Abstract
A number of studies have indicated that plasma membrane calcium ATPases (PMCAs) are expressed in the brain and spinal cord and could play important roles not only in the maintenance of cellular calcium homeostasis but also in the survival and function of central nervous system cells under pathological conditions. The different regional and cellular distributions of the various PMCA isoforms and splice variants in the nervous system and the diverse phenotypes of PMCA knockout mice support the notion that each isoform might play a distinct role. Especially in the spinal cord, the survival of neurons and, in particular, motor neurons could be dependent on PMCA2. This is indicated by the knockdown of PMCA2 in pure spinal cord neuronal cultures that leads to cell death via a decrease in collapsing response mediator protein 1 levels. Moreover, the progressive decline in the number of motor neurons in PMCA2-null mice and heterozygous mice further supports this notion. Therefore, the reported reduction in PMCA2 mRNA and protein levels in the inflamed spinal cord of mice affected by experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, and after spinal cord contusion injury, suggests that changes in PMCA2 expression could be a cause of neuronal pathology and death during inflammation and injury. Glutamate excitotoxicity mediated via kainate receptors has been implicated in the neuropathology of both EAE and spinal cord injury, and has been identified as a trigger that reduces PMCA2 levels in pure spinal cord neuronal cultures through degradation of the pump by calpain without affecting PMCA2 transcript levels. It remains to be determined which other stimuli modulate PMCA2 mRNA expression in the aforementioned pathological conditions of the spinal cord.
Keywords: Multiple sclerosis, Spinal cord injury, Neuroprotection, Neurodegeneration, Excitotoxicity, ATP2b2, Calpain, Glia
INTRODUCTION
Increasing evidence supports the notion that plasma membrane calcium ATPases (PMCAs) play critical roles in pathological conditions including those affecting the central nervous system[1,2]. In particular, investigations have shown that PMCA isoform 2 might be essential for the function and survival of spinal cord neurons, especially motor neurons[3,4]. The goal of this article is to summarize the major conceptual findings of studies that highlight the importance of PMCA2 in the spinal cord and to discuss the potential involvement of this calcium pump in neuronal degeneration in animal models of multiple sclerosis and spinal cord injury.
DISTRIBUTION OF PMCA ISOFORMS IN THE SPINAL CORD
There are four mammalian genes that encode the different PMCA isoforms (PMCA1-4)[5-7]. PMCA1 and PMCA4 are ubiquitously expressed throughout most tissues, including the brain and the spinal cord, whereas PMCA2 and -3 are especially abundant in the spinal cord and the brain[8-12].
The alternative splicing of the primary PMCA transcripts generates approximately 30 variants, each with a unique distribution, cellular localization, signaling cascade and functional characteristics[9,13]. Splicing at the C-terminal calmodulin binding domain, generates the a, b, c, d, e and g variants[14]. The a and b variants are associated with all four genes[14]. PMCA1a and -1c mRNAs are primarily localized to excitable tissues such as the brain and spinal cord, in addition to the heart and skeletal muscle, while the PMCA1b is found in most tissues[8]. The presence of PMCA2, PMCA3a and -3b mRNAs in the spinal cord has also been described[8]. Importantly, these investigators raised the possibility of additional PMCA2 and PMCA3 splice variants in the spinal cord as compared to the brain or other tissues. Thus, the diversity in PMCA splice variants might be higher in the spinal cord. Both PMCA4a and -4b variants are found in the spinal cord.
Studies on the cellular localization of PMCA mRNA and protein by use of in situ hybridization and immunocytochemistry, respectively, confirmed the presence of all isoforms in the spinal cord[12]. PMCA1, -2 and -3 mRNAs were expressed in neuronal cell bodies throughout the layers of the spinal cord whereas PMCA4 mRNA was localized to a small number of neurons in laminae I-V. The intensity of the PMCA2 signal was the strongest.
