Abstract
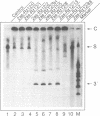
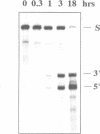
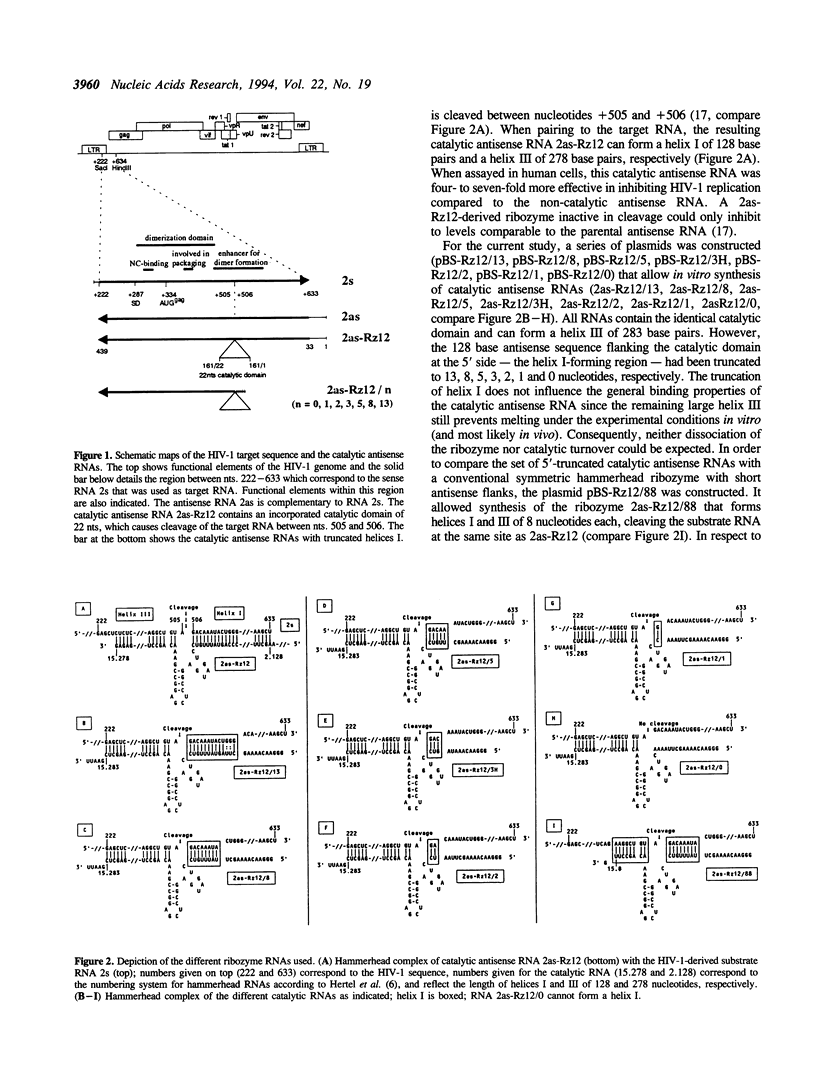
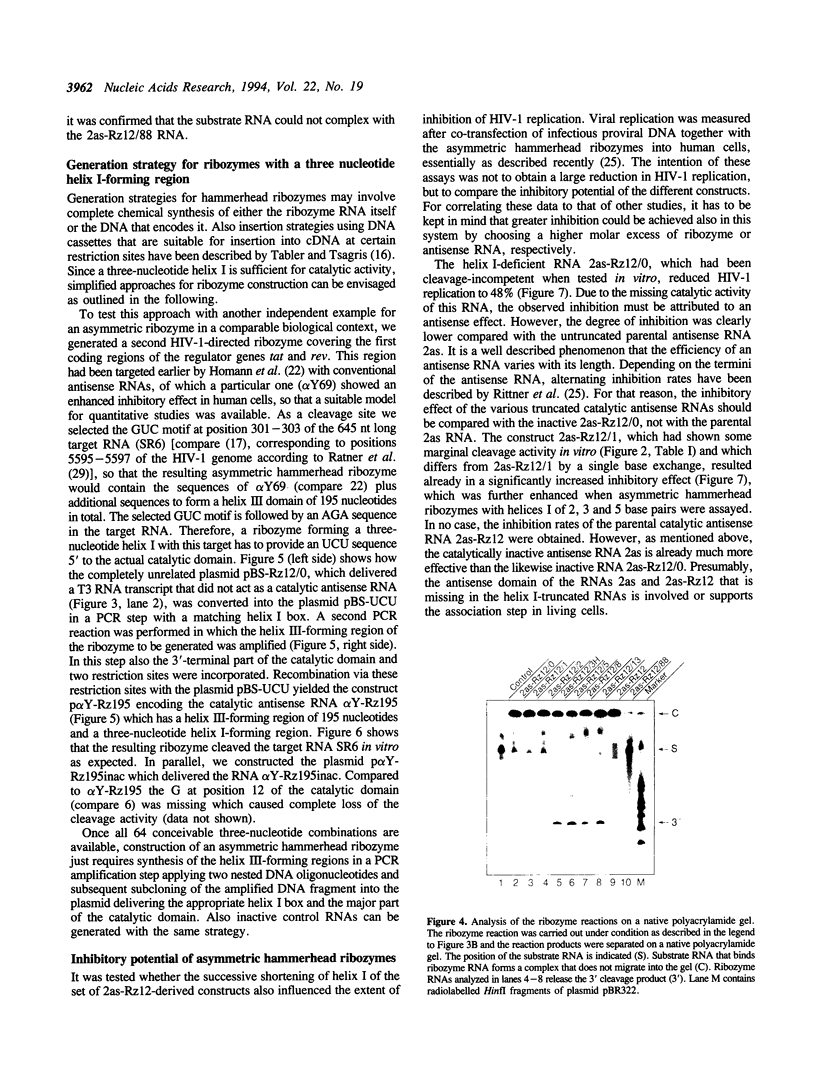
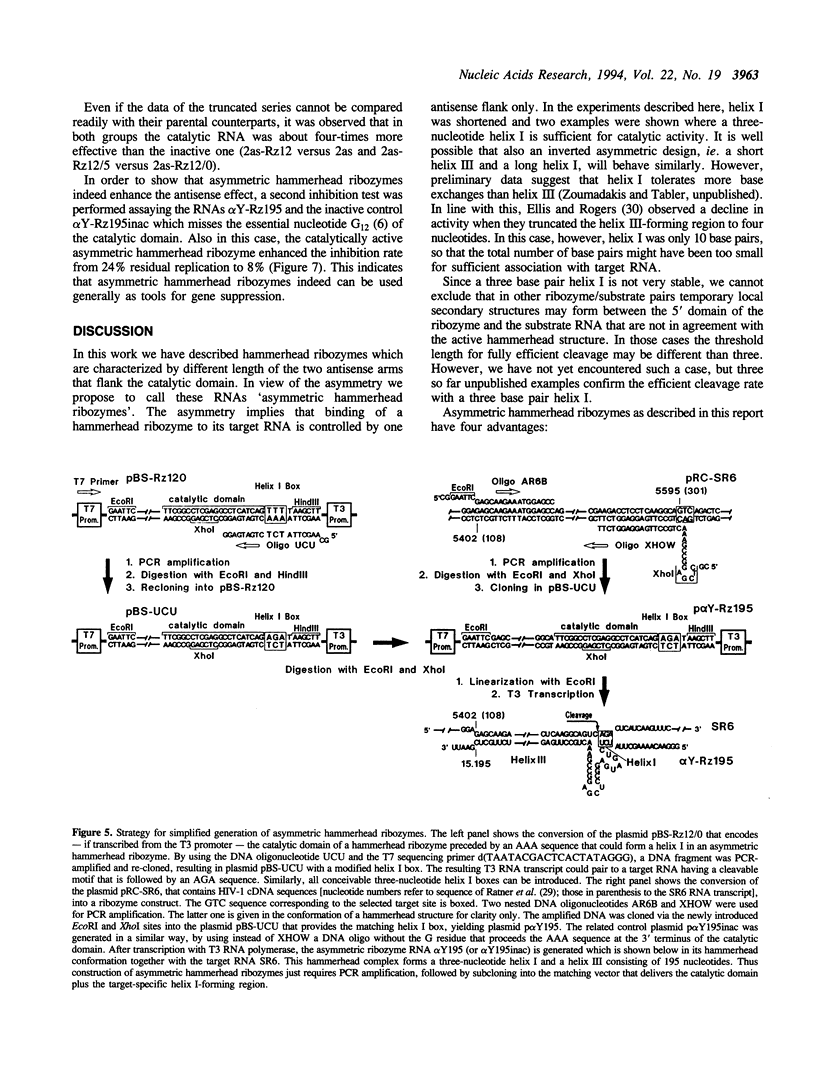
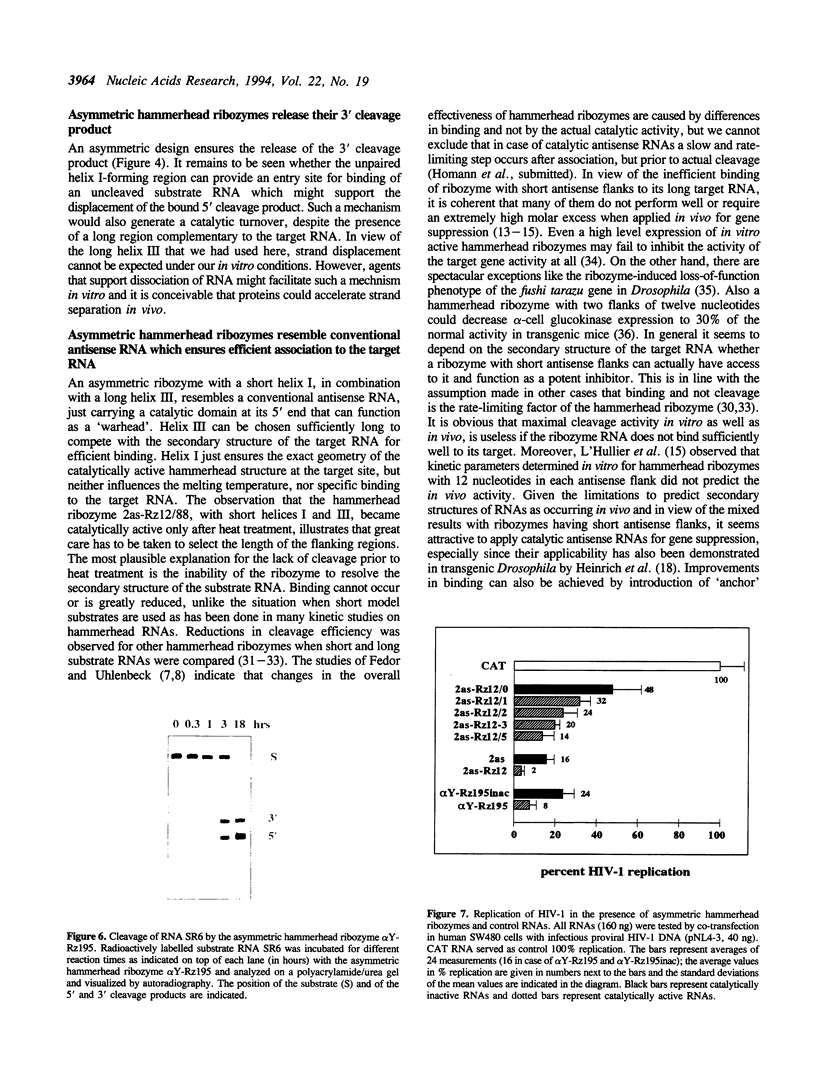
Trans-cleaving hammerhead ribozymes with long target-specific antisense sequences flanking the catalytic domain share some features with conventional antisense RNA and are therefore termed 'catalytic antisense RNAs'. Sequences 5' to the catalytic domain form helix I and sequences 3' to it form helix III when complexed with the target RNA. A catalytic antisense RNA of more than 400 nucleotides, and specific for the human immunodeficiency virus type 1 (HIV-1), was systematically truncated within the arm that constituted originally a helix I of 128 base pairs. The resulting ribozymes formed helices I of 13, 8, 5, 3, 2, 1 and 0 nucleotides, respectively, and a helix III of about 280 nucleotides. When their in vitro cleavage activity was compared with the original catalytic antisense RNA, it was found that a helix I of as little as three nucleotides was sufficient for full endonucleolytic activity. The catalytically active constructs inhibited HIV-1 replication about four-fold more effectively than the inactive ones when tested in human cells. A conventional hammerhead ribozyme having helices of just 8 nucleotides on either side failed to cleave the target RNA in vitro when tested under the conditions for catalytic antisense RNA. Cleavage activity could only be detected after heat-treatment of the ribozyme substrate mixture which indicates that hammerhead ribozymes with short arms do not associate as efficiently to the target RNA as catalytic antisense RNA. The requirement of just a three-nucleotide helix I allows simple PCR-based generation strategies for asymmetric hammerhead ribozymes. Advantages of an asymmetric design will be discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E., Pictet R., Grange T. Can hammerhead ribozymes be efficient tools to inactivate gene function? Nucleic Acids Res. 1994 Feb 11;22(3):293–300. doi: 10.1093/nar/22.