Abstract
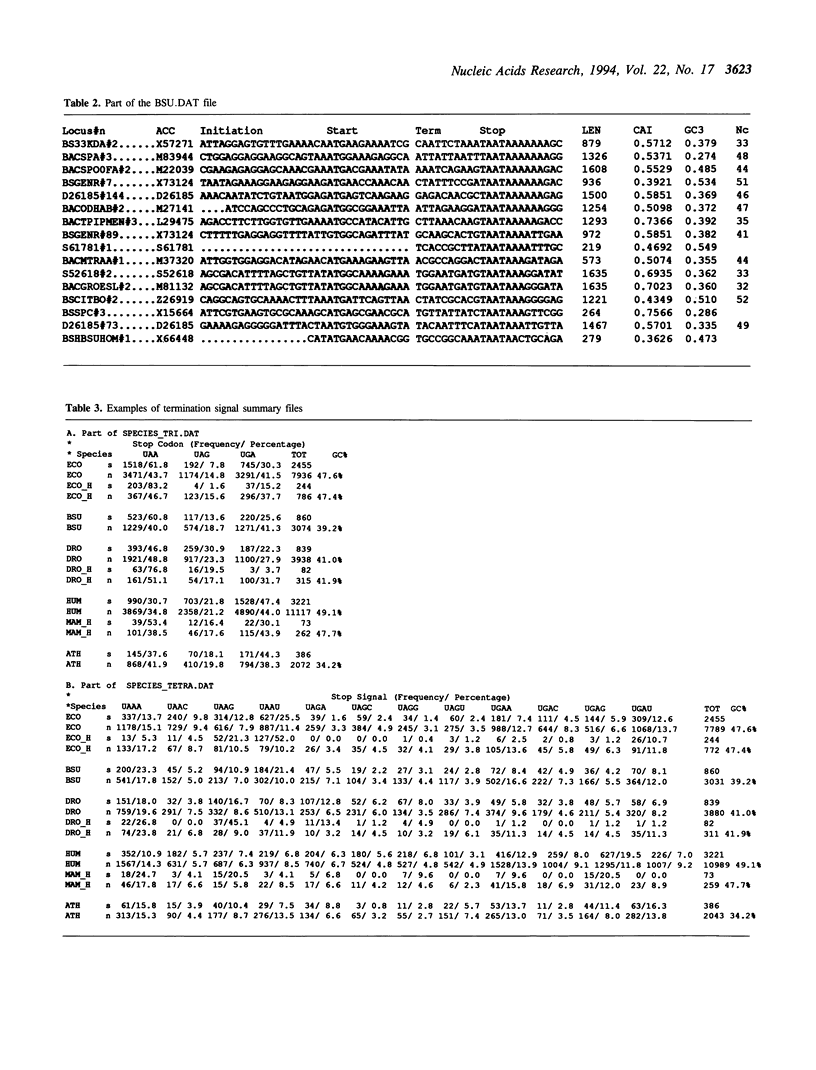
The TransTerm database of termination codon contexts has been extended to include sense codon usage, and initiation codon contexts. The database was constructed from 23,721 coding sequences from 93 organisms. The database contains: a) the sequence around the termination codon (-10, +10); b) the sequence around the initiation codon (-20, +10); c) the length, 'G+C%' of the third position of codons (GC3), the 'codon adaptation index' (CAI) and the 'effective number of codons' statistic (Nc); d) summary tables for each organism including total codon usage, stop codon and tetranucleotide stop-signal usage, and matrices tallying base frequencies at each position around the initiation and termination codons. The data are arranged to facilitate investigation of the relationships between the three phases of protein synthesis. The database is available electronically from EMBL.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson D., Lipman D. J., Ostell J. GenBank. Nucleic Acids Res. 1993 Jul 1;21(13):2963–2965. doi: 10.1093/nar/21.13.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Dalphin M. E., Stockwell P. A., Tate W. P. The translational termination signal database. Nucleic Acids Res. 1993 Jul 1;21(13):3119–3123. doi: 10.1093/nar/21.13.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Stockwell P. A., Trotman C. N., Tate W. P. Sequence analysis suggests that tetra-nucleotides signal the termination of protein synthesis in eukaryotes. Nucleic Acids Res. 1990 Nov 11;18(21):6339–6345. doi: 10.1093/nar/18.21.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Stockwell P. A., Trotman C. N., Tate W. P. The signal for the termination of protein synthesis in procaryotes. Nucleic Acids Res. 1990 Apr 25;18(8):2079–2086. doi: 10.1093/nar/18.8.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R., Ray S. C. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991 Jun 25;19(12):3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. W., Jukes T. H. Relationship between G + C in silent sites of codons and amino acid composition of human proteins. J Mol Evol. 1993 Mar;36(3):201–213. doi: 10.1007/BF00160475. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre-Walker A. DNA mismatch repair and synonymous codon evolution in mammals. Mol Biol Evol. 1994 Jan;11(1):88–98. doi: 10.1093/oxfordjournals.molbev.a040095. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland C. G. Major codon preference: theme and variations. Biochem Soc Trans. 1993 Nov;21(4):841–846. doi: 10.1042/bst0210841. [DOI] [PubMed] [Google Scholar]
- Lloyd A. T., Sharp P. M. Codon usage in Aspergillus nidulans. Mol Gen Genet. 1991 Nov;230(1-2):288–294. doi: 10.1007/BF00290679. [DOI] [PubMed] [Google Scholar]
- Martin R. On the relationship between preferred termination codon contexts and nonsense suppression in human cells. Nucleic Acids Res. 1994 Jan 11;22(1):15–19. doi: 10.1093/nar/22.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W. T., Curran J. F. Effects of the nucleotide 3' to an amber codon on ribosomal selection rates of suppressor tRNA and release factor-1. J Mol Biol. 1991 May 20;219(2):231–241. doi: 10.1016/0022-2836(91)90564-m. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987 Feb 11;15(3):1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Stenico M., Peden J. F., Lloyd A. T. Codon usage: mutational bias, translational selection, or both? Biochem Soc Trans. 1993 Nov;21(4):835–841. doi: 10.1042/bst0210835. [DOI] [PubMed] [Google Scholar]
- Sueoka N. Directional mutation pressure, selective constraints, and genetic equilibria. J Mol Evol. 1992 Feb;34(2):95–114. doi: 10.1007/BF00182387. [DOI] [PubMed] [Google Scholar]
- Tate W. P., Brown C. M. Translational termination: "stop" for protein synthesis or "pause" for regulation of gene expression. Biochemistry. 1992 Mar 10;31(9):2443–2450. doi: 10.1021/bi00124a001. [DOI] [PubMed] [Google Scholar]
- Tzareva N. V., Makhno V. I., Boni I. V. Ribosome-messenger recognition in the absence of the Shine-Dalgarno interactions. FEBS Lett. 1994 Jan 10;337(2):189–194. doi: 10.1016/0014-5793(94)80271-8. [DOI] [PubMed] [Google Scholar]
- Wada K., Wada Y., Ishibashi F., Gojobori T., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1992 May 11;20 (Suppl):2111–2118. doi: 10.1093/nar/20.suppl.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. The 'effective number of codons' used in a gene. Gene. 1990 Mar 1;87(1):23–29. doi: 10.1016/0378-1119(90)90491-9. [DOI] [PubMed] [Google Scholar]
- de Smit M. H., van Duin J. Translational initiation on structured messengers. Another role for the Shine-Dalgarno interaction. J Mol Biol. 1994 Jan 7;235(1):173–184. doi: 10.1016/s0022-2836(05)80024-5. [DOI] [PubMed] [Google Scholar]