Immunocytochemistry with antibodies specific to each isoform localized staining mostly to the neuropil, although PMCA4 immunoreactivity was also found in the cell bodies of neurons in laminae II-IV[12]. The distribution of the staining was distinct for each isoform. PMCA1 and PMCA3 immunoreactivity was found in the neuropil of all layers but was highest in laminae III and IV of the dorsal horn, whereas PMCA2 immunopositive processes were found in all layers except the superficial laminae of the dorsal horn. In contrast, PMCA4 staining was strongest in the superficial laminae of the dorsal horn. Therefore, it was suggested that PMCA2 expression is associated with myelinated thick fibers whereas PMCA4 is found in unmyelinated thin afferents. Taken together, these results indicate that PMCA isoforms and splice variants might play specific roles in the spinal cord.
A recent study analyzing the distribution of PMCA2a splice variants in the central nervous system has shown that PMCA2a staining is detected throughout the spinal cord, mostly in processes and the neuropil. These investigators did not detect PMCA2a staining in neuronal cell bodies, including motor neurons. However, unstained α-motor neuron-like cell bodies were surrounded by numerous PMCA2a-immunoreactive puncta[15]. Thus, PMCA2a could be critical for the extrusion of calcium at some of the presynaptic terminals projecting on α-motor neurons.
CRITICAL ROLE OF PMCA2 IN THE SURVIVAL OF SPINAL CORD NEURONS, IN VITRO AND IN VIVO
Whereas the discrete localization of the various PMCA isoforms in the spinal cord suggests specialized functions, information about their precise role is limited.
In vitro investigations have shown that PMCAs are pivotal for the survival of spinal cord neurons. Inhibition of all PMCA activity by addition of 5(6)-carboxyeosin to pure neuronal cultures leads to cell death. This is preceded by a delay in the clearance of depolarization-induced calcium transients, neuritic damage and induction of activated caspase-3[3]. These studies also indicated that most spinal cord neurons are dependent on PMCA activity for the effective clearance of depolarization-induced calcium transients, in vitro. However, about half of the cells showed higher sensitivity to inhibition of PMCA activity. In particular, motor neuron-like cells, identified by morphology, are most sensitive to blockade of PMCA activity as elevations in intracellular calcium levels after KCl-induced depolarization do not return to basal values in the presence of 20 μmol/L carboxyeosin. In accordance, in terms of survival, motor neurons are most vulnerable to inhibition of PMCA activity as compared to the total population of spinal cord neurons in the culture dish, since their number decreases after exposure to CE for a shorter time period.
Initially, it was believed that PMCAs play a minor role in calcium extrusion, especially as compared to the Na+/Ca2+ exchanger, another major calcium removal system. However, the activity of PMCAs can be modulated by a number of factors including calmodulin, phosphorylation by kinases, the protease calpain and caspases 1 and 3[16]. The studies of Kurnellas et al[3] indicate that PMCAs are essential not only for the maintenance of basal calcium levels but also for the clearance of elevated intracellular calcium after stimulation or depolarization of spinal cord neurons. Other investigators have also reported that PMCAs contribute significantly to the removal of calcium from different neuronal subtypes in the retina and the dorsal root ganglia[17-21].
Recent studies defined the specific contribution of PMCA2 to the survival of spinal cord neurons. Knockdown of PMCA2 by use of small interfering RNA (siRNA) was sufficient to cause death of spinal cord neurons, in vitro[4]. Thus, spinal cord neurons might be especially dependent on PMCA2 expression and activity for their normal and healthy function. The particular dependence of motor neurons on PMCA2 is illustrated by investigations on PMCA2-null mice and heterozygous mice[22]. Progressive loss of motor neurons in PMCA2-null mice and delayed loss of motor neurons in PMCA2-heterozygous mice have been reported[23]. Accordingly, motor unit number estimates, an electrophysiological approach that can detect early abnormalities in motor units, are lower in both PMCA2-null mice and heterozygous mice. In agreement with these findings, PMCA2-null mice exhibit reduced hindlimb grip strength.