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening G. Compilation of self-cleaving sequences from plant virus satellite RNAs and other sources. Methods Enzymol. 1989;180:546–558. doi: 10.1016/0076-6879(89)80123-5. [DOI] [PubMed] [Google Scholar]
- Cameron F. H., Jennings P. A. Specific gene suppression by engineered ribozymes in monkey cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9139–9143. doi: 10.1073/pnas.86.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Schaffner G., Birnstiel M. L. Ribozyme, antisense RNA, and antisense DNA inhibition of U7 small nuclear ribonucleoprotein-mediated histone pre-mRNA processing in vitro. Mol Cell Biol. 1989 Oct;9(10):4479–4487. doi: 10.1128/mcb.9.10.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisell P., Thompson S., James W. Inhibition of HIV-1 replication by ribozymes that show poor activity in vitro. Nucleic Acids Res. 1993 Nov 11;21(22):5251–5255. doi: 10.1093/nar/21.22.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman R. B. Cleavage of full-length beta APP mRNA by hammerhead ribozymes. Nucleic Acids Res. 1993 Aug 25;21(17):4119–4125. doi: 10.1093/nar/21.17.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Leiser M., Wu Y. J., Fusco-DeMane D., Emran O. A., Surana M., Jetton T. L., Magnuson M. A., Weir G., Fleischer N. Ribozyme-mediated attenuation of pancreatic beta-cell glucokinase expression in transgenic mice results in impaired glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2051–2055. doi: 10.1073/pnas.91.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J., Rogers J. Design and specificity of hammerhead ribozymes against calretinin mRNA. Nucleic Acids Res. 1993 Nov 11;21(22):5171–5178. doi: 10.1093/nar/21.22.