. N., Wootton J. C. SERPENT--an information storage and analysis resource for protein sequences. Comput Appl Biosci. 1992 Jun;8(3):295–296. doi: 10.1093/bioinformatics/8.3.295. [DOI] [PubMed] [Google Scholar]
- Attwood T. K., Beck M. E. PRINTS--a protein motif fingerprint database. Protein Eng. 1994 Jul;7(7):841–848. doi: 10.1093/protein/7.7.841. [DOI] [PubMed] [Google Scholar]
- Attwood T. K., Findlay J. B. Design of a discriminating fingerprint for G-protein-coupled receptors. Protein Eng. 1993 Feb;6(2):167–176. doi: 10.1093/protein/6.2.167. [DOI] [PubMed] [Google Scholar]
- Attwood T. K., Findlay J. B. Fingerprinting G-protein-coupled receptors. Protein Eng. 1994 Feb;7(2):195–203. doi: 10.1093/protein/7.2.195. [DOI] [PubMed] [Google Scholar]
- Bairoch A., Boeckmann B. The SWISS-PROT protein sequence data bank, recent developments. Nucleic Acids Res. 1993 Jul 1;21(13):3093–3096. doi: 10.1093/nar/21.13.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairoch A. The PROSITE dictionary of sites and patterns in proteins, its current status. Nucleic Acids Res. 1993 Jul 1;21(13):3097–3103. doi: 10.1093/nar/21.13.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker W. C., George D. G., Mewes H. W., Pfeiffer F., Tsugita A. The PIR-International databases. Nucleic Acids Res. 1993 Jul 1;21(13):3089–3092. doi: 10.1093/nar/21.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D., Lipman D. J., Ostell J. GenBank. Nucleic Acids Res. 1993 Jul 1;21(13):2963–2965. doi: 10.1093/nar/21.13.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasby A. J., Wootton J. C. Construction of validated, non-redundant composite protein sequence databases. Protein Eng. 1990 Jan;3(3):153–159. doi: 10.1093/protein/3.3.153. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Bairoch A., Attwood T. K., Michaels G. S. Proto-vav and gene expression. Nature. 1992 Jul 9;358(6382):113–113. doi: 10.1038/358113a0. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Satchwell S. C., Preddie E., Weston K. M., Barrell B. G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990 Apr 19;344(6268):774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Flower D. R., North A. C., Attwood T. K. Mouse oncogene protein 24p3 is a member of the lipocalin protein family. Biochem Biophys Res Commun. 1991 Oct 15;180(1):69–74. doi: 10.1016/s0006-291x(05)81256-2. [DOI] [PubMed] [Google Scholar]
- Flower D. R., North A. C., Attwood T. K. Structure and sequence relationships in the lipocalins and related proteins. Protein Sci. 1993 May;2(5):753–761. doi: 10.1002/pro.5560020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Homyak M., Edenfield J., Eisenberg D. Profile scanning for three-dimensional structural patterns in protein sequences. Comput Appl Biosci. 1988 Mar;4(1):61–66. doi: 10.1093/bioinformatics/4.1.61. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Automated assembly of protein blocks for database searching. Nucleic Acids Res. 1991 Dec 11;19(23):6565–6572. doi: 10.1093/nar/19.23.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Taylor W. R., Thornton J. M. A mutation data matrix for transmembrane proteins. FEBS Lett. 1994 Feb 21;339(3):269–275. doi: 10.1016/0014-5793(94)80429-x. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Taylor W. R., Thornton J. M. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992 Jun;8(3):275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Mehldau G., Myers G. A system for pattern matching applications on biosequences. Comput Appl Biosci. 1993 Jun;9(3):299–314. doi: 10.1093/bioinformatics/9.3.299. [DOI] [PubMed] [Google Scholar]
- Ogiwara A., Uchiyama I., Seto Y., Kanehisa M. Construction of a dictionary of sequence motifs that characterize groups of related proteins. Protein Eng. 1992 Sep;5(6):479–488. doi: 10.1093/protein/5.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry-Smith D. J., Attwood T. K. ADSP--a new package for computational sequence analysis. Comput Appl Biosci. 1992 Oct;8(5):451–459. doi: 10.1093/bioinformatics/8.5.451. [DOI] [PubMed] [Google Scholar]
- Parry-Smith D. J., Attwood T. K. SOMAP: a novel interactive approach to multiple protein sequences alignment. Comput Appl Biosci. 1991 Apr;7(2):233–235. doi: 10.1093/bioinformatics/7.2.233. [DOI] [PubMed] [Google Scholar]
- Pattabiraman N., Namboodiri K., Lowrey A., Gaber B. P. NRL-3D: a sequence-structure database derived from the protein data bank (PDB) and searchable within the PIR environment. Protein Seq Data Anal. 1990 Oct;3(5):387–405. [PubMed] [Google Scholar]
- Persson B., Argos P. Prediction of transmembrane segments in proteins utilising multiple sequence alignments. J Mol Biol. 1994 Mar 25;237(2):182–192. doi: 10.1006/jmbi.1994.1220. [DOI] [PubMed] [Google Scholar]
- Pongor S., Skerl V., Cserzö M., Hátsági Z., Simon G., Bevilacqua V. The SBASE protein domain library, release 2.0: a collection of annotated protein sequence segments. Nucleic Acids Res. 1993 Jul 1;21(13):3111–3115. doi: 10.1093/nar/21.13.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqi M. A., Sternberg M. J. Identification of sequence motifs from a set of proteins with related function. Protein Eng. 1994 Feb;7(2):165–171. doi: 10.1093/protein/7.2.165. [DOI] [PubMed] [Google Scholar]
- Seto Y., Ikeuchi Y., Kanehisa M. Fragment peptide library for classification and functional prediction of proteins. Proteins. 1990;8(4):341–351. doi: 10.1002/prot.340080408. [DOI] [PubMed] [Google Scholar]