MECHANISMS DOWNSTREAM TO PMCA2 THAT MEDIATE SPINAL CORD NEURONAL DEATH
What are the specific mechanisms that mediate cell death when PMCA2 expression is reduced in spinal cord neurons? This issue has been addressed by defining the proteome profile after knockdown of PMCA2 by siRNA in spinal cord neurons, in vitro. Collapsin response mediator protein 1 (CRMP1) has been identified as a candidate. Although the relationship between PMCA2 and CRMP1 is not yet defined, these studies have shown that CRMP1 levels are decreased after silencing of PMCA2 expression. This is followed by death of cultured spinal cord neurons[4]. In agreement with these findings, silencing of CRMP1 also leads to neuronal death. Further studies are necessary in order to determine why a reduction in PMCA2 levels is associated with a decrease in CRMP1 and how a fall in CRMP1 levels causes cell death. One possibility is perturbation of the cytoskeleton as some CRMPs have been implicated in microtubule assembly[24,25]. Dendritic anomalies in hippocampal neurons of CRMP1 knockout mice[26] and an involvement of CRMP1 in dendritic growth[27] have also been reported suggesting that reductions in CRMP1 could affect neurons in additional ways that are not yet fully defined but warrant further investigations.
REDUCTIONS IN SPINAL CORD PMCA2 LEVELS IN ANIMAL MODELS OF MULTIPLE SCLEROSIS AND SPINAL CORD INJURY
The importance of PMCA2 in pathological conditions of the spinal cord is illustrated by studies on two different animal models of human disease.
One of these animal models, experimental autoimmune encephalomyelitis (EAE), mimics features of multiple sclerosis[28-30]. EAE is induced by immunization of rats or mice with myelin components. Rodents affected by EAE develop progressive ascending paralysis starting from tail weakness and reaching quadriplegia. In most EAE models, the central nervous system region most affected is the spinal cord and especially the lumbar region. Histopathological landmarks include infiltration of inflammatory cells into the spinal cord, activation of resident glial cells, axonal damage, neuronal loss and in some models, demyelination of axons projecting from the brain into the spinal cord. A drop in spinal cord PMCA2 levels at onset of the first EAE symptom; i.e. tail weakness has been reported in both the rat and the mouse[10,31]. Most importantly, changes in PMCA2 expression were consistent with manifestation of clinical deficits. In the rat model, PMCA2 levels were restored to control values, just prior to remission from clinical deficits whereas in the mouse model, in which there is no functional remission, they remained low throughout the course of the disease[31]. In agreement with these results, recovery of function after administration of an α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate receptor antagonist, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), to mice with EAE, restored both PMCA2 and CRMP1 levels. This treatment has been shown to be effective in preventing the progression or reversing the symptoms and pathology of EAE[4,32,33]. Therefore, it has been hypothesized that before very late disease stages are reached, there could be a phase during which the decrease in PMCA2 can be reversed[4].
PMCA2 levels are also decreased after spinal cord injury. Gene profiling by microarray analysis showed a reduction in PMCA2 transcript levels in a segment containing the epicenter after spinal cord contusion injury in the rat[34]. PMCA2 mRNA expression was decreased as early as 6 h post injury and was further reduced at 48 h, the latest time examined. Tachibana et al[11] confirmed the reduction in PMCA2 mRNA levels in segments containing the epicenter at 24 h post-contusion injury by using cDNA microarray analysis and reverse transcription-polymerase chain reaction (RT-PCR). Other investigations demonstrated a decrease in PMCA2 in spinal cord segments at a distance from the epicenter, 48 h post-contusion injury using quantitative RT-PCR[35]. Thus, a reduction in PMCA2 could contribute to the aggravation of neuronal pathology and death after spinal cord injury.
In sum, these investigations raise the possibility that neuronal damage and loss in animal models of multiple sclerosis and spinal cord injury might share commonalties and could be partly mediated by a decrease in PMCA2 expression.