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Kinetics of intermolecular cleavage by hammerhead ribozymes. Biochemistry. 1992 Dec 8;31(48):12042–12054. doi: 10.1021/bi00163a012. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Substrate sequence effects on "hammerhead" RNA catalytic efficiency. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1668–1672. doi: 10.1073/pnas.87.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987 Jul 3;50(1):9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- Goodchild J., Kohli V. Ribozymes that cleave an RNA sequence from human immunodeficiency virus: the effect of flanking sequence on rate. Arch Biochem Biophys. 1991 Feb 1;284(2):386–391. doi: 10.1016/0003-9861(91)90313-8. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Heidenreich O., Eckstein F. Hammerhead ribozyme-mediated cleavage of the long terminal repeat RNA of human immunodeficiency virus type 1. J Biol Chem. 1992 Jan 25;267(3):1904–1909. [PubMed] [Google Scholar]
- Heinrich J. C., Tabler M., Louis C. Attenuation of white gene expression in transgenic Drosophila melanogaster: possible role of a catalytic antisense RNA. Dev Genet. 1993;14(4):258–265. doi: 10.1002/dvg.1020140403. [DOI] [PubMed] [Google Scholar]
- Hertel K. J., Herschlag D., Uhlenbeck O. C. A kinetic and thermodynamic framework for the hammerhead ribozyme reaction. Biochemistry. 1994 Mar 22;33(11):3374–3385. doi: 10.1021/bi00177a031. [DOI] [PubMed] [Google Scholar]
- Hertel K. J., Pardi A., Uhlenbeck O. C., Koizumi M., Ohtsuka E., Uesugi S., Cedergren R., Eckstein F., Gerlach W. L., Hodgson R. Numbering system for the hammerhead. Nucleic Acids Res. 1992 Jun 25;20(12):3252–3252. doi: 10.1093/nar/20.12.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M., Rittner K., Sczakiel G. Complementary large loops determine the rate of RNA duplex formation in vitro in the case of an effective antisense RNA directed against the human immunodeficiency virus type 1. J Mol Biol. 1993 Sep 5;233(1):7–15. doi: 10.1006/jmbi.1993.1480. [DOI] [PubMed] [Google Scholar]