TRIGGERS AND MECHANISMS MEDIATING A DECREASE IN PMCA2 LEVELS, IN VITRO
A number of candidates could induce the decrease in neuronal PMCA2 levels in inflammatory conditions of the spinal cord. Both in the case of EAE and spinal cord injury, glutamate excitotoxicity has been implicated in the damage of neuronal and non-neuronal cells[36,37]. Other studies showed a decrease in hippocampal PMCA2 after injection of kainic acid[38]. These findings prompted further investigations on the modulation of PMCA2 levels after exposure of spinal cord neuronal cultures to kainic acid. The concentrations employed were below the conventional amounts used to induce excitotoxicity. However, the cells were exposed to kainate continuously. Kainic acid decreased PMCA2 protein but not mRNA levels. The reduction was most likely the consequence of calpain-mediated degradation of PMCA2, as calpastatin, a calpain inhibitor, prevented the decrease[4]. Glutamate-induced, calpain-mediated loss of PMCA2 activity in hippocampal neurons has been reported[39]. However, this was ascribed to the internalization of the calcium pump.
Restoration of PMCA2 levels after administration of NBQX to mice with EAE further supports the notion that glutamate, acting via the AMPA/kainate receptors, could be a stimulus decreasing PMCA2 levels either by direct actions on neurons and/or indirectly, by stimulating other cells including glia which, in turn, could release substances that modulate PMCA2 levels. It is not yet known whether degradation by calpain is one of the mechanisms that accounts for the decrease in PMCA2 during EAE since the involvement of calpain has been shown only in spinal cord neurons in vitro, as described above. However, increases in calpain activity in EAE[40,41] and attenuation of axonal injury and EAE symptoms after inhibition of calpain have been reported[42]. Therefore, calpain could be a plausible candidate mediating the reduction in PMCA2 levels, at least partially, in the spinal cord during this disease. Glutamate excitotoxicity and calpain activation have also been implicated in spinal cord injury and could be factors contributing to the decrease in PMCA2[36,43].
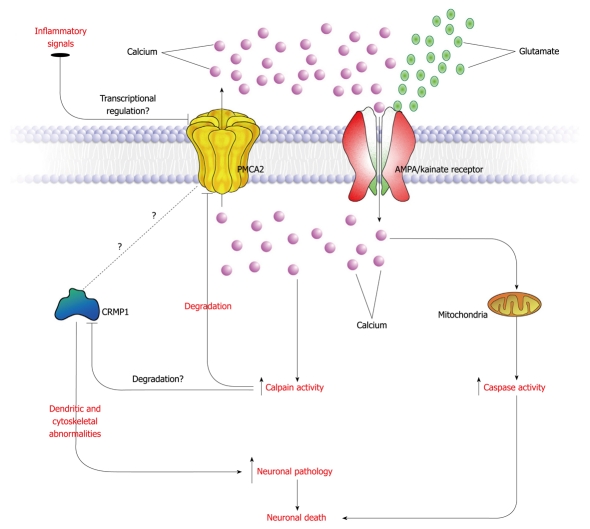
It is worth noting that both PMCA2 protein and mRNA levels are decreased in EAE[10,31]. Moreover, in spinal cord slice cultures, kainic acid causes a reduction in PMCA2 transcript levels[10]. This indicates that additional triggers and mechanisms might be responsible for the reduction in PMCA2 mRNA levels. In addition to glutamate, other substances released by inflammatory cells that infiltrate the spinal cord during EAE or after injury or factors produced by glia might be additional stimuli that modulate PMCA2 levels. A scheme that summarizes a proposed mechanism for PMCA2-mediated neurodegeneration is presented in Figure 1.
Figure 1.
Putative mechanism underlying plasma membrane calcium ATPase 2 (PMCA2)-mediated neuronal damage in the spinal cord during experimental autoimmune encephalomyelitis or after spinal cord injury. Excess glutamate in the affected spinal cord binds neuronal α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate receptors and initiates calcium influx. Inflammatory signals, released by activated glia or invading cells down regulate PMCA2 transcript levels attenuating calcium extrusion. The increased intracellular calcium levels activate proteases such as calpain and apoptotic mechanisms. Calpain-mediated degradation of PMCA2 further reduces PMCA2 levels. Decreased PMCA2 causes a decline in collapsin response mediator protein 1 (CRMP1) levels by mechanisms that are not yet identified. Changes in CRMP1 levels result in neuronal pathology leading to cell death.
CONCLUSION
The aforementioned investigations highlight the critical role of PMCA2 in motor neuron survival. In vitro studies indicate that additional spinal cord neuronal populations are also susceptible to reductions in PMCA2 activity. Further studies are necessary to identify precisely the various neuronal subtypes that are dependent on PMCA2. Comprehensive analysis of PMCA2-null and heterozygous mouse spinal cord and in vivo knockdown experiments could provide insights into these issues.
It is also important to demonstrate directly whether reductions in PMCA2 are causes of neuronal damage in vivo and if restoration of PMCA2 levels reverts neuronal pathology and prevents neuronal loss during EAE and after spinal cord injury. Finally, it is critical to determine the role of other PMCA isoforms and splice variants in the function of the spinal cord in health and disease.
Acknowledgments
We thank Arnaud Nicot, PhD and Emanuel E Strehler, PhD for critically reading the manuscript.
Footnotes
Supported by Grants No. NS046363 from the NIH and No. 08-3073-SCR-E-0 from NJCSCR
Peer reviewers: Tadahiro Numakawa, PhD, Department of Mental Disorder Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, 4-1-1, Ogawa-Higashi, Kodaira, Tokyo 187-8502, Japan; Maik Behrens, PhD, Department Molecular Genetics, German Institute of Human Nutrition Potsdam-Rehbruecke, Arthur-Scheunert-Allee 114-116, Nuthetal, 14558, Germany
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
References
- 1.Brini M. Plasma membrane Ca(2+)-ATPase: from a housekeeping function to a versatile signaling role. Pflugers Arch. 2009;457:657–664. doi: 10.1007/s00424-008-0505-6. [DOI] [PubMed] [Google Scholar]
- 2.Tempel BL, Shilling DJ. The plasma membrane calcium ATPase and disease. Subcell Biochem. 2007;45:365–383. doi: 10.1007/978-1-4020-6191-2_13. [DOI] [PubMed] [Google Scholar]
- 3.Kurnellas MP, Nicot A, Shull GE, Elkabes S. Plasma membrane calcium ATPase deficiency causes neuronal pathology in the spinal cord: a potential mechanism for neurodegeneration in multiple sclerosis and spinal cord injury. FASEB J. 2005;19:298–300. doi: 10.1096/fj.04-2549fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurnellas MP, Li H, Jain MR, Giraud SN, Nicot AB, Ratnayake A, Heary RF, Elkabes S. Reduced expression of plasma membrane calcium ATPase 2 and collapsin response mediator protein 1 promotes death of spinal cord neurons. Cell Death Differ. 2010:Epub ahead of print. doi: 10.1038/cdd.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerini D. The significance of the isoforms of plasma membrane calcium ATPase. Cell Tissue Res. 1998;292:191–197. doi: 10.1007/s004410051050. [DOI] [PubMed] [Google Scholar]
- 6.Olson S, Wang MG, Carafoli E, Strehler EE, McBride OW. Localization of two genes encoding plasma membrane Ca2(+)-transporting ATPases to human chromosomes 1q25-32 and 12q21-23. Genomics. 1991;9:629–641. doi: 10.1016/0888-7543(91)90356-j. [DOI] [PubMed] [Google Scholar]
- 7.Wang MG, Yi H, Hilfiker H, Carafoli E, Strehler EE, McBride OW. Localization of two genes encoding plasma membrane Ca2+ ATPases isoforms 2 (ATP2B2) and 3 (ATP2B3) to human chromosomes 3p26-->p25 and Xq28, respectively. Cytogenet Cell Genet. 1994;67:41–45. doi: 10.1159/000133794. [DOI] [PubMed] [Google Scholar]
- 8.Brandt P, Neve RL. Expression of plasma membrane calcium-pumping ATPase mRNAs in developing rat brain and adult brain subregions: evidence for stage-specific expression. J Neurochem. 1992;59:1566–1569. doi: 10.1111/j.1471-4159.1992.tb08476.x. [DOI] [PubMed] [Google Scholar]