- Homann M., Tzortzakaki S., Rittner K., Sczakiel G., Tabler M. Incorporation of the catalytic domain of a hammerhead ribozyme into antisense RNA enhances its inhibitory effect on the replication of human immunodeficiency virus type 1. Nucleic Acids Res. 1993 Jun 25;21(12):2809–2814. doi: 10.1093/nar/21.12.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M., Ohtsuka E. Effects of phosphorothioate and 2-amino groups in hammerhead ribozymes on cleavage rates and Mg2+ binding. Biochemistry. 1991 May 28;30(21):5145–5150. doi: 10.1021/bi00235a005. [DOI] [PubMed] [Google Scholar]
- L'Huillier P. J., Davis S. R., Bellamy A. R. Cytoplasmic delivery of ribozymes leads to efficient reduction in alpha-lactalbumin mRNA levels in C127I mouse cells. EMBO J. 1992 Dec;11(12):4411–4418. doi: 10.1002/j.1460-2075.1992.tb05541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini L., Axelos M., Lescure N., Yot P. Assaying synthetic ribozymes in plants: high-level expression of a functional hammerhead structure fails to inhibit target gene activity in transiently transformed protoplasts. Plant Mol Biol. 1992 Nov;20(4):715–731. doi: 10.1007/BF00046456. [DOI] [PubMed] [Google Scholar]
- Pachuk C. J., Yoon K., Moelling K., Coney L. R. Selective cleavage of bcr-abl chimeric RNAs by a ribozyme targeted to non-contiguous sequences. Nucleic Acids Res. 1994 Feb 11;22(3):301–307. doi: 10.1093/nar/22.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieken W. A., Olsen D. B., Benseler F., Aurup H., Eckstein F. Kinetic characterization of ribonuclease-resistant 2'-modified hammerhead ribozymes. Science. 1991 Jul 19;253(5017):314–317. doi: 10.1126/science.1857967. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rittner K., Burmester C., Sczakiel G. In vitro selection of fast-hybridizing and effective antisense RNAs directed against the human immunodeficiency virus type 1. Nucleic Acids Res. 1993 Mar 25;21(6):1381–1387. doi: 10.1093/nar/21.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner K., Sczakiel G. Identification and analysis of antisense RNA target regions of the human immunodeficiency virus type 1. Nucleic Acids Res. 1991 Apr 11;19(7):1421–1426. doi: 10.1093/nar/19.7.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczakiel G., Pawlita M., Kleinheinz A. Specific inhibition of human immunodeficiency virus type 1 replication by RNA transcribed in sense and antisense orientation from the 5'-leader/gag region. Biochem Biophys Res Commun. 1990 Jun 15;169(2):643–651. doi: 10.1016/0006-291x(90)90379-2. [DOI] [PubMed] [Google Scholar]
- Steinecke P., Herget T., Schreier P. H. Expression of a chimeric ribozyme gene results in endonucleolytic cleavage of target mRNA and a concomitant reduction of gene expression in vivo. EMBO J. 1992 Apr;11(4):1525–1530. doi: 10.1002/j.1460-2075.1992.tb05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Tsagris M. Catalytic antisense RNAs produced by incorporating ribozyme cassettes into cDNA. Gene. 1991 Dec 15;108(2):175–183. doi: 10.1016/0378-1119(91)90432-b. [DOI] [PubMed] [Google Scholar]
- Tsagris M., Tabler M., Mühlbach H. P., Sänger H. L. Linear oligomeric potato spindle tuber viroid (PSTV) RNAs are accurately processed in vitro to the monomeric circular viroid proper when incubated with a nuclear extract from healthy potato cells. EMBO J. 1987 Aug;6(8):2173–2183. doi: 10.1002/j.1460-2075.1987.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Zhao J. J., Pick L. Generating loss-of-function phenotypes of the fushi tarazu gene with a targeted ribozyme in Drosophila. Nature. 1993 Sep 30;365(6445):448–451. doi: 10.1038/365448a0. [DOI] [PubMed] [Google Scholar]