- 9.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Nicot A, Ratnakar PV, Ron Y, Chen CC, Elkabes S. Regulation of gene expression in experimental autoimmune encephalomyelitis indicates early neuronal dysfunction. Brain. 2003;126:398–412. doi: 10.1093/brain/awg041. [DOI] [PubMed] [Google Scholar]
- 11.Tachibana T, Noguchi K, Ruda MA. Analysis of gene expression following spinal cord injury in rat using complementary DNA microarray. Neurosci Lett. 2002;327:133–137. doi: 10.1016/s0304-3940(02)00375-0. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana T, Ogura H, Tokunaga A, Dai Y, Yamanaka H, Seino D, Noguchi K. Plasma membrane calcium ATPase expression in the rat spinal cord. Brain Res Mol Brain Res. 2004;131:26–32. doi: 10.1016/j.molbrainres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Di Leva F, Domi T, Fedrizzi L, Lim D, Carafoli E. The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch Biochem Biophys. 2008;476:65–74. doi: 10.1016/j.abb.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Zacharias DA, Dalrymple SJ, Strehler EE. Transcript distribution of plasma membrane Ca2+ pump isoforms and splice variants in the human brain. Brain Res Mol Brain Res. 1995;28:263–272. doi: 10.1016/0169-328x(94)00215-z. [DOI] [PubMed] [Google Scholar]
- 15.Burette AC, Strehler EE, Weinberg RJ. "Fast" plasma membrane calcium pump PMCA2a concentrates in GABAergic terminals in the adult rat brain. J Comp Neurol. 2009;512:500–513. doi: 10.1002/cne.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 17.Morgans CW, El Far O, Berntson A, Wässle H, Taylor WR. Calcium extrusion from mammalian photoreceptor terminals. J Neurosci. 1998;18:2467–2474. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–237. doi: 10.1016/s0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 19.Krizaj D, Demarco SJ, Johnson J, Strehler EE, Copenhagen DR. Cell-specific expression of plasma membrane calcium ATPase isoforms in retinal neurons. J Comp Neurol. 2002;451:1–21. doi: 10.1002/cne.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pottorf WJ, Thayer SA. Transient rise in intracellular calcium produces a long-lasting increase in plasma membrane calcium pump activity in rat sensory neurons. J Neurochem. 2002;83:1002–1008. doi: 10.1046/j.1471-4159.2002.01221.x. [DOI] [PubMed] [Google Scholar]
- 21.Thayer SA, Usachev YM, Pottorf WJ. Modulating Ca2+ clearance from neurons. Front Biosci. 2002;7:d1255–d1279. doi: 10.2741/A838. [DOI] [PubMed] [Google Scholar]
- 22.Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, et al. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- 23.Souayah N, Sharovetskaya A, Kurnellas MP, Myerson M, Deitch JS, Elkabes S. Reductions in motor unit number estimates (MUNE) precede motor neuron loss in the plasma membrane calcium ATPase 2 (PMCA2)-heterozygous mice. Exp Neurol. 2008;214:341–346. doi: 10.1016/j.expneurol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Y, Ihara Y. Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J Biol Chem. 2000;275:17917–17920. doi: 10.1074/jbc.C000179200. [DOI] [PubMed] [Google Scholar]
- 25.Fukata Y, Itoh TJ, Kimura T, Ménager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4:583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- 26.Su KY, Chien WL, Fu WM, Yu IS, Huang HP, Huang PH, Lin SR, Shih JY, Lin YL, Hsueh YP, et al. Mice deficient in collapsin response mediator protein-1 exhibit impaired long-term potentiation and impaired spatial learning and memory. J Neurosci. 2007;27:2513–2524. doi: 10.1523/JNEUROSCI.4497-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita N, Morita A, Uchida Y, Nakamura F, Usui H, Ohshima T, Taniguchi M, Honnorat J, Thomasset N, Takei K, et al. Regulation of spine development by semaphorin3A through cyclin-dependent kinase 5 phosphorylation of collapsin response mediator protein 1. J Neurosci. 2007;27:12546–12554. doi: 10.1523/JNEUROSCI.3463-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. doi: 10.1016/s0896-6273(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 29.Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 30.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 31.Nicot A, Kurnellas M, Elkabes S. Temporal pattern of plasma membrane calcium ATPase 2 expression in the spinal cord correlates with the course of clinical symptoms in two rodent models of autoimmune encephalomyelitis. Eur J Neurosci. 2005;21:2660–2670. doi: 10.1111/j.1460-9568.2005.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 33.Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- 34.Elkabes S, Nicot A. Aberrant calcium extrusion mechanisms may contribute to secondary spinal cord injury. J Neurol 2003; 250(S2): 179, Galante A, Soteropoulos P, Tolias P, Recce M, Young W, Hart RP. Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiol Genomics. 2001;7:201–213. doi: 10.1152/physiolgenomics.00074.2001. [DOI] [PubMed] [Google Scholar]
- 35.Elkabes S, Nicot A. Aberrant calcium extrusion mechanisms may contribute to secondary spinal cord injury. J Neurol. 2003;250 Suppl 2:179. [Google Scholar]
- 36.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 37.Werner P, Pitt D, Raine CS. Glutamate excitotoxicity--a mechanism for axonal damage and oligodendrocyte death in Multiple Sclerosis? J Neural Transm Suppl. 2000:375–385. doi: 10.1007/978-3-7091-6301-6_27. [DOI] [PubMed] [Google Scholar]
- 38.Garcia ML, Murray KD, Garcia VB, Strehler EE, Isackson PJ. Seizure-induced alterations of plasma membrane calcium ATPase isoforms 1, 2 and 3 mRNA and protein in rat hippocampus. Brain Res Mol Brain Res. 1997;45:230–238. doi: 10.1016/s0169-328x(96)00253-7. [DOI] [PubMed] [Google Scholar]
- 39.Pottorf WJ 2nd, Johanns TM, Derrington SM, Strehler EE, Enyedi A, Thayer SA. Glutamate-induced protease-mediated loss of plasma membrane Ca2+ pump activity in rat hippocampal neurons. J Neurochem. 2006;98:1646–1656. doi: 10.1111/j.1471-4159.2006.04063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guyton MK, Wingrave JM, Yallapragada AV, Wilford GG, Sribnick EA, Matzelle DD, Tyor WR, Ray SK, Banik NL. Upregulation of calpain correlates with increased neurodegeneration in acute experimental auto-immune encephalomyelitis. J Neurosci Res. 2005;81:53–61. doi: 10.1002/jnr.20470. [DOI] [PubMed] [Google Scholar]
- 41.Shields DC, Banik NL. Upregulation of calpain activity and expression in experimental allergic encephalomyelitis: a putative role for calpain in demyelination. Brain Res. 1998;794:68–74. doi: 10.1016/s0006-8993(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 42.Hassen GW, Feliberti J, Kesner L, Stracher A, Mokhtarian F. Prevention of axonal injury using calpain inhibitor in chronic progressive experimental autoimmune encephalomyelitis. Brain Res. 2008;1236:206–215. doi: 10.1016/j.brainres.2008.07.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ray SK, Matzelle DD, Sribnick EA, Guyton MK, Wingrave JM, Banik NL. Calpain inhibitor prevented apoptosis and maintained transcription of proteolipid protein and myelin basic protein genes in rat spinal cord injury. J Chem Neuroanat. 2003;26:119–124. doi: 10.1016/s0891-0618(03)00044-9. [DOI] [PubMed] [Google Scholar]